Abstract
Background
Multiple sclerosis is a human autoimmunological disease that causes neurodegeneration. One of the potential ways to stop its development is induction of oral tolerance, whose effect lies in decreasing immune response to the fed antigen. It was shown in animal models that administration of specific epitopes of the three main myelin proteins – myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP), and proteolipid protein (PLP) – results in induction of oral tolerance and suppression of disease symptoms. Use of bacterial cells to produce and deliver antigens to gut mucosa seems to be an attractive method for oral tolerance induction in treatment of diseases with autoimmune background.
Material/Methods
Synthetic genes of MOG35-55, MBP85-97, and PLP139-151 myelin epitopes were generated and cloned in Lactococcus lactis under a CcpA-regulated promoter. The tolerogenic effect of bacterial preparations was tested on experimental autoimmune encephalomyelitis, which is the animal model of MS. EAE was induced in rats by intradermal injection of guinea pig spinal cord homogenate into hind paws.
Results
Rats were administered preparations containing whole-cell lysates of L. lactis producing myelin antigens using different feeding schemes. Our study demonstrates that 20-fold, but not 4-fold, intragastric administration of autoantigen-expressing L. lactis cells under specific conditions reduces the clinical symptoms of EAE in rats.
Conclusions
The present study evaluated the use of myelin antigens produced in L. lactis in inhibiting the onset of experimental autoimmune encephalomyelitis in rats. Obtained results indicate that application of such recombinant cells can be an attractive method of oral tolerance induction.
MeSH Keywords: Encephalomyelitis, Autoimmune; Lactococcus lactis; Myelin Proteins
Background
Multiple sclerosis (MS) is an autoimmune disease that has a serious impact on physical abilities of the patient. It is postulated to involve cell-mediated and humoral responses directed against myelin proteins, including myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated glycoprotein (MAG), and myelin oligodendrocyte glycoprotein (MOG) [1–6]. This immunoactivity causes inflammation that leads to demyelination and axonal loss in the central nervous system (CNS) and results in slowing or even breaking neurotransmission [7]. The etiology of MS remains unknown and the commercially available medicines do not prevent disease development [8,9]. Currently, the most effective MS treatments are based on general immunosuppression or immunomodulation, which may lead to a number of adverse effects (e.g., common bacterial and viral infections, mood swings, high blood pressure, weight gain), including severe adverse effects such as progressive multifocal leukoencephalopathy. Data show that after 6 years of treatment, the disability of people with multiple sclerosis is the same as without drug therapy (Kwiatkowska-Patzer, personal communication).
An alternative treatment relies on induction of a state termed oral tolerance. Most simply, oral tolerance can be defined as the decrease of response to a previously fed antigen. It is a common physiological state preventing the formation of systemic immune responses to proteins ingested daily [10]. Preclinical trials show that on the same basis, oral administration of autoantigens induces a response suppressing experimental autoimmune diseases [11]. Studies using the experimental allergic encephalomyelitis (EAE), an animal model of MS, have shown that intradermal injection of myelin antigens such as MBP, MOG or PLP proteins with Freund’s adjuvant leads to development of acute paralysis [12–14]. However, oral administration of a myelin antigen or antigens before injection inhibits paralysis signs and immune cells infiltration as shown in histopathological studies [15,16]. Moreover, feeding with myelin antigens after the onset of EAE was determined to reduce disease development and facilitate remission [17]. Also, our own previous studies have demonstrated that intragastric administration of pig spinal cord hydrolysate, containing short peptide fragments of myelin proteins, induces oral tolerance in Wistar and Lewis rats with EAE [18–20]. Results indicated that the mixture of neuropeptides in the spinal cord hydrolysate given orally diminished immunological response to myelin antigens [21]. This initiated further studies on developing novel means of delivering myelin peptides to gut mucosa in combination with proficient production of selected autoantigens in bacterial cells.
Various studies show that peptides MOG35-55, MBP85-97, and PLP139-151 are epitopes that play a role in inhibiting autoantigen responses, both in MS patients and animals with EAE [17,22,23]. Therefore, they are generally considered as good antigens for oral tolerance induction. All of the above observations encourage continuing studies on the development of autoimmune disease treatment.
