Abstract
We examined hepatitis C virus (HCV) RNA levels in serum, peripheral blood mononuclear cells (PBMC), and the liver for 135 patients with chronic HCV infections, 44 of whom were human immunodeficiency virus (HIV) positive and treated with highly active antiretroviral therapy (group A), 66 of whom were HIV negative (group B), with abnormal serum alanine aminotransferase (ALT) values, and 25 of whom were HIV negative, with ALT values of ≤1.5 times the normal value (group C). Patients had not been treated with interferon, with or without ribavirin, at the time of the study. A statistically significant correlation between HCV RNA levels in the liver and serum was reproducibly documented, whereas this was inconsistent for serum and PBMC. A comparative evaluation of HCV RNA levels in the liver and PBMC showed significantly lower values for group A than for groups B and C (P < 0.01 and P < 0.0001, respectively). In contrast, HCV RNA levels in serum were significantly higher for group A than for group B (P < 0.001). A dissociation between HCV RNA levels in serum and the liver was found for patients with HIV-HCV coinfections. Although the relative contribution of extrahepatic reservoirs, including lymphoid cells, to HCV RNA levels in serum is unclear, it may be speculated that a low intrahepatic HCV burden is caused by restored immunocompetence after successful antiretroviral therapy in coinfected patients.
Although the introduction of highly active antiretroviral therapy (HAART) has significantly improved the life expectancies of patients with human immunodeficiency virus (HIV) infection, morbidity and mortality rates from liver disease caused by hepatitis C virus (HCV) still represent an overwhelming clinical problem for HIV-infected individuals (26). These patients usually respond poorly to treatment with alpha interferon, with or without ribavirin, which may also occasionally cause severe hepatotoxicity (5, 23). Moreover, there is evidence indicating that coinfection may accelerate the progression to severe forms of liver disease, such as fibrosing cholestatic hepatitis (29) and cirrhosis (12, 27). Impaired cell-mediated immune responses to HCV are currently thought to be an important cofactor in the failure to control virus-induced intrahepatic necroinflammatory processes (18). It has been suggested that a severe course of liver disease may also result from the restored immunocompetence induced by HAART (15).
Several lines of experimental evidence suggest that higher levels of HCV RNA in the serum are usually found in patients with HIV-HCV coinfections than in patients with HCV infections only (8, 31). Although the pathogenetic basis for this observation has never been fully elucidated, HIV-induced immune system deficiency is generally considered a potentially important factor (9). However, reproducible correlations between HCV RNA levels in serum and CD4+-T-cell counts have not been confirmed (6). For HIV-positive patients treated with HAART, controversial data showing no significant changes (35) or increased (30) or decreased (24) HCV RNA levels in serum have generally been reported. Although there is no convincing evidence in favor of a direct relationship between HCV RNA levels in the serum and the severity of liver lesions for a single HCV infection (14, 22), recent studies suggest an association between HCV RNA levels and the progression to full-blown AIDS (7), indicating that there is a synergism between the two viruses.
Intrahepatic HCV RNA levels in patients with chronic liver disease or in patients who have undergone transplants have been investigated in some studies (11, 22); however, only limited data are currently available for HIV-HCV-coinfected patients (20, 33). The aim of this study was to compare HCV viral load levels in HCV-infected versus HCV-HIV-coinfected persons and to assess quantitative differences in HCV viral burdens among the peripheral blood mononuclear cell (PBMC), liver, and serum compartments for these patient categories. HIV-coinfected patients had already been treated with HAART at the time of the study, thus ensuring adequate immune competence.
MATERIALS AND METHODS
Patients.
