Abstract
The aim of this study is to investigate chemical composition of acetone extracts of the lichens Parmelia arseneana and Acarospora fuscata and in vitro antioxidant, antimicrobial, and anticancer activities of these extracts and gyrophoric acid isolated from A. fuscata. The HPLC-UV method was used for the identification of secondary metabolites. Stictic acid, norstictic acid, gyrophoric acid, usnic acid, atranorin and chloroatranorin were identified in the A. fuscata. In P. arseneana, we detected stictic acid, norstictic acid, usnic acid and atranorin, while gyrophoric acid was not identified. Antioxidant activity was evaluated by measuring the scavenging capacity of tested samples on DPPH and superoxide anion radicals, reducing the power of samples and determination of total phenolic compounds in extracts. As a result of the study, gyrophoric acid was found to have the largest DPPH radical scavenging activity with an IC50 value of 105.75 µg/ml. Moreover, the tested samples had an effective superoxide anion radical scavenging and reducing power. The total content of phenol in extracts was determined as pyrocatechol equivalent. The antimicrobial activity was estimated by determination of the minimal inhibitory concentration by the broth microdilution method. The most active was also gyrophoric acid, with minimum inhibitory concentration values ranging from 0.019 to 1.25 mg/ml. Anticancer activity was tested against LS174 (human colon carcinoma cell line), A549 (human lung carcinoma cell line), Fem-x (malignant melanoma cell line), and a chronic myelogeneous leukaemia K562 cell line using the MTT method. Extract of P. arseneana expressed the strongest anticancer activity against all cell lines with IC50 values ranging from 11.61 to 47.06 µg/ml.
Keywords: HPLC analysis, biological activities, lichens, lichens compound
Introduction
Lichens are symbiotic organisms consisting of a fungus partner and a photosynthetic organism, either an alga or Cyanobacteria (Grube and Berg, 2010[11]; Bates et al., 2011[2]). More than 20,000 known species of lichens have been identified and inhabit diverse ecosystems ranging from Arctic tundra to desert climates (Oboh and Ademosun, 2006[26]). They are ubiquitous on barks, stems, leaves and in soil but often grow in habitats that are less favourable for higher plants (Vrablikova et al., 2006[35]). These organisms have historically been used as a cure for human diseases, food, dyes, in the production of alcohol and in the perfume industry (Bown, 2001[4]).
New studies have revealed that these slow-growing organisms produce a diverse array of secondary metabolites with different biological activities (Johnson et al., 2011[15]). Lichens are secondary metabolites, mostly synthesised from fungal metabolism. They are crystal deposits on the surface of hiphes, which poorly dilute in water and can usually be isolated from lichen by organic diluents (Otzurk et al., 1999[28]).
The biological potential of many lichens and their metabolites has largely remained unexplored. Thus, the aim of the present work was to identify the secondary metabolites of P. arseneana and A. fuscata by HPLC-UV and to evaluate the antioxidant capacity, antimicrobial and anticancer activities of the acetone extracts from this lichen as well as isolated gyrophoric acid.
Material and Methods
Lichen samples
Lichen samples of P. arseneana Gyeln, and A. fuscata (Nyl.) Arnold., were collected from Kopaonik, Serbia, in September 2012. The voucher specimen of the lichens (Voucher No. 113 and 119) was deposited at the Department of Biology and Ecology, Faculty of Science, University of Kragujevac, Serbia. The determination of the investigated lichens was accomplished using standard methods.
Preparation of the lichen extracts
Finely dry ground thalli of the examined lichens (100 g) were extracted using acetone in a Soxhlet extractor. The extracts were filtered and then concentrated under reduced pressure in a rotary evaporator. The dry extracts were stored at -18 °C until they were used in the tests. The extracts were dissolved in 5 % dimethyl sulphoxide (DMSO) for the experiments. DMSO was dissolved in sterile distilled water to the desired concentration.
High Performance Liquid Chromatography (HPLC) analysis
The acetone extracts of the tested lichens were re-dissolved in 500 µl of acetone and analysed on an Agilent Technologies HPLC instrument 1200 Series with C18 column (250 mm x 4.6 mm, 5 µm) and a UV spectrophotometric detector with detection at three wavelengths. The same compounds were detected in all selected wavelengths (254, 280 and 320 nm). Methanol–water–phosphoric acid (90:10:0.9, v/v/v) was used as a solvent. Methanol was of HPLC grade and was purchased from Merck (Darmstadt, Germany). Deionised water used throughout the experiments was generated by a Milli-Q academic water purification system (Milford, MA, USA). Phosphoric acid was an analytical-grade reagent. The flow rate was 1.0 ml/min and the sample injection volume was 10 µl. The standards used were obtained from the following sources: stictic acid (tR = 2.89 ± 0.20 min) was isolated from the lichen Xanthoparmelia conspersa, norstictic acid (tR = 3.41 ± 0.10 min) from the lichen Ramalina furinacea, gyrophoric acid (tR = 4.12 ± 0.10 min) from Umbilicaria fructulosa, and usnic acid (tR = 7.96±0.20 min), atranorin (tR = 8.56 ± 0.10 min) and chloroatranorin (tR = 10.90 ± 0.10 min) from the lichen Evernia prunastri. The standard samples were isolated in our laboratory and their structures were confirmed by spectral data.
