Abstract
Background:
Gastroesophageal reflux disease (GERD) is prevalent in morbidly obese patients, and its severity appears to correlate with body mass index (BMI).
Aim:
The aim of this study is to investigate the status of GERD after laparoscopic sleeve gastrectomy (LSG).
Materials and Methods:
A prospectively maintained database of all the patients who underwent LSG from February 2008 to May 2011 was reviewed.
Results:
A total of 131 patients were included. The mean age and the BMI of the patients were 49.4 years and 48.9 kg/m2, respectively. Prior to LSG, subjective reflux symptoms were reported in 67 (51%) patients. Anatomical presence of hiatal hernia was endoscopically confirmed in 35 (52%) patients who reported reflux symptoms prior to LSG. All these patients underwent simultaneous hiatal hernia repair during their LSG. The overall mean operative time was 106 min (range: 48-212 min). There were no intra- and 30-day postoperative complications. Out of the 67 preoperative reflux patients, 32 (47.7%) reported resolution of their symptoms after the operation, 20 (29.9%) reported clinical improvement, and 12 (22.2%) reported unchanged or persistent symptoms. Three patients developed new-onset reflux symptoms, which were easily controlled with proton pump inhibitors. No patient required conversion to gastric bypass or duodenal switch because of the severe reflux symptoms. At 18 months, the follow-up data were available in 60% of the total patients.
Conclusion:
LSG results in resolution or improvement of the reflux symptoms in a large number of patients. Proper patient selection, complete preoperative evaluation to identify the presence of hiatal hernia, and good surgical techniques are the keys to achieve optimal outcomes.
Keywords: Gastroesophageal reflux symptoms, hiatal hernia, laparoscopic sleeve gastrectomy (LSG)
Introduction
Gastroesophageal reflux disease (GERD) is prevalent in morbidly obese patients, reportedly as high as 70%, with a severity that appears to correlate to body mass index (BMI) and waist circumference.[1] Symptomatic hiatal hernia is recorded in 15% of patients with a BMI of >35kg/m2.[2] In the surgical literatures and clinical practice, it is well-known that Roux-en-Y gastric bypass (RYGB) produces marked clinical improvements in GERD symptoms. Many surgeons consider RYGB to be the ideal antireflux operation for obese patients with GERD.[3]
In the last decade, sleeve gastrectomy has gained worldwide popularity due to its excellent weight loss and metabolic results via a combination of restrictive and hormonal effects, while preserving gastrointestinal tract anatomy.[4] It is a technically less complex operation to perform, which carries less perioperative morbidity and mortality than RYGB. Sleeve gastrectomy also appears safer in the long term, with a lower risk of adverse nutritional consequences and surgical complications (specifically marginal ulceration and internal herniation). Concerns and debates, however, have been raised on the potential increased risk of de novo postoperative GERD, which is related to the gastric fundus removal, division of the gastroesophageal junction (GEJ) muscular fibers, reduced antral pump action, significantly reduced gastric reservoir volume, and the presence of high pressure zone in the proximal gastric sleeve.[5]
Some experts even consider GERD to be a contraindication for sleeve gastrectomy. In the 2012 International Sleeve Gastrectomy Expert Panel Consensus Statement, 57% of the panelists agreed that GERD is a relative contraindication for sleeve gastrectomy.[4] The second International Sleeve Gastrectomy Summit found that 6.5% (range: 0-85%) of the patients who have undergone sleeve gastrectomy experienced postoperative GERD.[6] However, the presently available data on the effect of sleeve gastrectomy on postoperative GERD are conflicting and difficult to interpret. The criteria used for the diagnosis of GERD are not always clear; routine use of preoperative endoscopy is nonstandard; the lack of objective evidences of GERD during pH study and manometry in many studies, and lastly, the surgical techniques used vary widely among the bariatric surgeons.
In morbidly obese patients, oftentimes RYGB is not feasible (in case of patients with extremely high BMI or extensive intra-abdominal adhesions from previous operations) or not suitable (Crohn's disease, pernicious anemia, etc.). These contraindications leave sleeve gastrectomy as the only viable surgical option, despite known concerns and debates of the possible GERD symptoms after operation. Therefore, in this study, we investigated the status of the reflux symptoms after laparoscopic sleeve gastrectomy (LSG) for the treatment of morbid obesity.