The following three groups of patients with chronic HCV infections were enrolled in this study: (i) 44 HAART-treated HIV-positive patients (group A), (ii) 66 HIV-negative patients with persistently high alanine aminotransferase (ALT) levels in the serum (group B), and (iii) 25 HIV-negative patients with ALT levels in serum of ≤1.5 times the upper limit of what is considered normal who were part of a long-term prospective survey (4) (group C). Patients in groups B and C were enrolled between February 1998 and December 1999, whereas patients in group A were enrolled between March 1999 and July 2000. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in the a priori approval by the Institutional Review Board of IRCCS Policlinico San Matteo. All patients gave their informed consent to donate part of the blood and liver tissue taken for diagnostic purposes to the research project. HIV-positive patients were referred to our center from ad hoc outpatient clinics for intravenous drug users where antiretroviral therapy was already instituted. The median estimated duration of HIV infection was 12 years (range, 2 to 16 years). For all patients, the inclusion criteria were the presence of circulating anti-HCV antibodies, a positive result for HCV RNA in serum by nested reverse transcription-PCR (RT-PCR), the availability of a diagnostic liver biopsy, and no previous treatment with interferon, with or without ribavirin. The Knodell score was employed to evaluate the histological grading score (histologic activity index [HAI] score) and the fibrosis stage (16). Additional requirements for HIV-infected patients were that they were between the ages of 18 and 60 years, consistently had HIV RNA levels in plasma of <12,000 copies/ml, and had ≥200 CD4+ T cells/μl in the 16 weeks preceding the biopsy procedure. All group A patients were treated with different combinations of antiretroviral drugs, including nucleoside reverse transcriptase inhibitors (zidovudine, didanosine, lamivudine, and stavudine), nonnucleoside reverse transcriptase inhibitors (efavirenz, nevirapine, and delavirdine), and protease inhibitors (PIs; saquinavir, indinavir, ritonavir, and nelfinavir), for a median time of 21 months (range, 2 to 51 months). Specifically, 30 of the 44 patients received a therapeutic regimen including PIs, whereas the remainder were treated with nucleoside reverse transcriptase inhibitors and nonnucleoside reverse transcriptase inhibitors. Additional data, such as the estimated duration of HIV infection as well as the HIV viral load and CD4+-T-cell count before the initiation of therapy, were available for 38 of the 44 patients. The median CD4+-T-cell count before the first line of antiretroviral therapy was 118 cells/μl of blood (range, 0 to 772 cells/μl), and the median HIV RNA level in plasma was 11,500 copies/ml (range, 83 to 1,000,000 copies/ml), whereas these values were 226 cells/μl (range, 8 to 812 cells/μl) and 5,257 copies/ml (range, 118 to 1,000,000 copies/ml), respectively, prior to the initiation of HAART.
HCV RNA quantification in liver, PBMC, and serum samples.
Fragments of diagnostic liver biopsies were used for HCV RNA quantification. Briefly, liver specimens were homogenized in a tissue grinder in the presence of 1.0 ml of guanidinium thiocyanate solution (RNAzol; TEL-TEST Inc., Friendswood, Tex.). The total RNA in the liver was then extracted according to the manufacturer's instructions and was resuspended in 20 μl of buffer. Finally, the total RNA yield was determined by spectrophotometry. HCV RNAs were then quantified by a modified RT-PCR method that amplified a 255-bp fragment of the 5′ untranslated region of the HCV genome (nucleotides 73 to 328) (13). The detection limit of this method was 10 copies of a plasmid carrying the amplification sequence spiked with either 10 μl of serum RNA, 0.5 μg of liver RNA, or 0.5 μg of PBMC RNA. Specifically, 2, 0.2, and 0.02 μl of liver RNA were subjected to RT for 20 min in the presence of 100 copies of in vitro-transcribed competitor RNA (24), 10 pmol of antisense primer, and 200 U of Moloney murine leukemia virus (Gibco-BRL, Life Technologies, Paisley, United Kingdom) in a final volume of 20 μl of a solution containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin, and a 625 μM concentration of each deoxynucleoside triphosphate. One-fourth the volume of each RT reaction was then subjected to PCR. The PCR mixture contained the following: 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin, a 200 μM concentration of each deoxynucleoside triphosphate, 0.5 μM (each) sense and antisense primers, and 1.5 U of Gold Taq polymerase (Applied Biosystems, Foster City, Calif.). The PCR thermal profile was as follows: 94°C for 10 min and 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, with an additional delay of 2 s at each cycle. PCR products were then visualized by ethidium bromide staining after electrophoretic separation in 8.5% polyacrylamide gels. The number of input HCV RNA copies was then quantified by means of gel densitometry (1). The results are expressed as numbers of HCV RNA copies per microgram of total liver RNA. Primers for HCV amplification were carefully selected for their specificity to avoid interference by nonspecific PCR products.