The isolation of gyrophoric acid from the lichen A. fuscata
The acetone extract of the lichen A. fuscata (500 mg) was dissolved in benzene and after filtration the residue was fractioned on a silica gel 60 column (under 0.063 mm; 230 mesh). The column was eluted with methanol-chloroform gradient solvent (20:1, 10:1 and 5:1) yielding nine fractions. The sixth eluted fraction of the lichen extract contained gyrophoric acid. This compound was used for the structural identification and determination of antioxidant, antimicrobial and cytotoxic activities. Gyrophoric acid was identified by the melting point, elemental analysis and 1H and 13C-NMR data (Huneck and Yoshimura, 1996[13]). The purity of the isolated lichen acid was determined by HPLC-DAD and amounted to 97.8 %.
Antioxidant activity
Scavenging DPPH radicals
The free radical scavenging activity of samples was measured by 1,1-diphenyl-2-picryl-hydrazil (DPPH). The method used is similar to the method previously used by some authors (Ibanez et al., 2003[14]; Dorman et al., 2004[8]) but was modified. Two millilitres of methanol solution of DPPH radical in the concentration of 0.05 mg/ml and 1 ml of test samples (1000, 500, 250, 125 and 62.5 µg/ml) were placed in cuvettes. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then, the absorbance was measured at 517 nm in a spectrophotometer (“Jenway” UK). Ascorbic acid was used as a positive control. The DPPH radical concentration was calculated using the following equation:
DPPH scavenging effect (%) = [(A0 - A1) / A0] x 100
where A0 is the absorbance of the negative control and A1 is the absorbance of reaction mixture or standard.
The inhibition concentration at 50 % inhibition (IC50) was the parameter used to compare the radical scavenging activity.
Reducing power
The reducing power of samples was determined according to the method of Oyaizu (1986[29]). One millilitre of test samples (1000, 500, 250, 125 and 62.5 µg/ml) was mixed with 2.5 ml of phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1 %). The mixtures were incubated at 50 °C for 20 min. Then, trichloroacetic acid (10 %, 2.5 ml) was added to the mixture and the sample was centrifuged. Finally, the upper layer was mixed with distilled water (2.5 ml) and ferric chloride (0.5 ml; 0.1 %). The absorbance of the solution was measured at 700 nm in spectrophotometer (“Jenway” UK). Higher absorbance of the reaction mixture indicated that the reducing power was increased. Ascorbic acid was used as a positive control.
Superoxide anion radical scavenging activity
The superoxide anion radical scavenging activity of samples was detected according to the method of Nishimiki et al. (1972[25]). Briefly, 0.1 ml of test samples (1000, 500, 250, 125 and 62.5 µg/ml) was mixed with 1 ml nitroblue tetrazolium (NBT) solution (156 µM in 0.1 M phosphate buffer, pH 7.4) and 1 ml nicotinamide adenine dinucleotide (NADH) solution (468 µM in 0.1 M phosphate buffer, pH 7.4). The reaction was started by adding 100 µL of phenazine methosulphate (PMS) solution (60 µM in 0.1 M phosphate buffer, pH 7.4). The mixture was incubated at room temperature for 5 min, and the absorbance was measured at 560 nm in spectrophotometer (“Jenway” UK) against blank samples. Decreased absorbance indicated increased superoxide anion radical scavenging activity. Ascorbic acid was used as a positive control. The percentage inhibition of superoxide anion generation was calculated using the following formula:
Superoxide anion scavenging activity (%) = [(A0 - A1) / A0] x 100
where A0 is the absorbance of the negative control and A1 is the absorbance of reaction mixture or standards.
The inhibition concentration at 50 % inhibition (IC50) was the parameter used to compare the radical scavenging activity.
Determination of total phenolic compounds
Total soluble phenolic compounds in the acetone extracts were determined with Folin-Ciocalteu reagent according to the method of Slinkard and Singleton (1997[33]), using pyrocatechol as a standard phenolic compound. Briefly, 1 ml of the extract (1 mg/ml) in a volumetric flask was diluted with distilled water (46 ml). One millilitre of Folin-Ciocalteu reagent was added and the content of the flask was mixed thoroughly. After 3 min, 3 ml of sodium carbonate (2 %) was added and then allowed to stand for 2 h with intermittent shaking. The absorbance was measured at 760 nm in in spectrophotometer (“Jenway” UK). The total concentration of phenolic compounds in the extract was determined as micrograms of pyrocatechol equivalent (PE) per milligram of dry extract by using an equation that was obtained from a standard pyrocatechol graph as follows:
Absorbance = 0.0057 x total phenols [µg PE/mg of dry extracts] - 0.1646 (R2 = 0.9203)
Antimicrobial activity
Microorganisms and media
The following bacteria were used as test organisms in this study: Bacillus mycoides (ATCC 6462), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922) and Klebsiella pneumoniae (ATCC 13883). All of the bacteria used were obtained from the American Type Culture Collection (ATCC). The fungi used as test organisms were: Aspergillus flavus (ATCC 9170), Aspergillus fumigatus (DBFS 310), Candida albicans (ATCC 10231), Penicillium purpurescens (DBFS 418) and Penicillium verrucosum (DBFS 262). They were from the American Type Culture Collection (ATCC) and the mycological collection maintained by the Mycological Laboratory within the Department of Biology of Kragujevac University's Faculty of Science (DBFS). Bacterial cultures were maintained on Müller-Hinton agar substrates (Torlak, Belgrade). Fungal cultures were maintained on potato dextrose (PD) agar and Sabourad dextrose (SD) agar (Torlak, Belgrade). All cultures were stored at 4 °C and sub-cultured every 15 days.