One of the strategies of delivering antigens to mucosal surfaces is the use of bacterial cells as carriers. Commonly, antigen delivery systems were based on attenuated pathogenic microorganisms or viruses such as Salmonella typhi or poliovirus [24,25]. An interesting alternative that has been gaining much interest in the last decades is the potential use of non-pathogenic bacteria as live oral vaccines. In this work we developed a system for production of selected epitopes from natural myelin proteins in a lactic acid bacterium (LAB). LAB are gram-positive, food-grade bacteria with various applications in food production and dairy fermentation [26,27]. Due to their recognized, non-pathogenic nature, LAB are of substantial interest for implementation in various research domains, including vaccine development, where they are considered as attractive antigen delivery vectors, and a good alternative to commonly used attenuated pathogens [28,29]. Lactococcus lactis is a model LAB that has been shown to efficiently produce heterologous proteins of different origin (e.g., viral, bacterial, mammalian) [30–33]. Numerous studies demonstrated that oral delivery of allergen- or autoantigen-expressing L. lactis cells can be a cost-effective and innovative means of inducing tolerance [34–36]. Among other evidence, it was shown that delivery of the ovalbumin-expressing L. lactis strain to the gut mucosal surface suppresses both systemic and local OVA-specific T-cell response [36].
These reports induced us to employ L. lactis as a host organism to produce myelin epitopes MOG35-55, MBP85-97, and PLP139-151 and deliver them to gut mucosa. For this, we generated synthetic genes of these peptide fragments and expressed them using the promoter region of the L. lactis ptcB gene (PptcB) inserted into the pIL253 vector. Previous studies have shown that the ptcB gene in L. lactis is engaged in sugar catabolism, and its promoter (PptcB) is regulated by CcpA, the catabolite control protein A [37]. The strength of PptcB was shown to vary depending on the sugar source in the medium. The highest promoter activation was determined to occur in the presence of cellobiose [37, Aleksandrzak-Piekarczyk – unpublished data]. Animal trials showed that oral administration of recombinant L. lactis cells encoding myelin epitopes can at certain doses inhibit progression of experimental autoimmune encephalomyelitis induced in rats. A scheme of antigen administration was also examined.
Material and Methods
Bacterial strains and plasmids
Strains and plasmids used in this work are listed in Table 1. Plasmid vector pIL253:PptcB, carrying the promoter region of L. lactis ptcB gene, was used in cloning procedures. L. lactis cells were grown at 30°C in M17 liquid medium supplemented with 0.5% cellobiose or on M17 solid medium containing 0.5% glucose [38]. Solid plate media contained 1.5% agar. When necessary the growth medium was supplemented with erythromycin at 5 μg/ml (for plasmid-carrying L. lactis derivatives).
Table 1.
Strains and plasmids.
| Strain | Genotype | Source |
|---|---|---|
| Lactococcus lactis IBB360 | Natural isolate | IBB PAS collection |
| Plasmid | ||
| pIL253:PptcB | EryR, pIL253-derivative with ptcB gene promoter region | IBB PAS collection |
DNA manipulations, transformation and sequencing
Standard DNA manipulations and cloning procedures were performed as described previously [39]. Transformation of L. lactis cells via electroporation was done as described elsewhere [40,41]. Digestions with restriction enzymes (Fermentas) were done according to the manufacturer’s instructions. DNA sequencing was done using a Big Dye sequencing kit. Sequences were analyzed with the BLAST program [42].
Cloning of MOG35-55, MBP85-97 and PLP139-151 synthetic gene fragments in L. lactis
Oligonucleotides used in this study are listed in Table 2. Two ‘LONG’ complementary oligonucleotides served as a template for amplification of neuropeptide-encoding sequences. Oligonucleotides were designed in accordance with the codon usage of L. lactis species to give the nucleotide sequence corresponding to the amino acid sequence of a given peptide fragment that is the most optimal for these bacteria. PCR reaction was performed with the Thermocycler apparatus using ExTaq DNA polymerase (TaKaRa) and, specific for each peptide, ‘SHORT’ primer pairs complementary to the 5′ ends of ‘LONG’ primers (Table 2). The resulting PCR products were then digested with PstI and SalI restriction enzymes (Fermentas) and ligated into the pIL253:PptcB vector cut with the same pair of enzymes. Ligated molecules were electroporated into L. lactis cells. Transformants were selected on M17 medium (Oxoid) supplemented with 1.5% agar, 0.5% glucose, and 5 μg/ml erythromycin to isolate cells carrying either the empty [pIL253:PptcB] or recombinant vectors. Colonies carrying single neuropeptide-containing plasmids were analyzed by ‘colony PCR’ technique using specific primers: ptcBfor/pILrev (Table 2). From confirmed proper recombinant cells, plasmid DNA was isolated using the Plasmid Mini Kit (A&A Biotechnology) and subjected to restriction analysis with HindIII enzyme (Fermentas). Obtained digestion pattern was compared to the pattern generated using the CloneManager 9 (Sci-Ed Software) program. Finally, nucleotide sequences of the cloned DNA fragments were examined for conformity with the nucleotide sequences of the synthetic myelin genes by DNA sequencing technique.
