Abstract
Human papillomavirus (HPV) has been found in cervical cancer, tonsillar cancer, and certain types of head and neck cancers. We report on a DNA microarray-based method for the simultaneous detection and typing of HPVs. The genotype spectrum discriminated by this HPV DNA microarray includes 15 high-risk HPV genotypes and 12 low-risk HPV genotypes. The HPV DNA microarray showed high degrees of specificity and reproducibility. We evaluated the performance of the HPV DNA microarray by application to three HPV-positive cell lines (HeLa, Caski, and SiHa cells) and two HPV-negative cell lines (C33A and A549 cells). The HPV DNA microarray successfully identified the known types of HPV present in the cell lines. The detection limit of the HPV DNA microarray was at least 100-fold higher than that of PCR. To assess the clinical applicability of the HPV DNA microarray, we performed the HPV genotyping assay with 73 nonmalignant and malignant samples from 39 tonsillar cancer patients. Twenty-five of the 39 (64.1%) malignant samples were positive for HPV, whereas 3 of 34 (8.8%) nonmalignant samples were positive for HPV. This result shows a preferential association of HPV with tonsillar carcinomas. The correlations of the presence of HPV with the grade of differentiation and risk factors were not significant. Our data show that the HPV DNA microarray may be useful for the diagnosis and typing of HPV in large-scale epidemiological studies.
Epidemiological and molecular studies have demonstrated that high-risk types of human papillomavirus (HPV) not only are etiologically related to the development of most cases of uterine cervical carcinoma (2, 8, 14, 16) but also are associated with certain types of carcinomas in the head and neck (7, 11). Until now, more than 100 different HPV genotypes have been identified on the basis of the DNA sequence of the L1, E6, and upstream regulatory regions (3, 17, 29). The mucosal HPV genotypes are generally classified into low-risk and high-risk groups on the basis of their association with malignant lesions and phylogenetic relationships (13, 15, 29). Furthermore, it has been demonstrated in tonsillar carcinoma that HPV types 16 and 33 express the E6 and E7 oncogenes and that transcription is localized in the cancer cells and does not occur in the surrounding stroma (24, 28). Because HPV genotyping information is clinically useful for prognosis and therapy based on the risk type, it is important that the HPV genotype be identified by as sensitive and as specific a method as possible.
At present, eight main strategies are used to detect and type various HPVs. All of these strategies have advantages and disadvantages, depending on their application (5, 10, 26). Several consensus PCR systems have been conveniently used in several large-scale epidemiological studies (9, 10, 19). However, consensus PCR products do not provide practical information for genotyping (26). Meanwhile, because it is difficult to design compatible multiple primer sets for genotype-specific PCR, the maximum number of HPVs detectable in a single assay is relatively limited (17). Although recent work has reported on HPV DNA microarray systems capable of typing multiple HPV genotypes (1, 4, 12, 16, 18), they still have technical limitations.
To overcome the existing limitations of the HPV detection and genotyping methodologies available, we report on an improved PCR-based HPV DNA microarray. The detection limit, reproducibility, and specificity of the HPV DNA microarray were estimated. To assess the applicability of the HPV DNA microarray in clinical practice, we performed DNA microarray hybridizations with samples from 39 Korean patients with tonsillar squamous carcinoma.
MATERIALS AND METHODS
Clinical samples and cell lines.
Five-micrometer sections of paraffin-embedded tonsillar carcinoma tissues from 39 patients diagnosed with tonsillar carcinoma were prepared. The genomic DNAs were isolated from microdissected nonmalignant and malignant tonsillar tissues of each patient in parallel. The cervical cell lines SiHa (HPV type 16 [HPV-16] positive), Caski (HPV-16 positive), HeLa (HPV-18 positive), and C33A and the lung cancer cell line A549 were kindly provided by the Cancer Metastasis Center of Yonsei University (Seoul, South Korea). Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% penicillin, and 1% streptomycin at 37°C with 5% CO2. The genomic DNA was prepared by using a Wizard Genomic DNA Purification kit (Promega Biosciences Inc., Madison, Wis.), according to the instructions of the manufacturer.
Construction of the HPV type-specific probes.
Type-specific 30-bp sequences of probes specific for HPV types 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 54, 56, and 58 were selected as reported previously (13). The DNA sequences of probes specific for HPV types 59, 62, 66, 67, 68, 69, 70, and 72 were obtained from a public HPV sequence database (http://hpv-web.lanl.gov/stdgen/virus/hpv), and their probe sequences were designed by multiple-sequence alignment analysis with the CLUSTAL X (version 1.81) program. The 30-bp type-specific probe sequences are listed in Table 1.
TABLE 1.