The sensitivity of microorganisms to tested samples was tested by determining the minimal inhibitory concentration (MIC).
Bacterial inoculi were obtained from bacterial cultures incubated for 24 h at 37 °C on Müller-Hinton agar substrate and brought up by dilution according to the 0.5 McFarland standard to approximately 108 CFU/ml. Suspensions of fungal spores were prepared from fresh mature (3- to 7-day-old) cultures that grew at 30 °C on a PD agar substrate. Spores were rinsed with sterile distilled water, used to determine turbidity spectrophotometrically at 530 nm, and then further diluted to approximately 106 CFU/ml according to the procedure recommended by NCCLS (1998[24]).
Minimal Inhibitory Concentration (MIC)
The minimal inhibitory concentration (MIC) was determined by the broth microdilution method with using 96-well micro-titre plates (Sarker et al., 2007[32]). A series of dilutions with concentrations ranging from 40 to 0.0047 mg/ml for extracts and component was used in the experiment against every microorganism tested. The starting solutions of test samples were obtained by measuring a certain quantity of samples and dissolving this in DMSO. Two-fold dilutions of test samples were prepared in Müller-Hinton broth for bacterial cultures and SD broth for fungal cultures. The minimal inhibitory concentration was determined with resazurin. Resazurin is an oxidation-reduction indicator used for the evaluation of microbial growth. It is a blue non-fluorescent dye that becomes pink and fluorescent when reduced to resorufin by oxidoreductases within viable cells. The boundary dilution without any changing colour of resazurin was defined as the mini-mal inhibitory concentration (MIC) for the tested microorganism at the given concentration. As a positive control of growth inhibition, streptomycin was used in the case of bacteria, and ketoconazole in the case of fungi. A DMSO solution was used as a negative control for the influence of the solvents. All experiments were performed in triplicate.
Cytotoxic activity
Cell culture
Human colon carcinoma LS174 cells, human lung carcinoma A549 cells, malignant melanoma Fem-x cells and chronic myelogeneous leukaemia K562 cells (American Type Culture Collection, USA) were cultured as a monolayer in the RPMI 1640 nutrient medium, with 10 % (inactivated at 56 °C) FBS, 3 mM of L-glutamine, and antibiotics, at 37 °C in humidified air atmosphere with 5 % CO2. For the growth of MDA-MB-361 and MDA-MB-453 cells, complete medium was enriched with 1.11 g/l glucose.
In vitro cytotoxic assay
In vitro assay for cytotoxic activity of investigated extract was performed when the cells reached 70-80 % confluence. Stock solution (50 mg/ml) of extract was dissolved in corresponding medium to the required working concentrations. Neoplastic LS174 cells (7000 cells per well), A549 cells (5000 cells per well), Fem-x cells (5000 cells per well) and K562 cells (5000 cells per well) were seeded into 96-well micro-titre plates, and 24 h later, after cell adherence, 5 different, double-diluted concentrations of investigated extract were added to the wells. Final concentrations of the extract were 200, 100, 50, 25, and 12.5 µg/ml, except for the control wells, where only nutrient medium was added. The cultures were incubated for the next 72 h. The effect on cancer cell survival was determined 72 h after the addition of extract, by the MTT test (Mosmann, 1983[23]). Briefly, 20 µl of MTT solution (5 mg/ml PBS) was added to each well and incubated for a further 4 h at 37 °C in 5 % CO2 and humidified air. Subsequently, 100 µl of 10 % SDS was added to solubilise the formazan crystals formed from MTT after the conversion by mitochondrial dehydrogenases of viable cells. Absorbencies proportional to the number of viable cells were measured using a microplate reader (Multiskan EX, Thermo Scientific, Finland) at 570 nm. Each experiment was performed in triplicate and independently repeated at least four times.
Flow cytometry analysis
Cellular DNA content and cell distribution were quantified by flow cytometry using propidium iodide (PI). Cells (3×105 cells/well) were seeded in 6-well plates and incubated with or without IC50 concentration of investigated extract for 24 h. After treatment, the cells were collected by trypsinisation, and fixed in ice-cold 70 % ethanol at -20 °C overnight. After fixation, the cells were washed in PBS and pellets obtained by centrifugation were treated with RNase (100 µg/ml) at 37 °C temperature for 30 min and then incubated with propidium iodide (PI) (40 µg/ml) for at least 30 min. DNA content and cell cycle distribution were analysed using a Becton Dickinson FAC-Scan flow cytometer. Flow cytometry analysis was performed using a CellQuestR (Becton Dickinson, San Jose, CA, USA), on a minimum of 10,000 cells per sample (Clothier, 1995[7]).
Statistical analyses
Statistical analyses were performed with the EXCEL and SPSS software packages. To determine the statistical significance of antioxidant activity, Student’s t-test was used. All values are expressed as mean ± SD of three parallel measurements.