Table 2.
Oligonucleotide sequences used in study.
| Neuropeptide | Oligonucleotide | Nucleotide sequence (forward/reverse) |
|---|---|---|
| Oligonucleotides („LONG”) used as DNA templates for amplification of synthetic neuropeptide genes | ||
| MOG35-55 | FLONGMOG35 | 5′ GTATTTCTATGGAAGTTGGATGGTATCGTTCACC ATTTTCACGTGTTGTTCATTTATATCGTAATGG 3′ |
| RLONGMOG35 | 5′ CCATTACGATATAAATGAACAACACGTGAAAAT GGTGAACGATACCATCCAACTTCCTAGAAATAC 3′ | |
| MBP85-97 | FLONGMBP85 | 5′ GTATTTCTATGCCAGGATCACGTCCACATTTAATTCGTTTATTTTCACGT 3′ |
| RLONGMBP85 | 5′ ACGTGAAAATAAACGAATTAAATGTGGACGTGATCCTGGCATAGAAATAC 3′ | |
| PLP139-151 | FLONGPLP139 | 5′ GTATTTCTATGCATTCATTAGGAAAATGGTTAGGACATCCAGATAAATTT 3′ |
| RLONGPLP139 | 5′ AAATTTATCTGGATGTCCTAACCATTTTCCTAATGAATGCATAGAAATAC 3′ | |
| Oligonucleotides (SHORT) used as primers for amplification of synthetic neuropeptide genes | ||
| Universal primer forward | FSHORTUniv | 5′ ACGCGTCGACAAGGAGTATTTCTATG 3′ |
| MOG35-55 | RSHORTMOG35 | 5′ AACTGCAGTTATTATTTTCCATTACGATA 3′ |
| MBP85-97 | RSHORTMBP85 | 5′ AACTGCAGTTATTAACGTGAAAATAAACG 3′ |
| PLP139-151 | RSHORTPLP139 | 5′ AACTGCAGTTATTAAAATTTATCTGG 3′ |
| Oligonucleotides used as amplification primers complementary to pIL253:PptcB | ||
| pILfor | 5′ TTAGAGTATACTTATTTGTCC 3′ | |
| pILrev | 5′ GGGATAGTAATTCATTCCTGG 3′ | |
| ptcBfor | 5′ GCGATTAATGTCGCCTAAAGGTTGC 3′ | |
| ptcBrev | 5′ AATCCCGGCGTTTTATAATTTAACGTTC 3′ | |
RBS and spacer sequence marked in bold, sequences recognized by restriction enzymes are underlined.
Transcriptional analysis of MOG35-55, MBP85-97 and PLP139-151 synthetic gene expression in L. lactis
Expression of the cloned neuropeptide synthetic gene sequences was analyzed by RT-PCR using SuperScriptIII (Invitrogen). Total RNA was isolated from recombinant L. lactis cells carrying individual neuropeptide synthetic genes using High Pure RNA isolation kit (Roche). To eliminate residual DNA, total RNA was additionally digested with DNase I (Sigma) and subjected to RT-PCR reaction using a reverse ‘SHORT’ primer specific for each neuropeptide-encoding gene (Table 2).
Preparation of recombinant L. lactis whole-cell extracts
Recombinant lactococci were prepared for intragastric administration as follows. After o/n incubation at 30°C, the bacterial culture was harvested (8 000 g, 10 min., 4°C) and washed once with 0.9% NaCl. Then, cells were suspended in 0.9% NaCl and disrupted 3 times for 1 min using the MiniBeadbeater (BioSpec Products) and 106-μm glass beads (Sigma). To determine the amount of myelin peptides that should be delivered to the gut mucosa to evoke tolerance, adequate dilutions of cell lysates, corresponding to doses 101–108 CFU (Colony Forming Units) per 0.5 ml were made. Single doses were frozen in Eppendorf tubes in liquid nitrogen and stored at −20°C until needed.