Blood specimens for HCV RNA quantification in PBMC and serum were obtained on the same days as the liver biopsies. PBMC were separated from whole blood in a Ficoll-Hypaque gradient and were extensively washed in phosphate-buffered saline to rule out the possibility of cell contamination by serum-derived viral particles. Total HCV RNAs were extracted by the RNAzol method performed according to the manufacturer's instructions. HCV RNAs were quantified in PBMC by quantitative RT-PCR as described above. The results are expressed as numbers of HCV RNA copies per microgram of total PBMC RNA. HCV RNA copy numbers in serum were determined by the Quantiplex HCV RNA 2.0 assay (Bayer, Eraigny, France). The results are expressed as numbers of HCV RNA copies per milliliter of serum. Preliminary experiments using patient sera showed a highly significant statistical correlation between the quantitative RT-PCR assay developed in our laboratory and the commercial Quantiplex signal amplification assay (R2 = 0.9603).
Quantification of HIV RNA in plasma and CD4+-T-cell counts.
HIV RNAs in plasma at the time of liver biopsies were quantified by the Quantiplex HIV-1 RNA 3.0 assay (Bayer). The results are expressed as numbers of HIV RNA copies per milliliter of plasma. For calculations of median HIV RNA values, results falling below the assay cutoff of 50 copies/ml were considered by convention to be 50% of the lower detection limit (25 copies/ml). Absolute CD4+-T-cell counts were determined for EDTA-treated peripheral blood samples by flow cytometry (Ortho Immunocount flow cytometer system and Cytoron Absolute; Ortho Diagnostic Systems Inc., Raritan, N.J.).
Statistical analysis.
The correlation between HCV RNA levels in liver, PBMC, and serum samples was determined by use of the nonparametric Spearman rank order correlation coefficient. Differences between groups of patients were analyzed by the Mann-Whitney U test for nonparametric data. Differences in HCV RNA levels with respect to biochemical and histological indexes of liver damage were analyzed by the Kruskal-Wallis test, while differences with respect to demographic characteristics, HCV genotype, HIV RNA levels, and CD4+-T-cell counts were analyzed by the Mann-Whitney U test. All statistical tests were two-sided, and P values of <0.05 were considered statistically significant.
RESULTS
Demographics, biochemical and histologic disease indexes, HCV genotypes, and HIV parameters.
As shown in Table 1, the median age of group A patients was significantly lower than those of group B (P < 0.001) and C (P < 0.001) patients. The median age for group C was also higher than that for group B (P < 0.05). The median ALT values as well as the degree of liver inflammation and fibrosis were similar for groups A and B (P > 0.05). Most patients in group C had normal ALT levels. There was a higher prevalence of genotypes 1 and 4 and a lower prevalence of genotypes 2 and 3 in group A (P = 0.011).
TABLE 1.
Characteristics of patients with HIV-HCV coinfections (group A), single HCV infections and abnormal ALT values (group B), and single HCV infections with ALT values of ≤1.5 times the upper normal limit (group C)
| Characteristica | Group A (n = 44) | Group B (n = 66) | Group C (n = 25) |
|---|---|---|---|
| Age (years) | |||
| Median | 37 | 48 | 57 |
| Range | 31-44 | 20-66 | 29-65 |
| Sex (no. of males/no. of females) | 36/8 | 46/20 | 9/16 |
| ALT value (IU/ml) | |||
| Median | 78 | 94 | 38 |
| Range | 33-270 | 62-697 | 10-56 |
| Pathological features (no. with features [%]) | |||
| No hepatitis, minimal changes | 11 (25.0) | 9 (13.6) | 13 (52.0) |
| Mild or moderate hepatitis | 29 (65.9) | 47 (71.2) | 12 (48.0) |
| Severe hepatitis or cirrhosis | 4 (9.1) | 10 (15.2) | 0 |
| HAI score | |||
| Median | 3 | 3 | 2 |
| Range | 0-12 | 0-12 | 0-5 |
| Fibrosis score | |||
| Median | 1 | 1 | 0 |
| Range | 0-4 | 0-4 | 0-2 |
| No. (%) of samples from HCV genotype | |||
| 1 | 27 (61.4) | 29 (43.9) | 14 (56.0) |
| 2 | 1 (2.3) | 24 (36.4) | 11 (44.0) |
| 3 | 9 (20.4) | 10 (15.1) | 0 |
| 4 | 7 (15.9) | 2 (3.0) | 0 |
| Mixture (types 1 and 2) | 0 | 1 (1.6) | 0 |
| HIV load in plasmab (copies/ml) | |||
| Median | 25 | ND | ND |
| Range | 25-10,939 | ND | ND |
| No. of CD4+ T cells/μl of bloodb | |||
| Median | 470 | ND | ND |
| Range | 196-1,198 | ND | ND |
ND, not done.