Materials and Methods
A prospectively maintained database of all the consecutive patients who underwent LSG from February 2008 to May 2011 was retrospectively reviewed. Prior to starting the study, approval from the institutional review board was obtained.
Patient demographic data include age, sex, gender, BMI, preoperative excess body weight, and the number of obesity-related comorbidities (e.g., diabetes mellitus, hypertension, hyperlipidemia, obstructive sleep apnea, pulmonary hypertension, osteoarthritis, pseudotumor cerebri, GERD, polycystic ovarian syndrome, nonalcoholic steatohepatitis, depression, and stress urinary incontinence). The pre- and postoperative symptoms of heartburn, reflux, or GERD were determined by the patient's subjective descriptions. The patients with severe reflux symptoms or known large hiatal hernias were offered to pursue RYGB. In addition to standard nutritional and psychiatric evaluations, we also performed routine preoperative esophagogastroduodenoscopy (EGD) in all the patients to rule out the presence of any subclinical esophageal and gastric disorders. Careful attention was paid to identify the presence of hiatal hernia during endoscopic retroflexion, which is uniformly repaired during the operation. In most of the cases, hiatal hernia repair was accomplished by the placement of several posterior interrupted nonabsorbable sutures. Routine mesh reinforcement was not part of the hiatal hernia repair. Loose sleeve gastrectomy was performed using a 36-Fr bougie (ConMed Endosurgery, Utica, New York, USA), starting approximately 4-6 cm from the pylorus. The staple line was either reinforced using Seamguard; (Gore, Flagstaff, AZ, US) or suture imbricated using Endo Stitch™ (Covidien, Norwalk, CT, US) based on the surgeon's preference. The major complications were defined as intraluminal/intra-abdominal bleeding, staple line leak, sleeve stricture, and other organ injury. All the patients were pre- and postoperatively interviewed to evaluate their reflux symptoms.
Results
A total of 131 patients, 98 women, and 33 men were included in this study. The mean age and the BMI of the patients were 49.4 years (range: 17-79 years) and 48.9 kg/m2 (range: 34-84 kg/m2), respectively, [Table 1]. The total number of obesity-related comorbidities was 6.2 (range: 3-10). Prior to LSG, the subjective reflux symptoms were reported in 67 (51%) patients. All the patients underwent routine preoperative upper endoscopy prior to their LSG. Anatomical presence of hiatal hernia was endoscopically confirmed in 35 (52%) patients who reported reflux symptoms prior to LSG. All these patients underwent simultaneous hiatal hernia repair during their LSG [Diagram 1]. The overall mean operative time was 106 min (range: 48-212 min). There were no major intra- and 30-day postoperative complications. Out of the 67 preoperative reflux patients, 32 (47.7%) reported resolution of their symptoms after the operation, 20 (29.9%) reported clinical improvement, and 12 (22.2%) reported unchanged or persistent symptoms. Three patients developed new-onset reflux symptoms, which were easily controlled with proton pump inhibitors [Figure 1]. No patient required conversion to RYGB or duodenal switch because of the severe reflux symptoms. At 18 months, the follow-up data were available in 60% of the total patients. The average excess body weight loss at 1 month, 3 months, 6 months, 9 months, 12 months, and 18 months were 19.3%, 31.2%, 44.5%, 50.2%, 57.1%, and 63.6%, respectively. No mortality occurred in this series.
Table 1.
Patients’ demographics
Diagram 1.
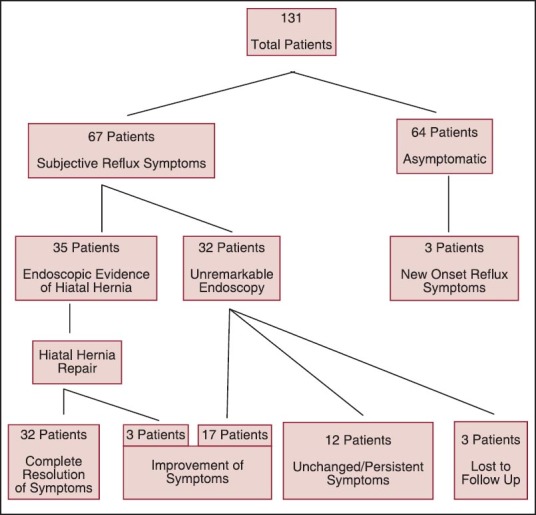
Patient distribution based on subjective and endoscopic findings
Figure 1.