The 30-bp sequences of the HPV type-specific probes
| HPV type | Sequence (5′-3′) | Position |
|---|---|---|
| 6 | ATCCGTAACTACATCTTCCACATACACCAA | 6807-6844 |
| 11 | ATCTGTGTCTAAATCTGCTACATACACTAA | 6799-6828 |
| 16 | GTCATTATGTGCTGCCATATCTACTTCAGA | 6662-6691 |
| 18 | TGCTTCTACACAGTCTCCTGTACCTGGGCA | 6647-6676 |
| 31 | TGTTTGTGCTGCAATTGCAAACAGTGATAC | 6583-6612 |
| 33 | TTTATGCACACAAGTAACTAGTGACAGTAC | 6622-6651 |
| 34 | TACACAATCCACAAGTACAACTGCACCATA | 6539-6568 |
| 35 | GTCTGTGTGTTCTGCTGTGTCTTCTAGTGA | 6602-6531 |
| 39 | TCTACCTCTATAGAGTCTTCCATACCTTCT | 6672-6702 |
| 40 | GCTGCCACACAGTCCCCCACACCAACCCCA | 6808-6837 |
| 42 | CTGCAACATCTGGTGATACATATACAGCTG | 6876-6905 |
| 43 | TCTACTGACCCTACTGTGCCCAGTACATAT | 94-123 (MY)a |
| 44 | GCCACTACACAGTCCCCTCCGTCTACATAT | 6720-6749 |
| 45 | ACACAAAATCCTGTGCCAAGTACATATGAC | 6658-6687 |
| 51 | AGCACTGCCACTGCTGCGGTTTCCCCAACA | 6553-6583 |
| 52 | TGCTGAGGTTAAAAAGGAAAGCACATATAA | 6692-6721 |
| 54 | TACAGCATCCACGCAGGATAGCTTTAATAA | 6633-6662 |
| 56 | GTACTGCTACAGAACAGTTAAGTAAATATG | 6627-6656 |
| 58 | ACTGAAGTAACTAAGGAAGGTACATATAAA | 6678-6707 |
| 59 | CTACTACTCTCTATTCCTAATGTATACACA | 6656-6676 |
| 62 | CTGCTGCAGCAGAATACACGGCTACCAACT | 101-130 (MY)a |
| 66 | TTAACTAAATATGATGCCCGTGAAATCAAT | 6694-6723 |
| 67 | AATCAGAGGCTACATACAAAAATGAAAACT | 6664-6693 |
| 68 | AATCAGCTGTACCAAATATTTATGATCCTA | 101-130 (MY)a |
| 69 | TCTGCATCTGCCACTTTTAAACCATCAGAT | 6594-6623 |
| 70 | GCCATACCTGCTGTATATAGCCCTACAAAG | 6634-6663 |
| 72 | TCTGTATCAGAATATACAGCTTCTAATTTT | 6843-6872 |
The nucleotide sequences indicate the positions in ∼450 bp of DNA derived from MYH-PCR.
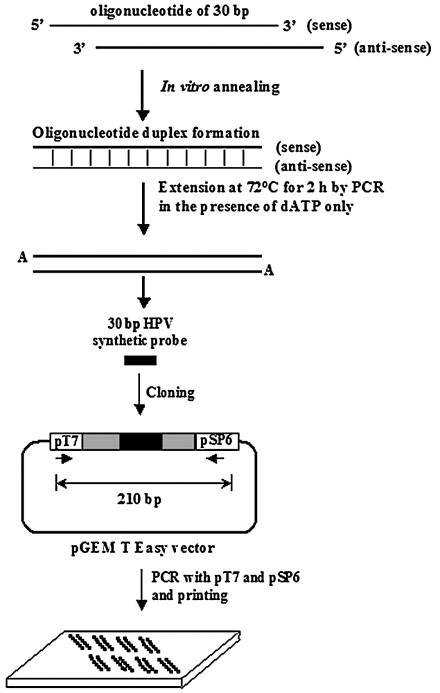
The protocol for HPV-specific probe synthesis is outlined in Fig. 1. To facilitate amplification and binding to the solid support of the HPV probes, we cloned the 30-bp DNAs into plasmid vectors. Equal amounts (5 μg) of each complementary oligonucleotide probe were mixed and completely denatured by heating at 100°C for 3 min. The denatured oligonucleotides were left at room temperature for 1 h to make duplex oligonucleotides. To clone the HPV-specific probes, 2 μg of each of the duplex oligonucleotides was subjected to an extension procedure in order to add an adenine base to both ends by PCR for 2 h in the presence of dATP only. The adenine-tailed HPV probes were cloned into the pGEM T Easy vector (Promega Biosciences Inc.). The sequences of the 27 HPV type-specific probes were confirmed by DNA sequencing with an automatic DNA sequencer (ABI 3700; Applied Biosystems, Foster City, Calif.). The ∼180-bp positive control probe, the sequence of which corresponds to the L1 region that is highly conserved among most known types of genital HPVs, was amplified with primers MY11 and GP6+ from the genomic DNA of the Caski (HPV-16 positive) and HeLa (HPV-18 positive) cell lines and cloned into the pGEM T Easy vector.
FIG. 1.
Protocol for HPV-specific probe synthesis by oligonucleotide shuffling (25). Oligonucleotides were synthesized by the standard phosphoramidite method. Equal amounts (5 μg each) of two complementary oligonucleotides corresponding to 27 HPV probes were combined and subsequently heated at 100°C for 3 min. The denatured oligonucleotides were left at room temperature for 1 h to allow annealing. Details are described in Materials and Methods.
The PCR control, ∼550 bp of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA, was amplified from the genomic DNA of Caski cells by reverse transcription-PCR with GAPDH-specific primers GAPDH-LEFT and GAPDH-RIGHT. To use GAPDH cDNA as an indicator of the adequacy of PCR for the amplification of HPV DNA by MYH-PCR, we added sequences specific for the MY09 and MY11 primers to both ends of the GAPDH cDNA. The GAPDH cDNA clone carrying the MY09 and MY11 primer sequences was cloned into the pGEM T Easy vector.
A negative control probe (180 bp) carrying a partial Escherichia coli lacZ gene of the pGEM T Easy vector was amplified from the multiple-cloning site of the pGEM T Easy vector. The standard PCR for preparation of the HPV DNA microarray was simply performed with the pSP6 and pT7 primer sets. The PCR mixture (50 μl) contained 2.5 mM deoxynucleoside triphosphates, primers pSP6 and pT7 (25 pmol each), 1× PCR buffer, and 5 U of Taq polymerase (Solgent Co., Taejon, South Korea). The amplification steps were as follows: 95°C for 10 min and 30 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min. This was followed by a final extension for 10 min. PCR was performed in a Primus-HP thermal cycler (MWG Biotechnology, Ebersberg, Germany). All the primer sequences used for the PCR are listed in Table 2.