Value at the time of liver biopsy.
HIV infections in group A patients were well controlled by HAART, as documented by the very low median HIV RNA level in plasma (25 HIV RNA copies/ml; range, 25 to 10,939) and the high median CD4+-T-cell count (470 CD4+ T cells/μl of blood; range, 196 to 1,198) at the time of liver biopsy.
Quantification of HCV RNA in liver, PBMC, and serum.
Liver and serum samples were available from all patients, whereas PBMC samples were obtained from 40 of 44 (90.9%) patients in group A, 51 of 66 (77.2%) patients in group B, and all 25 (100%) patients in group C.
Upon stratification of each of the three groups of patients by demographic characteristic (age, >40 years or ≤40 years), HCV genotype (types 1 and 4 or types 2 and 3), histological activity grade (HAI score of 0 to 3, 4 to 9, or >9), and degree of fibrosis (0 to 1, 2 to 3, or 4), no significant difference between strata was detected for HCV RNA levels in the liver, PBMC, or serum. For group A, there were no significant differences in HCV RNA levels in the liver, PBMC, and serum between patients with or without detectable HIV RNA levels and high (>350) or low (≤350) CD4+-T-cell counts (data not shown). In addition, for group A, no statistically significant differences in HCV RNA levels in the liver, PBMC, and serum were detected between patients with an estimated duration of HIV infection of 10 years or less and those infected more than 10 years earlier, between patients treated for 12 months or less and those treated for more than 12 months, and between patients treated with therapeutic regimens which did or did not include PIs.
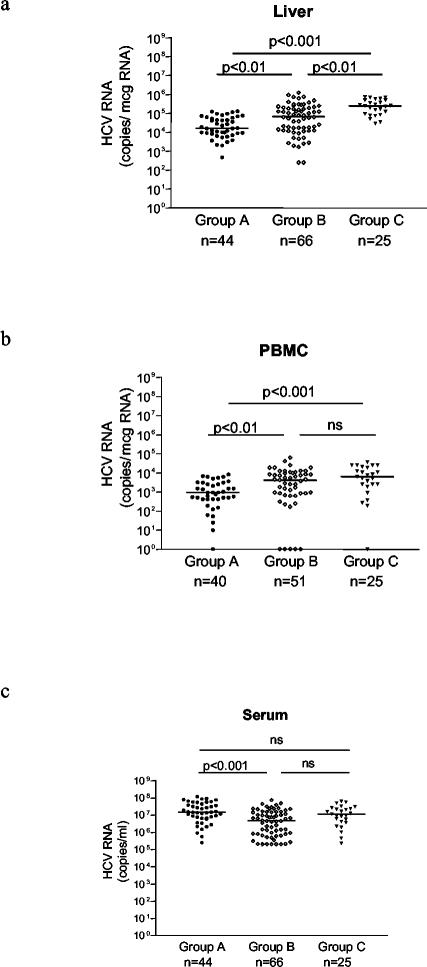
A comparison of HCV RNA levels in the liver, PBMC, and serum among patients from groups A, B, and C showed statistically significant differences. As shown in Fig. 1a, the median hepatic HCV RNA level was significantly lower for group A (16,146 HCV RNA copies/μg of total liver RNA; range, 469 to 131,578) than for groups B (68,750 HCV RNA copies/μg of total liver RNA; range, 250 to 1,250,000) and C (250,000 HCV RNA copies/μg of total liver RNA; range, 30,000 to 750,000). Hepatic HCV RNA levels were also significantly lower for group B than for group C (Fig. 1a). Similarly, the median HCV RNA level in PBMC was significantly lower for group A (937 HCV RNA copies/μg of total PBMC RNA; range, 0 to 8,333) than for groups B (4,166 HCV RNA copies/μg of total PBMC RNA; range, 0 to 62,500) and C (6,250 HCV RNA copies/μg of total PBMC RNA; range, 0 to 35,714) (Fig. 1b). Preliminary experiments on specimens obtained from four patients to define the subpopulation(s) of PBMC harboring HCV RNA indicated that the virus was predominantly localized in the B-cell subset (data not shown).