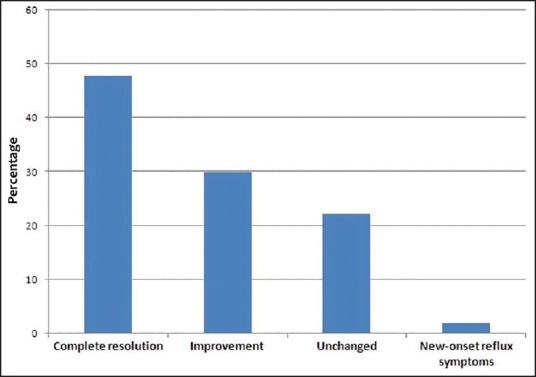
Gastroesophageal reflux symptoms after LSG
Discussion
A systematic review by Chiu et al. examined 15 studies, which included GERD as either a primary or a secondary outcome measure after sleeve gastrectomy. Out of these studies, four were found to have an increased prevalence of GERD while seven showed a reduced prevalence of GERD after sleeve gastrectomy.[7,8] Despite the conflicting concerns of the increased GERD symptoms postoperatively, LSG continues to gain popularity in the United States and worldwide, surpassing the current gold standard RYGB. Many experts believe that correctly fashioned and well-performed sleeve gastrectomy should not cause postoperative GERD symptoms.[9]
In a sleeve gastrectomy series by Daes et al., hiatal hernia was intraoperatively detected in approximately 25% of the patients.[9] Hiatal hernia is commonly associated with GERD. Therefore, Daes et al. and Soricelli et al.,[2,9,10] have suggested the importance of careful identification of hiatal hernia and repair of the crural defect during LSG in order to reduce the incidence of postoperative reflux and esophagitis. About a quarter of their patients underwent concomitant hiatal hernia repair. With appropriate closure of the crural defects, only 1.5% of the patients experienced symptoms of GERD in the follow-up at 6-12 months.[9] Later in their experience, with more complete removal of the gastric fundus, routine correction of hiatal hernia, and avoidance of narrowing/torsion of the sleeve, a sharp decrease occurred in the need for postoperative endoscopy to investigate the symptoms of GERD and food intolerance. The patients with severe persistent GERD underwent remedial operations, which included repair of hiatal hernia and reformation of the sleeve. These result in excellent symptomatic relief without any complication. In this study, we found parallel results using similar preoperative workups and operative principles.
Several contributing factors to the success or failure after sleeve gastrectomy have been described and debated in the surgical literatures. Yehoshua et al.[11] reported that intraluminal basal pressure in the gastric sleeve is similar to the stomach, but the pressures were significantly higher on occlusion and filling with saline that implies much less distensibility of the sleeve. Higher pressures and lesser distensibility explain the adverse impact on GERD symptoms after sleeve gastrectomy. Once the stomach becomes full (approximately 100-200 mL in volume), the intraluminal pressure increases according to Laplace's law. Part of the ingested bolus impacts against the elevated gastric pressure and regurgitates back into the distal esophagus (no signs of esophageal acidification in this situation). This phenomenon, which is related to transient intraluminal stasis and not de novo GERD, is sometimes incorrectly interpreted at standard pH monitoring as GERD. Himpens et al.[12] observed that increasing sleeve compliance with the passage of time contributes to gradual symptomatic improvements in many patients, up to the third postoperative year.