TABLE 2.
PCR primers used in this study
| Primer name and HPV type | Sequence (5′-3′)a | Position |
|---|---|---|
| MY09 | CGT CCM ARR GGA WAC TGA TC | 7033-7014 in HPV-16 |
| MY11 | GCM CAG GGW CAT AAY AAT GG | 6582-6601 in HPV-16 |
| GP6+ | GAA AAA TAA ACT GTA AAT CA | 6765-6746 in HPV-16 |
| HMB01b | GCG ACC CAA TGC AAA TTG GT | |
| GAPDH-LEFT | TCA ACG GAT TTG GTC GTA TT | 77-96 |
| GAPDH-RIGHT | TAG AGG CAG GGA TGA TGT TC | 664-645 |
| pSP6 | ATT TAG GTG ACA CTA TAG AA | 139-158 in pGEM T Easy |
| pT7 | TAA TAC GAC TCA CTA TAG GG | 2999-3 in pGEM T Easy |
| TOPO-LEFT | AGC TTG GTA CCG AGC TCG GAT | 255-235 in pCR2.1-TOPO |
| TOPO-RIGHT | CGA ATT GGG CCC TCT AGA TGC | 363-343 in pCR2.1-TOPO |
| MY09GAP | CGT CCM ARR GGA WAC TGA TC TCA ACG GAT TTG GTC GTA TT | |
| MY11GAP | GCM CAG GGW CAT AAY AAT GG TAG AGG CAG GGA TGA TGT TC |
Nucleotide abbreviations are according to the International Union of Pure and Applied Chemistry system of nomenclature: M, A + C; R, A + G; W, A + T; Y, C + T.
HMB01 is directed to the minus strand of HPV-51.
Target probe labeling by PCR.
The MYH-PCR (9, 19) was used for amplification of HPV DNA from the cell lines and tonsillar tissue. A standard PCR was carried out with a 50-μl mixture containing the primer set MY09, MY11, and HMB01 (25 pmol each of primers MY09 and MY11 and 5 pmol of HMB01); 10 pg of a PCR control DNA (GAPDH); 5 μl of tonsillar tissue DNA or 1 ng of cell line genomic DNA; a mixture of deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dUTP, 10 mM each) containing 1 μl of cyanine 5 (Cy5)-dUTP (25 nM; Pharmacia); and 5 U of Taq polymerase (Solgent Co.) in the presence of 1× PCR buffer. To avoid contamination of the template with DNA from a previous PCR, we pretreated the PCR mixture with uracil N′-glycosylase (0.5 U; Perkin-Elmer) at 50°C for 5 min before PCR (14). The amplification cycles were carried out as follows: 95°C for 10 min and 35 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min. This was followed by a final extension for 10 min, and the PCR products labeled with Cy5 were purified by using a PCR purification kit (Qiagen), as recommended by the manufacturer. The purified amplicons were stored at −20°C under darkness until use. The amount of DNA added to a PCR mixture represents 1 to 5% of the DNA obtained from the tonsillar tissues. Negative and positive controls were included in all PCR experiments.
Design of HPV DNA microarray.
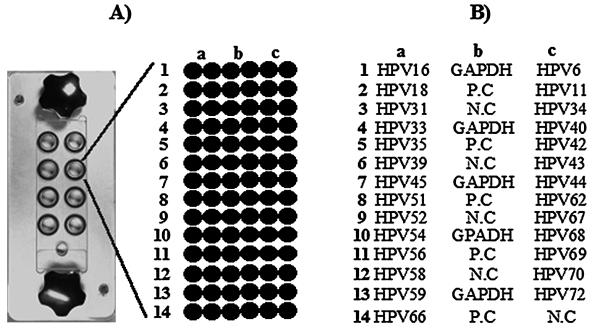
Twenty-seven HPV-specific probes, GAPDH cDNA-specific probes, HPV DNA-positive control probes, and negative control probes were printed on DNA microarray slides with a robotic microspotting microarrayer (OmniGrid II; GeneMachine, Ann Arbor, Mich.). All amplified probes were dissolved in 10 μl of 50% dimethyl sulfoxide (AMRESCO) at final concentrations of 200 to 240 ng/μl. As shown in Fig. 2, the average size of the spots was 250 μm. Amine-coated GAPS II slides (Corning Co., Corning, N.Y.) were used for the microarray.
FIG. 2.
Microarray design for HPV genotyping. (A) Eight-well platform hybridization reaction chamber. Each well contains 84 probes corresponding to the PCR control, the HPV-positive control, the HPV-negative control, and type-specific probes. (B) Schematic diagram of the HPV DNA microarray probe positions. GAPDH cDNA, HPV-positive controls (P.C), and HPV-negative controls (N.C) were spotted on the center of the slide. Each HPV type-specific probe was printed on the both sides, as shown, and all probes were printed in duplicate.
Microarray hybridization and HPV genotyping.
HPV DNA microarray hybridization was performed as described previously (27). HPV DNA microarray hybridization was carried out at 55°C for 2 h in an eight-well platform hybridization chamber (GenomicTree, Inc.). The hybridization mixture (100 μl) contained half (25 μl) of the amplified target probe, 3.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5 μg of salmon sperm DNA (Gibco BRL, Paisley, United Kingdom), and 0.2% sodium dodecyl sulfate. It was heated for 2 min at 95°C and immediately applied onto the HPV DNA microarray.