FIG. 1.
HCV RNA levels in liver (a), PBMC (b), and serum (c) from patients with HIV-HCV coinfections (group A), HCV infections only, with abnormal ALT levels (group B), and HCV infections only, with ALT values of ≤1.5 times the upper limit of normal (group C). Horizontal lines represent medians.
In contrast, HCV RNA levels in the serum showed an opposite trend, being significantly higher in patients from group A (14,810,000 HCV RNA copies/ml; range, 252,000 to 118,900,000) than in patients from group B (4,729,500 HCV RNA copies/ml; range, 199,000 to 74,620,000), although they were not significantly different from those in patients from group C (11,530,000 HCV RNA copies/ml; range, 230,000 to 63,470,000) (Fig. 1c).
Correlation of HCV RNA levels in liver, serum, and PBMC.
For all three groups of patients, a statistically significant correlation was found between HCV RNA levels in the liver and the serum, although the correlation was less consistent between HCV RNA levels in the serum or liver and those in PBMC (Table 2).
TABLE 2.
Correlation of HCV RNA levels in different compartments for the three groups of patients
| Group | Correlation for HCV RNA loada
|
|||||
|---|---|---|---|---|---|---|
| Serum vs liver
|
Serum vs PBMC
|
Liver vs PBMC
|
||||
| r | P value | r | P value | r | P value | |
| A | 0.6657 | <0.0001 | 0.3754 | 0.0170 | 0.2706 | 0.0912 |
| B | 0.5176 | <0.0001 | 0.5004 | 0.0002 | 0.3560 | 0.0104 |
| C | 0.5197 | 0.0078 | 0.3700 | 0.0687 | 0.4276 | 0.0330 |
HCV RNA loads were calculated in copies per milliliter for serum samples and in copies per microgram of RNA for liver and PBMC samples.
DISCUSSION
In this study, we have reported a systematic quantitative analysis of HCV RNA in different body compartments (serum, liver, and PBMC) for patients with HCV infection only or with HCV-HIV coinfection. Statistically significant differences emerged from the evaluation of a substantial number of patients in each category. However, even though differences between the groups were statistically significant, a considerable overlap was consistently documented among patient categories.
We were able to confirm previous surveys that reported a significant correlation between HCV RNA levels in the liver and the serum for HCV-infected patients (11, 22) and for a small group of HIV-HCV-coinfected patients (33). However, for the present study, coinfected patients were referred to us while they were already on antiretroviral treatment, thus significantly reducing the effect of HIV-induced immune system suppression on HCV replication, which may be a profound influence (3, 30). Because of this, it is important to emphasize that >50% of the patients had virtually undetectable HIV levels and only six patients had HIV titers exceeding 1,000 copies/ml.
The discrepancy between low hepatic HCV RNA concentrations and high HCV RNA concentrations in the serum for HIV-HCV-coinfected patients may have several explanations. Increased HCV RNA levels in the serum suggest that there is an enhanced release of HCV virions into the circulation or enhanced viral replication in extrahepatic sites. However, the former hypothesis is probably untenable, since at steady state there should be equilibrium between virus production and clearance. On the other hand, recent findings suggested that the suppression of HIV-1 RNA and increases in CD4+ cell counts induced by HAART are indeed associated with significant increases in HCV RNA that are sustained over time (3). An alternative, and probably more plausible, explanation is that since HCV appears to replicate in certain subsets of PBMC (19), under basal conditions of coinfection, HIV and HCV would compete for this reservoir. With the successful clearance of HIV-infected lymphocytes, HCV RNA levels in serum would increase as more susceptible target cells become available.
Low hepatic HCV RNA levels may instead result from successful therapeutic responses to antiretroviral drugs. Indeed, it has been shown that the CD4+-T-cell reconstitution induced by HAART is responsible for better control of hepatic HCV replication, and vigorous CD4+-T-cell responses are associated with slowly progressive or asymptomatic chronic liver disease (2). It is well known that therapeutic regimens which include PIs significantly improve T-cell responses in HIV-infected subjects by enhancing CD4+- and CD8+-T-cell counts. Indeed, the sustained disappearance of HCV RNA from patients responding to HAART, most likely through the restoration of cytolytic T-cell responses, has been repeatedly reported (10, 24, 34).