Results
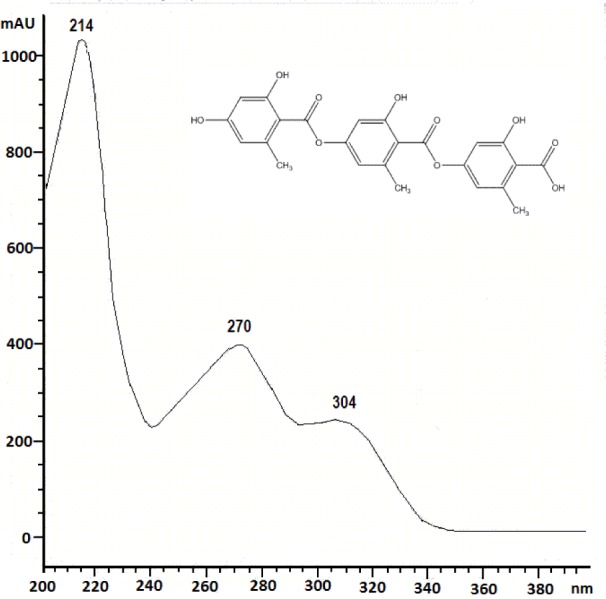
The present paper deals with the phytochemical analysis of acetone extracts from the species A. fuscata and P. arseneana, and their antioxidant, antimicrobial and anti-cancer activities. Figures 1(Fig. 1) and 2(Fig. 2) show the HPLC chromatograms of the standards and the acetone extracts from the species A. fuscata and P. arseneana. Six metabolites, stictic acid, norstictic acid, gyrophoric acid, usnic acid atranorin and chloroatranorin, were identified in A. fuscata. On the other hand, the HPLC chromatogram for P. arseneana showed the presence of stictic acid, norstictic acid, usnic acid and atranorin, with gyrophoric acid not being identified. Identification of these compounds was achieved by comparison of their tR values with the standard substances previously isolated from lichens. Table 1(Tab. 1) shows the retention time of the detected lichen substances and their absorbance maxima (nm). Gyrophoric acid (4-[4-(2,4-dihydroxy-6-methylbenzoyl)oxy-2-hydroxy-6-methylbenzoyl]oxy-2-hydroxy-6-methylbenzoic acid is very interesting because the activity of tridepsides has not been well studied. Because of that, after HPLC analysis of the tested lichen extracts, gyrophoric acid (tR = 4.18 min) was isolated from the acetone extracts of A. fuscata and used for antioxidant, antimicrobial and anti-tumour activities. Gyrophoric acid has specific UV spectra with absorption maxima at 214, 270 and 304 nm (Figure 3(Fig. 3)).
Figure 1. Chromatogram of the standards used for the identification of compounds present in Acarospora fuscata and Parmelia arseneana.
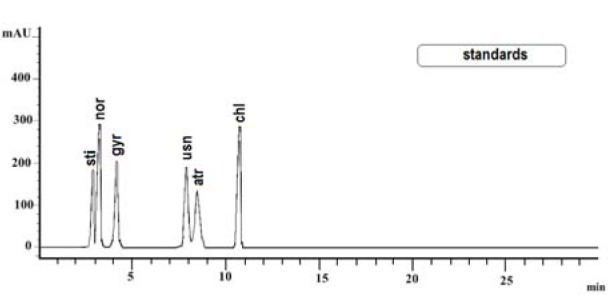
Figure 2. The HPLC chromatogram acquired at 254 nm of the extracts from Acarospora fuscata and Parmelia arseneana.
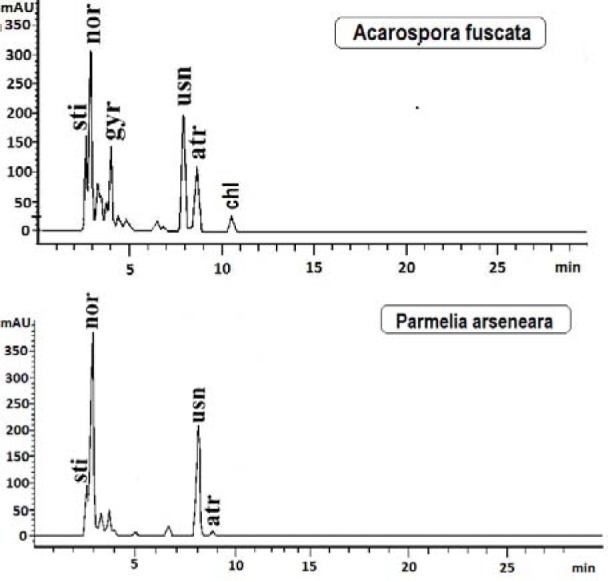
Table 1. Retention time of the examined lichen substances and their absorbance maxima (nm).
Figure 3. UV spectrum of gyrophoric acid.
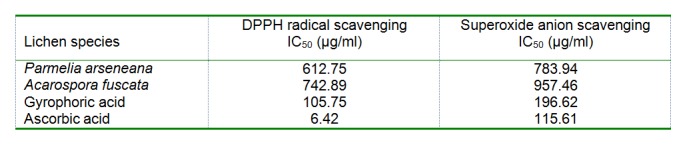
The scavenging DPPH radicals of the studied samples are shown in Table 2(Tab. 2). The IC50 values of both extracts and compounds ranged from 105.75 to 742.89 µg/ml. There was a statistically significant difference between samples and control (P<0.05). Among the tested extracts, acetone extract from P. arseneana showed the largest DPPH radical scavenging activity (IC50 = 612.75 µg/ml). The isolated lichen component demonstrated very strong DPPH radical scavenging activity, greater than those from extracts. The IC50 value for gyrophoric acid was 105.75 µg/ml.
Table 2. DPPH radical scavenging activity and superoxide anion scavenging activity of acetone extracts of Parmelia arseneana and Acarospora fuscata and isolated gyrophoric acid.