To determine the HPV genotype, the HPV DNA microarray was imaged with an Axon 4000B scanner (Axon Instruments, Union City, Calif.) and image analysis was performed. The signal intensities were measured and analyzed by using GenePix Pro (version 4.0) software. The HPV genotype was determined by showing that a probe specific for a given HPV type displayed a reliable signal. We considered 55% pixels in a spot showing a signal 1.5-fold higher than that of the local background as a reliable signal.
Test of HPV DNA microarray specificity.
To test the specificities of the HPV DNA-specific probes, we performed HPV DNA microarray hybridizations with the type-specific probe clones labeled with fluorescent Cy5-dUTP. Twenty-seven HPV type-specific probes were cloned into a TA site of a different cloning vector, TOPO pCR2.1 (Invitrogen, Breda, The Netherlands). One nanogram (∼2.33 × 108 copies) of each cloned HPV probe was amplified with primers TOPO-LEFT and TOPO-RIGHT, together with 10 pg of the plasmid containing the GAPDH cDNA-specific probe, in the presence of Cy5-dUTP. Each product was independently hybridized onto an array of the 27 immobilized HPV-specific probes, and the hybridization results were evaluated by scanning the microarrays.
Test of HPV DNA microarray reproducibility.
To examine the consistency of MYH-PCR-mediated microarray hybridization, we performed a standard MYH-PCR in replicates in the presence of Cy5-dUTP using 1 ng of genomic DNA from a Caski cell (HPV-16 positive). Each 20% volume of the amplified products was hybridized on the HPV DNA microarray. Data are represented as the mean intensities of positive signals, with standard deviations, from three independent experiments.
Detection limit of HPV DNA microarray.
To assess the detection limit of the HPV DNA microarray, we performed a serial dilution test with plasmid DNA containing cloned HPV-16. The plasmid copy numbers were calculated from optical density measurements, and dilution series containing 1011 to 100 copies of HPV DNA were made. The standard PCR mixture contained 10 pg of the plasmid with the GAPDH cDNA-specific probe, 2 ng of HPV-negative C33A genomic DNA, Cy5-dUTP, and a serial diluent of the plasmid with the probe for HPV-16 in order to mimic the complex nucleic acid environment present during amplification of genomic DNA. After amplification, each 20% volume of PCR product was analyzed by agarose gel electrophoresis and hybridization by the HPV DNA microarray.
Statistical analysis.
Pearson's χ2 test was used to compare the correlation of the presence of HPV with the grade of differentiation, risk factors, and malignancy by the Excel program (Microsoft, Redmond, Wash.). Differences with P values of <0.01 were regarded as significant.
RESULTS
Design and specificity of HPV DNA microarray.
Our HPV DNA microarray contained 27 HPV type-specific probes and 3 kinds of probes as controls, including PCR-positive, HPV-positive, and negative controls, as shown in Fig. 2. The PCR-positive control determines the adequacy of the PCR for the target specimen. Since we selected the 180-bp sequence as an HPV-positive control probe corresponding to the highly conserved region of the L1 gene, which is common among most known types of HPV, the positive control is capable of detecting any type of HPV, if any is present.
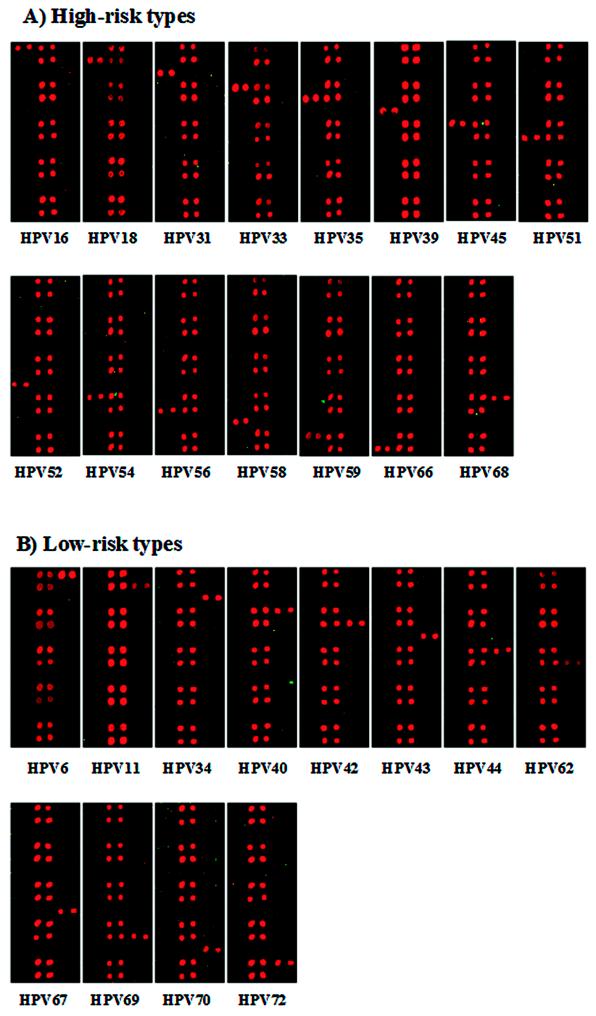
To evaluate the specificity of the HPV type-specific probes, we performed HPV DNA microarray hybridization with PCR amplicons from plasmids containing probes for the 27 HPV types. As shown in Fig. 3, all HPV-specific probes hybridized specifically to the corresponding targets of each of the HPV genotypes, and no cross-hybridization with other HPV types was observed. The hybridization signal intensities varied due to slight differences in probe sequences and amounts of PCR amplicons. Nonetheless, assignment to a genotype was not significantly affected.
FIG. 3.