The correlation of HCV RNA levels in PBMC with those in the liver and serum for the three groups of patients was less consistent. Controversial data on the actual ability of HCV to replicate in PBMC have been reported (17, 21), suggesting a limited contribution of PBMC to the total HCV RNA burden. In addition, no clear-cut differences between the three groups of patients examined were observed. However, it was not possible to distinguish between active viral replication and the mere presence of HCV RNA in lymphoid cells, as we did not specifically investigate the presence of the HCV RNA minus strand, which some investigators believe is indicative of viral replication in this cellular compartment (22). Preliminary evidence suggests that the HCV E2 envelope protein directly interacts with CD81, a putative HCV receptor (25), on B-cell membranes by inducing cross-linking of the B-cell coreceptor complex with a membrane immunoglobulin (28). This may significantly lower the B-cell activation threshold in the absence of viral replication. In this regard, note that, in agreement with these findings, our study showed that HCV RNA was predominantly localized in the B-cell subset and recent data have reproducibly shown productive HCV infections of B-cell lines isolated from patients with chronic HCV infection (32). Further studies are needed to fully elucidate the mechanisms of HCV pathogenesis and to clarify the role of virus replication in different compartments in both HIV-seronegative and HIV-seropositive individuals.
Acknowledgments
We thank Lara Firmo for editorial assistance.
This work was supported by the Italian Ministry of Health, Ricerca Finalizzata (grants N. ICS 030.4/RF00.62, N. 08920401*, and N. 103) and Ricerca Corrente (grants N. 80221 and N. 203), and the Italian Ministry of Education, University and Research (grant N. MM06261448_003). Support was also provided by an unrestricted research grant from Schering-Plough Italy and by Fondazione Oretta Bartolomei Corsi, Florence, Italy.
REFERENCES
- 1.Bagnarelli, P., A. Valenza, S. Menzo, A. Manzin, G. Scalise, P. E. Varaldo, and M. Clementi. 1994. Dynamics of molecular parameters of human immunodeficiency virus type 1 activity in vivo. J. Virol. 68:2495-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botarelli, P., M. R. Brunetto, M. A. Minutello, P. Calvo, D. Unutmaz, A. J. Weiner, Q. L. Choo, J. R. Shuster, G. Kuo, F. Bonino, M. Houghton, and S. Abrignagni. 1993. T-lymphocyte response to hepatitis C virus in different clinical courses of infection. Gastroenterology 104:580-587. [DOI] [PubMed] [Google Scholar]
- 3.Chung, R. T., S. R. Evans, Y. Yang, D. Theodore, H. Valdez, R. Clark, C. Shikuma, T. Nevin, and K. E. Sherman. 2002. Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS 16:1915-1923. [DOI] [PubMed] [Google Scholar]
- 4.Cividini, A., C. Rebucci, E. Silini, and M. U. Mondelli. 2001. Is the natural history of hepatitis C virus carriers with normal aminotransferase really benign? Gastroenterology 121:1526-1527. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, C. L., M. A. Parbhakar, and J. B. Angel. 2002. Hepatotoxicity associated with antiretroviral therapy containing dual versus single protease inhibitors in individuals coinfected with hepatitis C virus and human immunodeficiency virus. Clin. Infect. Dis. 34:1259-1263. [DOI] [PubMed] [Google Scholar]
- 6.Cribier, B., D. Rey, C. Schmitt, J. M. Lang, A. Kirn, and F. Stoll-Keller. 1995. High hepatitis C viremia and impaired antibody response in patients coinfected with HIV. AIDS 9:1131-1136. [DOI] [PubMed] [Google Scholar]