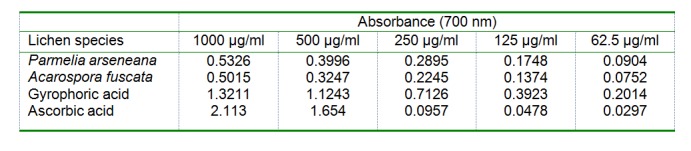
The results of the reducing power assay of the tested extracts and compound are summarised in Table 3(Tab. 3). Higher absorbance indicates higher reducing power. As shown in Table 3(Tab. 3), gyrophoric acid showed the highest reducing power.
Table 3. Reducing power of acetone extracts of Parmelia arseneana and Acarospora fuscata and isolated gyrophoric acid.
The scavenging of superoxide anion radicals by the tested lichen extracts and compounds is shown in Table 2(Tab. 2). There was a statistically significant difference between samples and control (P<0.05). The isolated lichen component showed the highest superoxide anion radical scavenging activity (the IC50 was 196.62 µg/ml). The scavenging activities of tested extracts were somewhat lower.
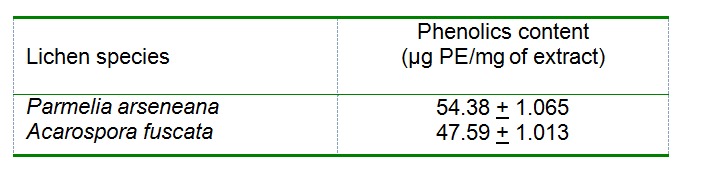
The total phenolic contents of the tested lichen extracts are given in Table 4(Tab. 4). The total phenolics contents of the acetone extracts of P. arseneana and A. fuscata were 54.38 and 47.59 µg PE/mg, respectively.
Table 4. Total phenolics content of acetone extracts of Parmelia arseneana and Acarospora fuscata.
Various antioxidant activities were compared to ascorbic acid. The results showed that standard antioxidants had stronger activity than tested samples.
The antimicrobial activities of the lichen extracts and isolated component against the test microorganisms are shown in Table 5(Tab. 5). The extract from P. arseneana showed moderate antibacterial and antifungal activity. They inhibited the microorganisms tested at concentrations from 0.078 to 10 mg/ml. Extracts from A. fuscata also inhibited all of the tested microorganisms, but at higher concentrations. The strongest antimicrobial activity was found in gyrophoric acid, which inhibited all of the species of bacteria and fungi in extremely low amounts (Table 5(Tab. 5)).
Table 5. Minimum inhibitory concentration (MIC) of acetone extracts of Parmelia arseneana and .
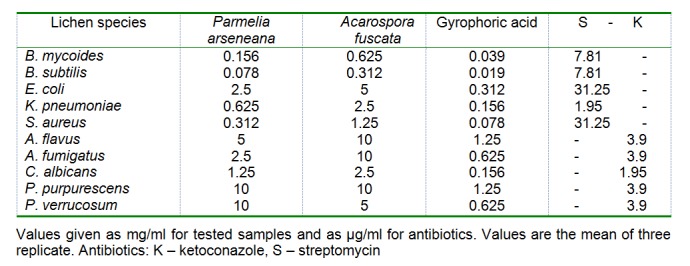
Acarospora fuscata and isolated gyrophoric acid
The antimicrobial activity was compared with the standard antibiotics streptomycin (for bacteria) and ketoconazole (for fungi). The results showed that standard antibiotics had stronger activity than tested samples, as shown in Table 5(Tab. 5). In the negative control, DMSO had no inhibitory effect on the tested organisms.
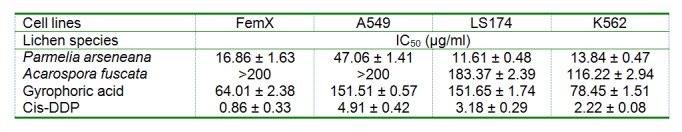
The cytotoxic activity of the tested lichen extracts and compounds against the target cell lines is shown in Table 6(Tab. 6). The P. arseneana extract exhibited remarkable cytotoxic effect against LS174, K562 and Fem-x cells (IC50 11.61, 13.84 and 16.86 µg/ml, respectively) and moderate cytotoxic effect when screened against A549 cells (IC50 47.06 µg/ml). In contrast, the extract of the A. fuscata displayed some mild effect against K562 cells, very weak to LS174 cells and no activity against the A549 and Fem-x cells (IC50 > 200 mg/ml). Also, gyrophoric acid, as a typical secondary metabolite of lichens, showed very weak activity against A549 and 174 cells and exerted a moderate cytotoxic effect against Fem-x and K562 cells (IC50 64.01 and 78.45 µg/ml, respectively). As shown in the table, the positive control (cis-DDP) had a significantly better cytotoxic activity than the tested samples.
Table 6. Growth inhibitory effects of acetone extracts of Parmelia arseneana and Acarospora fuscata and isolated gyrophoric acid on FemX, A549, LS174 and K562 cell lines.