Reduced acid production due to the reduction of G cell and parietal cell masses after sleeve gastrectomy could account for some of the beneficial effects seen on GERD. There is no published study that has specifically examined and objectively documented this aspect in morbidly obese patients undergoing sleeve gastrectomy.[8]
Delayed gastric emptying is associated with the development of GERD.[13,14] Alterations in gastric emptying after sleeve gastrectomy could, therefore, be potentially important in determining GERD symptoms postoperatively. Melissas et al.[15] proposed that faster gastric emptying for solids but slower for liquids after sleeve gastrectomy could be due to excision of the fundus, absence of receptive relaxation, and alterations in contractility of the proximal stomach. They further added that distension of the antrum due to absence of the fundus could be the dominant mechanism behind reduced hunger and increased satiety in these patients. In their study, only one out of eight patients with preexisting GERD reported increased symptoms and two patients developed new GERD symptoms after sleeve gastrectomy. This clearly indicates that there are other factors besides gastric emptying that play a role in the development of postoperative GERD symptoms.
Distance from the pylorus, bougie size, and distance from the angle of His have been widely discussed in the literature and meetings. These technical factors may be determinants for the development of GERD after sleeve gastrectomy. It is conceivable that the proximity of initial staple firing to the pylorus and use of small caliber bougie lead to the reduced compliance of the gastric sleeve and therefore, increased the incidence of the reflux. Carter and Keider et al.[16,17] reported significantly increased postoperative reflux symptoms in patients who underwent sleeve gastrectomy using 32-34-Fr bougies. A small bougie size (<40 Fr) may reduce the final luminal volume and potentially increase the risk of developing GERD. Starting distance of 1-2 cm from the pylorus also seems to contribute to the increased reflux symptoms, likely caused by the relative functional obstruction in the distal sleeve. Using traditional manometry, Braghetto et al.[18] concluded that lower esophageal sphincter pressure was reduced after sleeve gastrectomy due to the division of the sling muscular fibers at the GEJ when stapling in proximity to the angle of His. Therefore, it is advisable to drive the last stapler to the left, aiming to keep it at least 1 cm away from the GEJ.
In our study, 60% of the patients were available for follow-up in 18 months. The average excess body weight loss at 1 month, 3 months, 6 months, 9 months, 12 months, and 18 months were 19.3%, 31.2%, 44.5%, 50.2%, 57.1%, and 63.6%, respectively. This is comparable to the weight loss result reported in the bariatric literature. We did not find any major complications specific to the sleeve gastrectomy such as intraluminal/intra-abdominal bleeding, staple line leak, sleeve stricture, and other organ injury. The three patients who postoperatively developed new-onset reflux symptoms were completely managed with oral proton pump inhibitor without any need for reoperation. These patients could have had undetected esophageal motility disorder preoperatively, which became unmasked after sleeve gastrectomy. Finally, appropriate patient selection, complete preoperative evaluation that includes aggressive identification of hiatal hernia, and proper surgical techniques as described above are the keys to avoid complications and achieve good outcomes.
Limitations of our study include subjective measurements of the GERD symptoms based on individual complaints, which could widely vary among the patients. The pre- and postoperative objective measurements, using 24-h pH study to determine the DeMeester scores, and a high resolution esophageal manometry were not available. We had 18-month follow-up data in 60% of the patients, which could probably be improved with more aggressive follow-ups. Further studies, which include objective measurements of the pre- and postoperative reflux symptoms, would be important to better characterize the factors leading to either suboptimal or excellent outcomes after LSG.