Specificities of the type-specific probes for the 27 HPV types amplified from plasmids. (A) Hybridization results with high-risk type-specific probes; (B) hybridization results with low-risk type-specific probes. Each HPV type is indicated at the bottom.
Detection limit and reproducibility of the HPV DNA microarray.
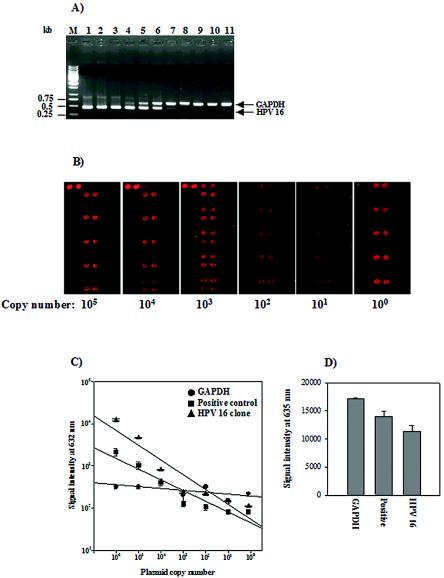
To assess the detection limit of the HPV DNA microarray, the serially diluted plasmid templates and a fixed amount of PCR control template were used for the end point dilution test, as described in Materials and Methods. After amplification, each 20% volume of the PCR products was analyzed by agarose gel electrophoresis and hybridization to the HPV DNA microarray (Fig. 4B and C). The HPV DNA microarray showed a highly significant linear relationship between the log of the input HPV copy number and the log of the signal intensities. The HPV DNA microarray efficiently detected 100 copies of the HPV starting template, while MYH-PCR detected 104 copies of the plasmid with the HPV-16-specific probe by agarose gel analysis. This result indicates that the detection limit of the HPV DNA microarray is at least 100-fold higher than that of the conventional MYH-PCR method under our analysis conditions. The signals for the HPV-positive control and HPV-16 varied with the HPV copy number, while the signals for the PCR control were steadily maintained (Fig. 4C).
FIG. 4.
Detection limit and reproducibility of the HPV DNA microarray. (A) Agarose gel electrophoresis of the MYH-PCR products with a serial dilution of the plasmid with the HPV-16-specific probe. Ten microliters of each 50-μl PCR product was separated on a 1.0% agarose gel. The input copy numbers of the plasmid with the HPV-16-specific probe are as follows in the indicated lanes: M, 1-kb ladder; 1, 1010 copies; 2, 109 copies; 3, 108 copies; 4, 107 copies; 5, 106 copies; 6, 105 copies; 7, 104 copies; 8, 103 copies; 9, 102 copies; 10, 10 copies; 11, 1 copy. (B) HPV DNA microarray hybridization results with the amplicon obtained by MYH-PCR of the plasmid with the HPV-16-specific probe. Ten microliters of the 50-μl PCR products derived from 105 to 100 copies were hybridized with HPV DNA on the microarray for 2 h at 55°C. The starting plasmid copy number is shown at the bottom. (C) Standard curves of signal intensities for HPV-16 with serial dilutions of 106 to 100 copies. Linear regression was based on the titration series for the plasmid with the HPV-16-specific probe, and each curve is based on the average of three replicates. (D) Reproducibility of the HPV DNA microarray. Three independent experiments were performed with the genomic DNA of Caski cells. The mean ± standard deviation was calculated from the signal intensity of each probe at 635 nm. Each probe is indicated at the bottom: GAPDH, PCR control; Positive, HPV-positive control; HPV-16, HPV-16-specific probe.
In addition, to test the consistency of HPV DNA microarray hybridization, we performed three independent microarray experiments with the same amount of DNA derived from the Caski cell line carrying HPV-16. The relative standard deviations of positive signals from the PCR control, the HPV control, and the HPV-16-specific probes were within 10% of the mean intensities (Fig. 4D). The results revealed that HPV DNA microarray hybridization shows a high degree of reproducibility.
HPV DNA microarray analysis with cell lines.
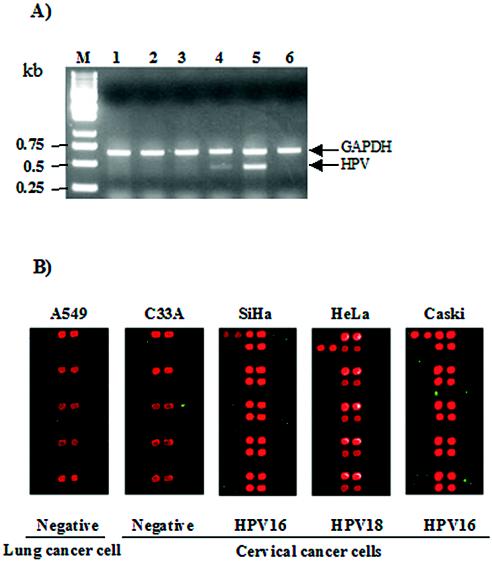
To address the applicability of the HPV DNA microarray, we carried out HPV DNA microarray hybridization with HPV-positive and -negative cell lines. The most representative HPV-positive cell lines, SiHa (HPV-16 positive; 1 to 10 copies per cell), HeLa (HPV-18 positive; 10 to 50 copies/cell), and Caski (HPV-16 positive; 60 to 600 copies/cell) cells, were examined. The results of the PCR and hybridization images with these cell lines are shown in Fig. 5. The MYH-PCR was performed in the presence of 1 ng of each template DNA, and the 20% volumes of the PCR products were separated on an agarose gel (Fig. 5A). The HPV-negative A549 and C33A cell lines showed no HPV DNA on the gel. The amounts of the PCR products for the HeLa and Caski cell lines are dependent on the viral copy number, while we did not observe any significant PCR product from SiHa cells carrying HPV at low copy numbers. The same amounts of PCR products were hybridized with the HPV DNA microarray (Fig. 5B). Positive signals appeared for the PCR control, the positive control, and the type-specific probes, as expected. We found that the PCR products of the SiHa and Caski cell genomic DNAs specifically hybridized to the HPV-16-specific probes and the PCR product of the HeLa cell genomic DNA hybridized to the HPV-18-specific probes. Hybridization signals were recorded only for the PCR controls for HPV-negative cell lines. The signal intensities of the HPV type-specific probes were related to the HPV copy number present in the cell lines and closely matched the PCR results.