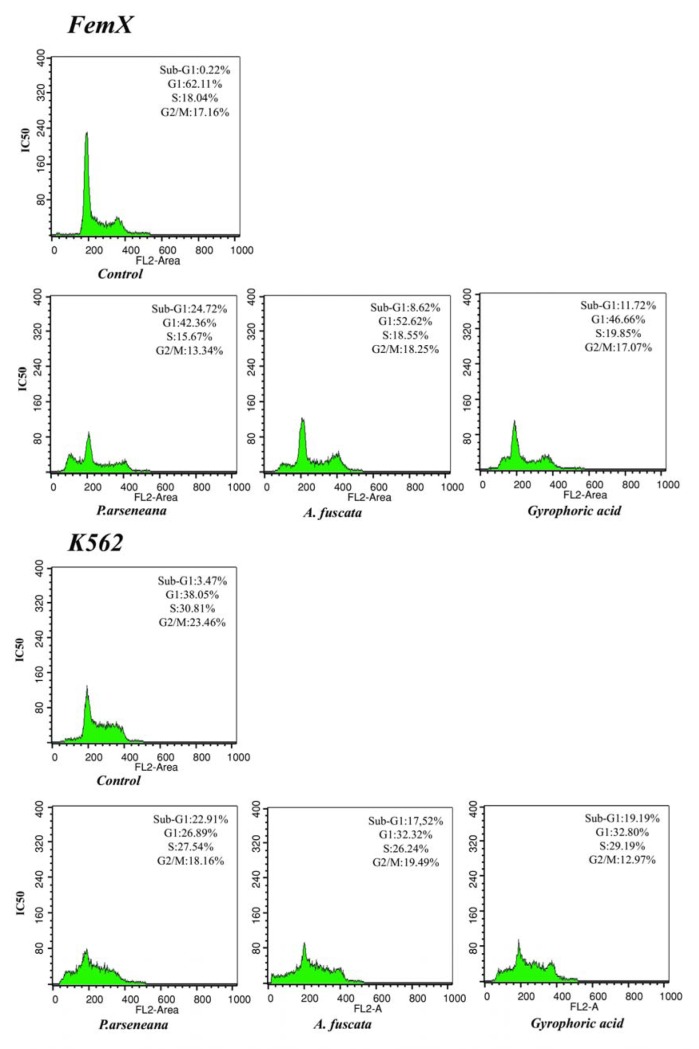
Flow cytometry analysis performed on Fem-x and K562 cells after 24 h of incubation with investigated extracts or gyrophoric acid (IC50 (µg/ml)) indicated an alteration in the percentage of cells in each stage of the cell cycle as compared to the control. The results show a typical pattern of DNA content, reflecting marked apoptosis of sub-G1 phase in malignant cells and the corresponding change in reducing the percentage of cells in G0/G1 and S-G2/M phases of the cell cycle, as shown in Figure 4(Fig. 4).
Figure 4. Cell-cycle distribution after 24 h of continuous action of (IC50) P. arseneana, A. fuscata and Gyrophoric acid in FemX and K562 cell lines. Representative histograms of cell-cycle distribution of malignant cells measured by flow cytometric analysis of DNA content after treatment.
Discussion
Antioxidant, antimicrobial and anticancer activities of some lichen extracts and lichen metabolites were previously shown in our papers (Kosanic et al., 2013[16]; Manojlovic et al., 2012[20]; Rankovic et al., 2012[31]). In the present study, the chemical composition of the acetone extracts of the lichens P. arseneana and A. fuscata and in vitro antioxidant, antimicrobial, and anticancer activities of these extracts and gyrophoric acid from A. fuscata were examined.
The tested lichen extracts and isolated compounds have a strong antioxidant activity against various oxidative systems in vitro. The strong antioxidant activity of tested samples is correlated with a high content of total phenols. In fact, it was observed that the examined lichen extracts containing the higher content of phenols exerted a stronger radical scavenging effect, suggesting that phenolics are the main agents responsible for their antioxidant activity. These results mostly agree with the literature, where we can find a number of reports for the antioxidant activity of extracts with high content of phenolic compounds (Behera et al., 2009[3]). In most lichens, phenols are important antioxidants because of their ability to scavenge free radicals such as singlet oxygen, superoxide, and hydroxyl radicals (Kosanic et al., 2012[18]). However, some authors believe that the antioxidant activity of extracts may not necessarily be correlated with the content of polyphenolics, suggesting that the antioxidant activity of different lichens may also depend on other, non-phenol components (Odabasoglu et al., 2004[27]).
The antioxidant effect of some other lichens was also studied by other researchers. For example, Kosanic et al. (2013[16]) found an antioxidant activity for the acetone extracts of the lichen Evernia prunastri and Pseudoevernia furfuraceae and their evernic acid and physodic acid constituents. Manojlovic et al. (2012[22]) explored the antioxidant properties of Umbilicaria cylindrica. Praveen Kumar et al. (2010[30]) found an antioxidant activity for the extracts of the lichen Ramalina hossei and Ramalina conduplicans. Compared with their results, the results of this research suggest that the tested samples showed a relatively powerful antioxidant activity.
In our experiments, the tested lichen extracts show a relatively strong antimicrobial activity but the antimicrobial activity of their component was much stronger. This means that lichen components are responsible for the antimicrobial activity of lichens. Differences in antimicrobial activity of different species of lichens are probably a consequence of the presence of different components with antimicrobial activity (Rankovic et al., 2012[31]). However, it is necessary to understand that extracts are mixtures of natural compounds, and their antimicrobial activity is not only a result of the different activities of individual components but may be the result of their interactions, which can have different effects on the overall activity of extracts.