- 7.Daar, E. S., H. Lynn, S. Donfield, E. Gomperts, S. J. O'Brien, M. W. Hilgartner, W. K. Hoots, D. Chernoff, S. Arkin, W. Y. Wong, and C. A. Winkler. 2001. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J. Infect. Dis. 183:589-595. [DOI] [PubMed] [Google Scholar]
- 8.Di Martino, V., P. Rufat, N. Boyer, P. Renard, F. Degos, M. Martinot-Peignoux, S. Matheron, V. Le Moing, F. Vachon, C. Degott, D. Valla, and P. Marcellin. 2001. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology 34:1193-1199. [DOI] [PubMed] [Google Scholar]
- 9.Eyster, M. E., M. W. Fried, A. M. Di Bisceglie, and J. J. Goedert. 1994. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Blood 84:1020-1023. [PubMed] [Google Scholar]
- 10.Fialaire, P., C. Payan, D. Vitour, J. M. Chennebault, J. Loison, E. Pichard, and F. Lunel. 1999. Sustained disappearance of hepatitis C viremia in patients receiving protease inhibitor treatment for human immunodeficiency virus infection. J. Infect. Dis. 180:574-575. [DOI] [PubMed] [Google Scholar]
- 11.Gervais, A., M. Martinot, N. Boyer, A. Auperin, W. Le Breton, C. Degott, D. Valla, and P. Marcellin. 2001. Quantitation of hepatic hepatitis C virus RNA in patients with chronic hepatitis C. Relationship with severity of disease, viral genotype and response to treatment. J. Hepatol. 35:399-405. [DOI] [PubMed] [Google Scholar]
- 12.Graham, C. S., L. R. Baden, E. Yu, J. M. Mrus, J. Carnie, T. Heeren, and M. J. Koziel. 2001. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin. Infect. Dis. 33:562-569. [DOI] [PubMed] [Google Scholar]
- 13.Grassi, G., G. Pozzato, M. Moretti, and M. Giacca. 1995. Quantitative analysis of hepatitis C virus RNA in biopsies by competitive reverse transcription and polymerase chain reaction. J. Hepatol. 23:403-411. [DOI] [PubMed] [Google Scholar]
- 14.Gretch, D., L. Corey, J. Wilson, C. dela Rosa, R. Willson, R. Carithers, Jr., M. Busch, J. Hart, M. Sayers, and J. Han. 1994. Assessment of hepatitis C virus RNA levels by quantitative competitive RNA polymerase chain reaction: high-titer viremia correlates with advanced stage of disease. J. Infect. Dis. 169:1219-1225. [DOI] [PubMed] [Google Scholar]
- 15.John, M., J. Flexman, and M. A. French. 1998. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease? AIDS 12:2289-2293. [DOI] [PubMed] [Google Scholar]
- 16.Knodell, R. G., K. G. Ishak, W. C. Black, T. S. Chen, R. Craig, N. Kaplowitz, T. W. Kiernan, and J. Wollman. 1981. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431-435. [DOI] [PubMed] [Google Scholar]
- 17.Laskus, T., M. Radkowski, A. Piasek, M. Nowicki, A. Horban, J. Cianciara, and J. Rakela. 2000. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: evidence of active replication in monocytes/macrophages and lymphocytes. J. Infect. Dis. 181:442-448. [DOI] [PubMed] [Google Scholar]
- 18.Lauer, G. M., T. N. Nguyen, C. L. Day, G. K. Robbins, T. Flynn, K. McGowan, E. S. Rosenberg, M. Lucas, P. Klenerman, R. T. Chung, and B. D. Walker. 2002. Human immunodeficiency virus type 1 hepatitis C virus coinfection: intraindividual comparison of cellular immune responses against two persistent viruses. J. Virol. 76:2817-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerat, H., S. Rumin, F. Habersetzer, F. Berby, M. A. Trabaud, C. Trepò, and G. Inchauspé. 1998. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood 91:3841-3849. [PubMed] [Google Scholar]
- 20.Neau, D., P. Trimoulet, M. Winnock, B. Le Bail, E. Schvoerer, E. Legrand, J. M. Ragnaud, M. Dupon, H. Fleury, and M. E. Lafon. 2001. Impact of protease inhibitors on intrahepatic hepatitis C virus viral load. AIDS 15:1736-1738. [DOI] [PubMed] [Google Scholar]
- 21.Negro, F., and M. Levrero. 1998. Does the hepatitis C virus replicate in cells of the hematopoietic lineage? Hepatology 28:261-264. [DOI] [PubMed] [Google Scholar]