FIG. 5.
HPV genotyping results with the cell lines. (A) Agarose gel electrophoresis of the amplified products from the cell lines. Ten microliters of each 50-μl PCR product was separated on a 1.0% agarose gel. Lanes: M, 1-kb ladder; 1, A549 cells; 2, C33A cells; 3, SiHa cells; 4, HeLa cells; 5, Caski cells; 6, PCR control. (B) Ten microliters of each 50-μl PCR product was hybridized with the HPV DNA microarray at 55°C for 2 h. HPV-negative cell lines A549 and C33A showed hybridization signals with the PCR controls only. HPV-positive hybridization signals were shown with the SiHa (HPV-16 positive), HeLa (HPV-18 positive), and Caski (HPV-16 positive) cell lines. The cell lines are indicated at the top, and the HPV types are indicated at the bottom.
Prevalence of HPV in tonsillar squamous carcinoma.
To examine the clinical performance of the HPV DNA microarray, we performed HPV DNA microarray experiments with 73 clinical tonsillar tissue samples from 39 patients with tonsillar squamous carcinoma. Of the 39 patients (mean age, 58.2 years; age range, 32 to 77 years), 35 were men (mean age, 58.9 years; age range, 32 to 77 years) and 4 were women (mean age, 52 years; age range, 40 to 62 years). Thirty-nine of the 73 specimens were malignant tonsillar squamous epithelia and 34 were from an adjacent nonmalignant part. As shown in Table 3, the tonsillar carcinoma tissues of 25 (64.1%) of the 39 patients were found to be HPV positive, while nonmalignant tonsillar squamous epithelia from 3 (8.8%) of 34 patients were HPV positive. HPV-16 was the most frequent HPV type found in the HPV-positive carcinomas (23 of 25 samples) and nonmalignant epithelia (3 of 3 samples). One patient was found to be infected with HPV-33, and another patient was found to be coinfected with HPV-6 and HPV-58. The results showed significant hybridization signals and a high degree of specificity to probes specific for the corresponding genotype. We carried out the χ2 test (P < 0.01) to determine the correlation of HPV infection with malignancy, the grade of differentiation, and two risk factors (Table 3). The results revealed that the presence of HPV showed no significant correlation with histology (P = 0.603), smoking (P = 0.43), or drinking of alcohol (P = 0.04), while the presence of HPV was significantly correlated with malignancy (P = 0.001).
TABLE 3.
Correlation of HPV prevalence with clinical data in tonsillar carcinomasa
| Malignancyb
|
Histologyc
|
Smokingd
|
Drinkinge
|
||||
|---|---|---|---|---|---|---|---|
| Type | Frequencyf | Type | Frequency | Amt | Frequency | Amt | Frequency |
| T | 25/39 (64.1) | WD | 2/3 (66.67) | − | 3/5 (60.0) | − | 1/5 (20.0) |
| N | 3/34 (8.8) | MD | 15/25 (60.0) | + | 3/6 (50.0) | + | 7/8 (87.5) |
| PD | 8/11 (72.7) | ++ | 21/28 (75.0) | ++ | 18/26 (69.2) | ||
P values were calculated by the χ2 test and were considered statistically significant if the P value was <0.01. The P values were 0.001, 0.603, 0.43, and 0.04 for the malignancy, histology, smoking, and drinking groups, respectively.
N, nontumor; T, tumor.
Cell tumor differentiation grade according to histopathology: MD, moderate differentiation; PD, poor differentiation; WD, good differentiation.
−, nonsmoker; +, smoker for less than 20 years; ++, smoker for more than 20 years.
−, nondrinker; +, less than one alcoholic drink per week; ++, more than one alcoholic drink per week.
Frequency data are presented as number of samples HPV DNA positive/total number of samples tested (percent).
DISCUSSION
It is important that HPV genotypes be determined by as elaborate a method as possible, because the HPV genotype provides information useful for the prognosis of malignant degeneration. Although PCR is known to be the most sensitive method for the detection of HPV infection in clinical samples (17, 23), it has some drawbacks, in that false-positive results because of amplification of related organisms can occur and false-negative amplifications may result from variations in the sequences of the primer binding sites for the target region of the virus. Furthermore, in order to type the consensus amplicon, additional labor-intensive procedures, such as sequencing or type-specific PCR, are required (27). Recently, several studies involving genotyping of HPV with type-specific oligonucleotides and by DNA microarray analysis have been reported (1, 4, 12, 16). However, those reports did not address technical details, including type specificity and reproducibility in studies with type-specific oligonucleotides, nor did they present evidence of the clinical performance of DNA microarray analysis. In this work, we report on a more useful MYH-PCR-mediated HPV DNA microarray system capable of genotyping 15 high-risk HPV types and 12 low-risk HPV types.