Conclusions
In our series, substantial prevalence of GERD symptoms and hiatal hernia is seen in morbidly obese patients who underwent LSG for the treatment of their morbid obesity. Despite mixed initial reports regarding the increased GERD symptoms following sleeve gastrectomy, we found a significant number of patients resolving or improving their reflux symptoms after LSG. We believe that appropriate patient selection, complete preoperative evaluation that includes aggressive identification of hiatal hernia, and proper surgical techniques are crucial to achieve optimal patient outcomes. Further studies with objective measurements of the pre- and postoperative reflux symptoms are necessary.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Prachand VN, Alverdy JC. Gastroesophageal reflux disease and severe obesity: Fundoplication or bariatric surgery? Wold J Gastroenterol. 2010;16:3757–61. doi: 10.3748/wjg.v16.i30.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soricelli E, Iossa A, Casella G, Abbatini F, Cali B, Basso N. Sleeve gastrectomy and crural repair in obese patients with gastroesophageal reflux disease and/or hiatal hernia. Surg Obes Relat Dis. 2013;9:356–61. doi: 10.1016/j.soard.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Varela JE, Hinojosa MW, Nguyen NT. Laparoscopic fundoplication compared with laparoscopic gastric bypass in morbidly obese patients with gastroesophageal reflux disease. Surg Obes Relat Dis. 2009;5:139–43. doi: 10.1016/j.soard.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, et al. International Sleeve Gastrectomy Expert Panel. International Sleeve Gastrectomy Expert Panel Consensus Statement: Best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8–19. doi: 10.1016/j.soard.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Del Genio G, Tolone S, Limongelli P, Brusciano L, D'Alessandro A, Docimo G, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg. 2014;24:71–7. doi: 10.1007/s11695-013-1046-4. [DOI] [PubMed] [Google Scholar]
- 6.Gagner M, Deitel M, Kalberer TL, Erickson AL, Crosby RD. The Second International Consensus Summit for Sleeve Gastrectomy, March 19-21, 2009. Surg Obes Relat Dis. 2009;5:476–85. doi: 10.1016/j.soard.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Chiu S, Birch DW, Shi X, Sharma AM, Karmali S. Effect of sleeve gastrectomy on gastroesophageal reflux disease: A systematic review. Surg Obes Relat Dis. 2011;7:510–5. doi: 10.1016/j.soard.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Mahawar KK, Jennings N, Balupuri S, Small PK. Sleeve gastrectomy and gastro-oesophageal reflux disease: A complex relationship. Obes Surg. 2013;23:987–91. doi: 10.1007/s11695-013-0899-x. [DOI] [PubMed] [Google Scholar]
- 9.Daes J, Jimenez ME, Said N, Daza JC, Dennis R. Laparoscopic sleeve gastrectomy: Symptoms of gastroesophageal reflux can be reduced by changes in surgical technique. Obes Surg. 2012;22:1874–9. doi: 10.1007/s11695-012-0746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soricelli E, Casella G, Rizzello M, Calì B, Alessandri G, Basso N. Initial experience with laparoscopic crural closure in the management of hiatal hernia in obese patients undergoing sleeve gastrectomy. Obes Surg. 2010;20:1149–53. doi: 10.1007/s11695-009-0056-8. [DOI] [PubMed] [Google Scholar]
- 11.Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen J, et al. Laparoscopic sleeve gastrectomy - volume and pressure assessment. Obes Surg. 2008;18:1083–8. doi: 10.1007/s11695-008-9576-x. [DOI] [PubMed] [Google Scholar]
- 12.Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: Results after 1 and 3 years. Obes Surg. 2006;16:1450–6. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 13.McCallum RW, Berkowitz DM, Lerner E. Gastric emptying in patients with gastroesophageal reflux. Gastroenterology. 1981;80:285–91. [PubMed] [Google Scholar]
- 14.Maddern GJ, Chatterton BE, Collins PJ, Horowitz M, Shearman DJ, Jamieson GG. Solid and liquid gastric emptying in patients with gastro-oesophageal reflux. Br J Surg. 1985;72:344–7. doi: 10.1002/bjs.1800720505. [DOI] [PubMed] [Google Scholar]
- 15.Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, et al. Sleeve gastrectomy: A restrictive procedure? Obes Surg. 2007;17:57–62. doi: 10.1007/s11695-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 16.Carter PR, LeBlanc KA, Hausmann MG, Kleinpeter KP, deBarros SN, Jones SM. Association between gastroesophageal reflux disease and laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:569–72. doi: 10.1016/j.soard.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Keidar A, Appelbaum L, Schweiger C, Elazary R, Baltasar A. Dilated upper sleeve can be associated with severe postoperative gastroesophageal dysmotility and reflux. Obes Surg. 2010;20:140–7. doi: 10.1007/s11695-009-0032-3. [DOI] [PubMed] [Google Scholar]
- 18.Braghetto I, Lanzarini E, Korn O, Valladares H, Molina JC, Henriquez A. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg. 2010;20:357–62. doi: 10.1007/s11695-009-0040-3. [DOI] [PubMed] [Google Scholar]