The intensity of the antimicrobial effect depended on the species of organism tested. The extracts and compounds used in this study had a stronger antibacterial than anti-fungal activity. This observation is in accordance with other studies (Yang and Anderson, 1999[36]; Kosanic et al., 2012[17]; Licina et al., 2013[19]) focused on antimicrobial activity, which have demonstrated that bacteria are more sensitive to the antimicrobial activity than the fungi due to differences in the composition and permeability of the cell wall. The cell wall of Gram-positive bacteria is made of peptidoglucanes and teichoic acids, while the cell wall of Gram-negative bacteria is made of peptidoglucanes, lipopolysaccharides and lipoproteins (Heijenoort, 2001[12]; Kosanic et al., 2012[17]). The cell wall of fungi is poorly permeable and consists of polysaccharides such as hitchin and glucan (Farkaš, 2003[9]).
Numerous lichens and lichen compounds were screened for antimicrobial activity in search of the new antimicrobial agents (Rankovic et al., 2012[31]; Goel et al., 2011[10]), but in this study, for the first time, the antimicrobial activity of the acetone extracts of P. arseneana and A. fuscata was investigated. In correlation with the results obtained in experiments with other lichens, we noticed that the studied samples showed relatively strong antimicrobial activity.
In the present study, the results clearly demonstrate that tested samples showed a significant cytotoxic effect on the tested cancer cell lines. Some literature data reported that lichen components are responsible for the anticancer activities of lichens (Bucar et al., 2004[5]; Burlando et al., 2009[6]). However, it is difficult to determine the contribution of individual components for the overall anticancer effect. Often, the activity of the extracts may be the result of synergistic or antagonistic effect of several compounds. Our results are in accordance with the previously obtained data on gyrophoric acid cytotoxic activity in vitro (Backorová et al., 2012[1]).
The importance of lichens as anticancer agents is confirmed in recent years, which suggests that lichens can be used as biological agents in the treatment of cancer. The mechanism of action of the tested extracts and their compound is yet to be tested. Thus, further research will be necessary for fractionation in order to identify compounds responsible for the observed anti-tumour effects, and to establish the opportunities for reinforcement activities as well as to improve the selectivity.
Until now, only a few researchers proved that lichens have anticancer activity. Kosanic et al. (2013[16]) reported a significant anticancer effect for Evernia prunastri and Pseudoevernia furfuraceae. Manojlovic et al. (2010[21]) explored anticancer properties of Thamnolia vermicularis. Triggiani et al. (2009[34]) found strong anticancer activity for Xanthoria parietina.
The obtained data indicate that P. arseneana extract has significant important biological properties and remarkable cytotoxic activity, since they display IC50 values in a range comparable to that of the anti-tumour drug cisplatin. Finally, P. arseneana extracts are considered as agent with potential anti-tumour activity, and could therefore be a candidate for further stages of screening in vitro and/or in vivo.
In conclusion, it can be stated that tested lichen extracts and their compound had a certain level of antioxidant, antimicrobial and anticancer activities in vitro. On the basis of these results, lichens appear to be good natural antioxidant, antimicrobial and anticancer agents. Furthermore, it could be of significance in the food industry as well as to control various human, animal and plant diseases. Further studies should be performed to search new compounds from lichens that exhibit strong antioxidant, antimicrobial and anticancer activities.
Acknowledgements
This work was financed in part by the Ministry of Science, Technology, and Development of the Republic of Serbia and was carried out within the framework of projects no. 173032, 175011 and 172015.
References
- 1.Bačkorova M, Jendželovsky R, Kello M, Bačkor M, Mikeš J, Fedoročko P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol In Vitro. 2012;26:462–468. doi: 10.1016/j.tiv.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Bates ST, Cropsey GW, Caporaso JG, Knight R, Fierer N. Bacterial communities associated with the lichen symbiosis. Appl Environ Microbiol. 2011;77:1309–1314. doi: 10.1128/AEM.02257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behera BC, Verma N, Sonone A, Makhija U. Optimization of culture conditions for lichen Usnea ghattensis G. awasthi to increase biomass and antioxidant metabolite production. Food Technol Biotech. 2009;47:7–12. [Google Scholar]
- 4.Bown D. Encyclopaedia of herbs and their uses. London: Dorling Kindersley; 2001. [Google Scholar]
- 5.Bucar F, Schneider I, Ogmundsdottir H, Ingolfsdottir K. Antiproliferative lichen compounds with inhibitory activity on 12(S)-HETE production in human platelets. Phytomedicine. 2004;11:602–606. doi: 10.1016/j.phymed.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Burlando B, Ranzato E, Volante A, Appendino G, Pollastro F, Verotta L. Anti-proliferative effects on tumour cells and promotion of keratinocyte wound healing by different lichen compounds. Planta Med. 2009;75:607–613. doi: 10.1055/s-0029-1185329. [DOI] [PubMed] [Google Scholar]
- 7.Clothier RH. The FRAME cytotoxicity test. Methods Mol Biol. 1995;43:109–18. doi: 10.1385/0-89603-282-5:109. [DOI] [PubMed] [Google Scholar]