We chose the hypervariable region within the L1 genes of the 27 HPV types for use as type-specific probes for HPV genotyping and synthesized 30-bp oligonucleotides whose sequences were specific for the 27 types. All oligonucleotides selected contained more than six mismatches with the corresponding regions of the other HPVs. Although a high copy number, equal to 108 copies of the HPV-specific probe on the plasmid used as the template, was used for the PCR, a signal derived from cross-hybridization was not observed. This result indicates that even high yields of HPV DNA from clinical samples will not induce cross-hybridization. The chance of cross-hybridization in assays with cervical swab specimens and other types of clinical samples can thus be ignored. We also measured the detection limit of the HPV DNA microarray. HPV DNA microarray hybridization with the same amount of starting template showed a higher detection limit than MYH-PCR alone. In addition, we tested the reproducibilities of the HPV DNA microarray experiments. The relative standard deviations of positive signals for each probe were found to be within 10% of the mean values. This result indicates that the hybridization results showed a high degree of reproducibility.
The preclinical performance of the HPV DNA microarray was evaluated by testing the cell lines A549 (HPV negative), C33A (HPV negative), SiHa (HPV-16), HeLa (HPV-18 positive), and Caski (HPV-16 positive). In the microarray hybridization experiments with HPV-positive cell lines, hybridization signals appeared for each known type of HPV probe and HPV-positive controls, while HPV-negative cell lines did not show any positive hybridization signal with the type-specific probes. The signal intensities of the type-specific probes are related to the HPV copy number present in the cells. This result indicates that the HPV DNA microarray is able to determine the relative viral loads by the differences in hybridization signals.
On the basis of the results obtained with the HPV DNA microarray and the different cell lines, we examined the HPV DNA microarray in clinical practice. We performed HPV genotyping with 34 samples of nonmalignant tissues and 39 samples of malignant tissues from 39 Korean patients with tonsillar squamous carcinoma. It is known that the frequency of HPV identified in patients with head and neck cancer varies widely (2 to 76%), depending on clinical preparation of the patients, the materials and methods used for analysis, as well as the number of cases included (6, 20, 21, 24). In this work, HPV DNA was found in 25 (64.1%) of the 39 carcinoma tissue samples. Recently, an increasing number of reports have shown that among all head and neck carcinomas the high-risk HPV type HPV-16 is the most frequent HPV type detected in tonsillar carcinomas (45 to 70%) (20-22). Our data also showed that the most frequent HPV type in tonsillar carcinomas is HPV-16. In addition, we have analyzed the correlation of the HPV infection status with malignancy, the grade of differentiation, and two risk factors, including smoking and drinking. The results showed that the presence of HPV is not correlated with the grade of differentiation or risk factors, while it was closely correlated with malignancy, as shown in Table 3. Our results support the hypothesis that the presence of HPV is closely associated with tonsillar carcinomas. In the HPV DNA microarray hybridization experiments with clinical samples, we did not observe any false-positive or -negative signals compared with the results of detection by PCR under our experimental conditions. Because the use of an extremely sensitive and reliable method is required for the diagnosis of HPV infection in clinical practice, the use of the HPV DNA microarray technology has distinct advantages.
In conclusion, we developed a microarray-based system for HPV genotyping. Our data demonstrate that HPV DNA microarray analysis coupled with MYH-PCR can be applied to HPV detection and genotyping. This diagnostic tool will undoubtedly be useful for the clinical diagnosis of HPV infection and large-scale epidemiological studies.
Acknowledgments
We thank Chiwang Yoon and Daekyung Yoon (GenomicTree, Inc.) for fabrication of the HPV DNA microarrays.
REFERENCES
- 1.An, H. J., N. H. Cho, S. Y. Lee, I. H. Kim, C. Lee, S. J. Kim, M. S. Mun, S. H. Kim, and J. K. Jeong. 2003. Correlation of cervical carcinoma and precancerous lesions with human papillomavirus (HPV) genotypes detected with the HPV DNA chip microarray method. Cancer 97:1672-1680. [DOI] [PubMed] [Google Scholar]
- 2.Bernard, H. U., S. Y. Chan, M. M. Manos, C. K. Ong, L. L. Villa, H. Delius, C. L. Peyton, H. M. Bauer, and C. M. Wheeler. 1994. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J. Infect. Dis. 170:1077-1085. [DOI] [PubMed] [Google Scholar]
- 3.Chan, S. Y., H. Delius, A. L. Halpern, and H. U. Bernard. 1995. Analysis of genomic sequences of 95 papillomavirus types: uniting typing, phylogeny, and taxonomy. J. Virol. 69:3074-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho, N. H., H. J. An, J. K. Jeong, S. Kang, J. W. Kim, Y. T. Kim, and T. K. Park. 2003. Genotyping of 22 human papillomavirus types by DNA chip in Korean women: comparison with cytologic diagnosis. Am. J. Obstet. Gynecol. 188:56-62. [DOI] [PubMed] [Google Scholar]
- 5.Cox, J. T., A. T. Lorincz, M. H. Schiffman, M. E. Sherman, A. Cullen, and R. J. Kurman. 1995. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am. J. Obstet. Gynecol. 172:946-954. [DOI] [PubMed] [Google Scholar]