- 22.Negro, F., K. Krawczynski, R. Quadri, L. Rubbia-Brandt, M. U. Mondelli, J. P. Zarski, and A. Hadengue. 1999. Detection of genomic- and minus-strand of hepatitis C virus RNA in the liver of chronic hepatitis C patients by strand-specific semiquantitative reverse-transcriptase polymerase chain reaction. Hepatology 29:536-542. [DOI] [PubMed] [Google Scholar]
- 23.Nunez, M., P. Rios, L. Martin-Carbonero, M. Pérez-Olmeda, J. Gonzales-Lahoz, and V. Soriano. 2002. Role of hepatitis C virus genotype in the development of severe transaminase elevation after the introduction of antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 30:65-68. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Olmeda, M., J. Garcia-Samaniego, and V. Soriano. 2000. Hepatitis C viremia in HIV-HCV co-infected patients having immune restoration with highly active antiretroviral therapy. AIDS 14:212. [DOI] [PubMed] [Google Scholar]
- 25.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Rosado, R., M. Pérez-Olmeda, J. Garcia-Samaniego, and V. Soriano. 2001. Management of hepatitis C in HIV-infected persons. Antivir. Res. 52:189-198. [DOI] [PubMed] [Google Scholar]
- 27.Romeo, R., M. G. Rumi, M. F. Donato, M. A. Cargnel, P. Vigano, M. U. Mondelli, B. Cesana, and M. Colombo. 2000. Hepatitis C is more severe in drug users with human immunodeficiency virus infection. J. Viral Hepat. 7:297-301. [DOI] [PubMed] [Google Scholar]
- 28.Rosa, D., G. Saletti, G. Pozzato, and S. Abrignani. 2001. HCV can activate B cells via CD81 engagement: a molecular mechanism for B cell autoreactivity and cryoglobulinemia in HCV infection. Hepatology 34:252A. [Google Scholar]
- 29.Rosenberg, P. M., J. J. Farrell, D. R. Abraczinskas, F. M. Graeme-Cook, J. L. Dienstag, and R. T. Chung. 2002. Rapidly progressive fibrosing cholestatic hepatitis-hepatitis C virus in HIV coinfection. Am. J. Gastroenterol. 97:478-483. [DOI] [PubMed] [Google Scholar]
- 30.Rutschmann, O. T., F. Negro, B. Hirschel, A. Hadengue, D. Anwar, and L. H. Perrin. 1998. Impact of treatment with human immunodeficiency virus (HIV) protease inhibitors on hepatitis C viremia in patients coinfected with HIV. J. Infect. Dis. 177:783-785. [DOI] [PubMed] [Google Scholar]
- 31.Soriano, V., R. Rodriguez-Rosado, and J. Garcia-Samaniego. 1999. Management of chronic hepatitis C in HIV-infected patients. AIDS 13:539-546. [DOI] [PubMed] [Google Scholar]
- 32.Sung, V. M., S. Shimodaira, A. L. Doughty, G. R. Picchio, H. Can, T. S. Yen, K. L. Lindsay, A. M. Levine, and M. M. Lai. 2003. Establishment of B-cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro: the apoptotic effects of virus infection. J. Virol. 77:2134-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trimoulet, P., D. Neau, B. Le Bail, A. Rullier, M. Winnock, T. Galperine, E. Legrand, E. Schvoerer, M. Dupon, J. M. Ragnaud, P. Bioulac-Sage, G. Chene, H. Fleury, and M. E. Lafon. 2002. Intrahepatic HCV RNA loads in 37 HIV-HCV co-infected patients with controlled HIV infection. J. Med. Virol. 67:143-151. [DOI] [PubMed] [Google Scholar]
- 34.Yokozaki, S., J. Takamatsu, I. Nakano, Y. Katano, H. Toyoda, K. Hayashi, T. Hayakawa, and Y. Fukuda. 2000. Immunologic dynamics in hemophiliac patients infected with hepatitis C virus and human immunodeficiency virus: influence of antiretroviral therapy. Blood 96:4293-4299. [PubMed] [Google Scholar]
- 35.Zylberberg, H., M. L. Chaix, C. Rabian, C. Rouzioux, B. Aulong, C. Brechot, J. P. Viard, and S. Pol. 1998. Tritherapy for human immunodeficiency virus infection does not modify replication of hepatitis C virus in coinfected subjects. Clin. Infect. Dis. 26:1104-1106. [DOI] [PubMed] [Google Scholar]