- 8.Dorman HJ, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J Agr Food Chem. 2004;52:762–70. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- 9.Farkaš V. Structure and biosynthesis of fungal cell walls: Methodological approaches. Folia Microbiol. 2003;48:469–478. doi: 10.1007/BF02931327. [DOI] [PubMed] [Google Scholar]
- 10.Goel M, Dureja P, Rani A, Uniyal PL, Laatsch H. Isolation, characterisation and antifungal activity of major constituents of the Himalayan lichen Parmelia reticulata Tayl.†. J Agr Food Chem. 2011;59:2299–2307. doi: 10.1021/jf1049613. [DOI] [PubMed] [Google Scholar]
- 11.Grube M, Berg G. Microbial consortia of bacteria and fungi with focus on the lichen symbiosis. Fungal Biol Rev. 2010;23:72–85. [Google Scholar]
- 12.Heijenoort J. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology. 2001;11:25–36. doi: 10.1093/glycob/11.3.25r. [DOI] [PubMed] [Google Scholar]
- 13.Huneck S, Yoshimura I. Identification of lichen substances. Berlin: Springer-Verlag; 1996. [Google Scholar]
- 14.Ibanez E, Kubatova A, Senorans FJ, Cavero S, Reglero G, Hawthorne SB. Subcritical water extraction of antioxidant compounds from rosemary plants. J Agr Food Chem. 2003;51:375–382. doi: 10.1021/jf025878j. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CJ, Bennett JP, Biro SM, Duque-Velasquez JC, Rodriguez CM, Bessen RA, et al. Degradation of the disease-associated prion protein by a serine protease from lichens. PLoS One. 2011;6:e19836. doi: 10.1371/journal.pone.0019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosanić M, Manojlović N, Janković S, Stanojković T, Ranković B. Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem Toxicol. 2013;53:112–118. doi: 10.1016/j.fct.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Kosanić M, Ranković B, Stanojković T. Antioxidant, antimicrobial and anticancer activity of 3 Umbilicaria species. J Food Sci. 2012;77:T20–T25. doi: 10.1111/j.1750-3841.2011.02459.x. [DOI] [PubMed] [Google Scholar]
- 18.Kosanić M, Ranković B, Stanojković T. Antioxidant, antimicrobial, and anticancer activities of three Parmelia species. J Sci Food Agr. 2012;92:1909–1916. doi: 10.1002/jsfa.5559. [DOI] [PubMed] [Google Scholar]
- 19.Ličina B, Stefanović O, Vasić S, Radojević I, Dekić M, Čomić Lj. Biological activities of the extracts from wild growing Origanum vulgare L. Food Control. 2013;33:498–504. [Google Scholar]
- 20.Manojlović N, Ranković B, Kosanić M, Vasiljević P, Stanojković T. Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine. 2012;19:1166–1172. doi: 10.1016/j.phymed.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Manojlović N, Vasiljević P, Jusković M, Najman S, Janković S, Milenkovic-Andjelković A. HPLC analysis and cytotoxic potential of extracts from the lichen, Thamnolia vermicularis var. Subuliformis. J Med Plants Res. 2010;4:817–823. [Google Scholar]
- 22.Manojlović NT, Vasiljević PJ, Masković PZ, Jusković M, Bogdanović-Dusanović G. Chemical composition, antioxidant and antimicrobial activities of lichen Umbilicaria cylindrica (L.) delise (Umbilicariaceae) Evid Based Compl Alt. 2012;2012:1–8. doi: 10.1155/2012/452431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.NCCLS (National Committee for Clinical Laboratory Standards) Reference method for broth dilution antifungal susceptibility. Testing of conidium-forming filamentous fungi: Proposed Standard M38-P. Wayne, PA: NCCLS; 1998. [Google Scholar]
- 25.Nishimiki M, Rao NA, Yagi K. The occurrence of super-oxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–853. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 26.Oboh G, Ademosun AO. Comparative studies on the ability of crude polyphenols from some Nigerian citrus peels to prevent lipid peroxidation - in vitro. Asian J Biochem. 2006;1:169–177. [Google Scholar]
- 27.Odabasoglu F, Aslan A, Cakir A, Suleyman H, Karagoz Y, Halici M, Bayir Y. Comparison of antioxidant activity and phenolic content of three lichen species. Phytother Res. 2004;18:938–941. doi: 10.1002/ptr.1488. [DOI] [PubMed] [Google Scholar]
- 28.Otzurk S, Guvenc S, Arikan N, Yylmaz O. Effect of usnic acid on mitotic index in root tips of Allium cepa L. Lagascalia. 1999;21:47–52. [Google Scholar]
- 29.Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–314. [Google Scholar]
- 30.Praveen Kumar SV, Prashith Kekuda TR, Vinayaka KS, Sudharshan SJ. Anthelmintic and antioxidant efficacy of two macrolichens of Ramalinaceae. Pharmacogn J. 2010;1:4. [Google Scholar]
- 31.Ranković B, Kosanić M, Stanojković T, Vasiljević P, Manojlović N. Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int J Mol Sci. 2012;13:14707–14722. doi: 10.3390/ijms131114707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slinkard K, Singleton VL. Total phenolic analyses: automation and comparison with manual method. Am J Enol Viticult. 1997;28:49–55. [Google Scholar]
- 34.Triggiani D, Ceccarelli D, Tiezzi A, Pisani T, Munzi S, Gaggi, et al. Antiproliferative activity of lichen extracts on murine myeloma cells. Biologia. 2009;64:59–62. [Google Scholar]
- 35.Vrablikova H, McEvoy M, Solhaug KA, Bartak M, Gauslaa Y. Annual variation in photoacclimation and photoprotection of the photobiont in the foliose lichen Xanthoria parietina. J Photochem Photobiol B. 2006;83:151–162. doi: 10.1016/j.jphotobiol.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Anderson EJ. Antimicrobial activity of a porcine myeloperozidase against plant phatgenic bacteria and fungi. J Appl Microbiol. 1999;86:211–220. [Google Scholar]