- 6.Friesland, S., H. Mellin, E. Munck-Wikland, A. Nilsson, J. Lindholm, T. Dalianis, and R. Lewensohn. 2001. Human papilloma virus (HPV) and p53 immunostaining in advanced tonsillar carcinoma—relation to radiotherapy response and survival. Anticancer Res. 21:529-534. [PubMed] [Google Scholar]
- 7.Frisch, M., and R. Biggar. 1999. Aetiological parallel between tonsillar and anogenital squamous-cell carcinomas. Lancet 354:1442-1443. [DOI] [PubMed] [Google Scholar]
- 8.Gaarenstroom, K. N., P. Merkert, J. M. M. Walboomers, A. J. C. van den Brule, P. J. F. van Bommel, C. J. L. M. Meijer, F. J. Voorhorst, P. Kenemans, and T. J. M. Helmerhorst. 1994. Human papillomavirus DNA and genotypes: prognostic factors for progression of cervical intraepithelial neoplasia. Int. J. Gynecol. Cancer 4:73-78. [DOI] [PubMed] [Google Scholar]
- 9.Graviit, P. E., C. L. Peyton, T. Q. Alessi, C. M. Wheeler, F. Coutlée, A. Hildesheim, M. H. Schiffman, D. R. Scott, and R. J. Apple. 2000. Improved amplification of genital human papillomavirus. J. Clin. Microbiol. 38:357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart, K. W., O. M. Williams, N. Thelwell, A. N. Fiander, T. Brown, L. K. Borysiewicz, and C. M. Gelder. 2001. Novel method for detection, typing, and quantification of human papillomavirus in clinical samples. J. Clin. Microbiol. 39:3204-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemminski, K., C. Dong, and M. Frisch. 2000. Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur. J. Cancer Prev. 9:433-437. [DOI] [PubMed] [Google Scholar]
- 12.Hwang, T. S., J. K. Jeong, M. Park, H. S. Han, H. K. Choi, and T. S. Park. 2003. Detection and typing of HPV genotypes in various cervical lesions by HPV oligonucleotide microarray. Gynecol. Oncol. 90:51-56. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs, M. V., A. M. de R. Husman, A. J. C. van den Brule, P. J. F. Snijders, C. J. L. Miejer, and J. M. M. Walboomers. 1995. Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. J. Clin. Microbiol. 33:901-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefsson, A., K. Livak, and U. Gyllensten. 1999. Detection and quantitation of human papillomavirus by using the fluorescent 5′ exonuclease assay. J. Clin. Microbiol. 37:490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataja, V., S. Syrjanen, R. Mantyhjarvi, M. Yliskoski, S. Saarikoski, and K. Syrjanen. 1992. Prognositic factors for cervical human papillomavirus infections. Sex. Transm. Dis. 19:154-160. [DOI] [PubMed] [Google Scholar]
- 16.Kim, C. J., J. K. Jeong, M. Park, T. S. Park, T. C. Park, S. E. Namkoong, and J. S. Park. 2003. HPV oligonucleotide microarray-based detection of HPV genotypes in cervical neoplastic lesions. Gynecol. Oncol. 89:210-217. [DOI] [PubMed] [Google Scholar]
- 17.Kleter, B., L. van Doorn, L. Schrauwen, A. Molijn, S. Sastrowijoto, J. ter Schegget, J. Lindeman, B. ter Harmsel, M. Burger, and W. Quint. 1999. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J. Clin. Microbiol. 37:2508-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, C. H., W. L. Ma, R. Shi, Y. Q. Ou, B. Zhang, and W. L. Zheng. 2003. Possibility of using DNA chip technology for diagnosis of human papillomavirus. J. Biochem. Mol. Biol. 36:349-353. [DOI] [PubMed] [Google Scholar]
- 19.Manos, M. M., Y. Ting, D. K. Wright, A. J. Lewis, T. R. Broker, and S. M. Wolinsky. 1989. The use of polymerase chain reaction amplification for the detection of genital human papillomavirus. Cancer Cells 7:209-214. [Google Scholar]
- 20.Mckaig, R. G., R. S. Baric, and A. F. Olshan. 1997. Human papillomavirus and head and neck cancer: epidemiology and molecular biology. Head Neck 20:250-265. [DOI] [PubMed] [Google Scholar]
- 21.Mellin, H., S. Friesland, R. Lewensohn, T. Dallianis, and E. Munck-Wikland. 2000. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int. J. Cancer 89:300-304. [PubMed] [Google Scholar]
- 22.Mellin, H., L. Dahlgren, E. Munck-Wikland, J. Lindholm, H. Rabbani, M. Kalantari, and E. Dalianis. 2002. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int. J. Cancer 102:152-158. [DOI] [PubMed] [Google Scholar]
- 23.Qu, W., G. Jiang. Y. Cruz, C. J. Chang, G. Y. F. Ho, R. S. Klein, and R. D. Burk. 1997. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J. Clin. Microbiol. 35:1304-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snijders, P. J. F., F. V. Cromme, A. J. C. van den Brule, H. F. J. Schrijnemakers, G. B. Snow, C. J. L. M. Meijer, and J. M. M. Walboomers. 1992. Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int. J. Cancer 51:845-850. [DOI] [PubMed] [Google Scholar]
- 25.Stemmer, W. P. C., A. Crameri, D. H. Kim, T. M. Brennan, and H. L. Heyneker. 1995. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene 164:49-53. [DOI] [PubMed] [Google Scholar]
- 26.Vernon, S. D., E. R. Unger, and D. Williams. 2000. Comparison of human papillomavirus detection and typing by cycle sequencing, line blotting, and hybrid capture. J. Clin. Microbiol. 38:651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, D., L. Coscoy, M. Zylberberg, P. C. Avila, H. A. Boushey, D. Ganem, and J. L. DeRisi. 2002. Microarray-based detection and genotyping of viral pathogens. Proc. Natl. Acad. Sci. USA 26:15687-15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilczynski, S. P., B. T. Y. Lin, X. Xie Yuan, and I. B. Paz. 1998. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinomas. Am. J. Pathol. 152:145-156. [PMC free article] [PubMed] [Google Scholar]
- 29.zur Hausen, H. 1996. Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta 1288:F55-F78. [DOI] [PubMed] [Google Scholar]