Tolerance induction in rats with EAE using whole-cell extracts of L. lactis producing MOG35-55, MBP85-97 and PLP139-151
Female Lewis rats, 180–200 g, were fed intragastrically with a gauged pointed needle, four times during one week (every second day) or once a day for 20 consecutive days (from day −10 to +9). Preparations given to rats contained whole-cell L. lactis lysates diluted in 0.5 ml PBS accordingly to give 101–108 CFU per feeding. EAE was evoked a week after the last feeding in respect to the 4-day scheme or on day 10 of the 20-day schedule. The day of immunization is marked as 0 DPI – day post immunization. To induce EAE, rats received intradermal injection of 50% guinea pig spinal cord homogenate in PBS, with Freund ’s adjuvant (1:1) and Mycobacterium tuberculosis (4 mg/ml), into hind paws (100 μl/paw). Rats were weighed every day and clinical symptoms were evaluated from the day they first occurred, using a 5-grade scale: 1 – limp tail, 2 – hind leg weakness, 3 – hind leg paralysis and incontinence, 4 – front and hind leg weakness or quadriplegia, 5 – death. On day 14 after immunization (DPI) animals were sacrificed.
Analysis of the therapeutic effect of L. lactis-produced peptide preparations on rats
The efficiency of bacterial preparations were assessed by calculating (i) mean score of clinical symptoms, and (ii) body mass, reflecting the overall condition of the animal. First clinical symptoms of EAE were observed at the 11 DPI. Peak of clinical symptoms was noted between 11–14 DPI. The mean clinical score was calculated for this time range. Body mass index was counted as the ratio of body mass at the end of experiment [14 DPI] to body mass at the day of immunization [0 DPI]. Body mass at 0 DPI was considered as 1. Statistical evaluations were made using the Mann-Whitney test. A value of p less than 0.05 was considered as statistically significant.
Experiments on animals have been carried out in accordance with the EU Directive 2010/63/EU for animal experiments and approved by the IV Local Ethics Committee for Animal Experiments in Warsaw (Act no. 66/2010 made on 08.10.2010).
Results
Designing of synthetic MOG35-55, MBP85-97 and PLP139-151 gene sequences
Synthetic genes encoding human-derived MOG35-55 (from MOG protein; gi: 56388814) MBP85-97 (from MBP protein; gi: 54038405AAH84713.1) and PLP139-151 (from PLP protein; gi: 13591880) epitopes were obtained by PCR method. For each peptide fragment, two complementary oligonucleotides (for/rev ‘LONG’) were used as template. To ensure efficient gene expression associated with faster translation rates, all ‘LONG’ primers used in this study were designed taking into account optimal codon usage in L. lactis, obtained from the Codon Usage Database (www.kazusa.or.jp). Additionally, ‘LONG’ primers contained at their 5′ ends sequences specific for the Lactococcus lactis RBS (ribosome-binding site; AAGGAG) consensus sequence recognized by the translation machinery and ‘spacer’ sequence (TATTTCT) localized between the RBS region and the translation START codon. The forward ‘LONG’ primer used to amplify the PLP139-151-encoding gene was modified by introducing a sequence corresponding to the translation START codon (ATG), just before the original peptide sequence. Genes encoding myelin peptide fragments were generated by two oligonucleotides (for/rev ‘SHORT’) homologous to the extremities of the ‘LONG’ primers (Table 2).
Cloning of MOG35-55-, MBP85-97- and PLP139-151-encoding genes in L. lactis
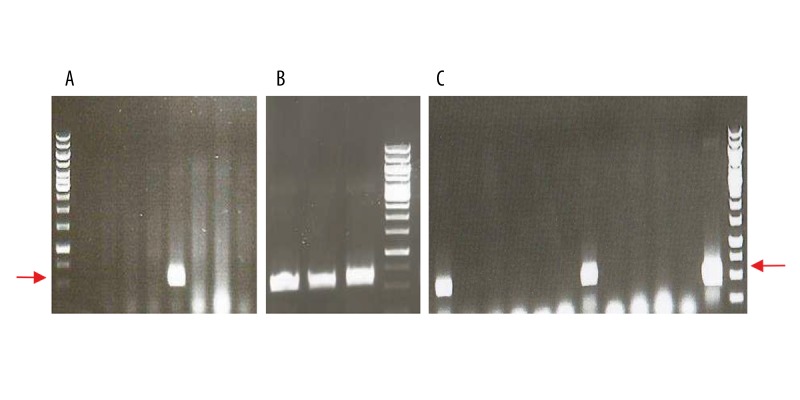
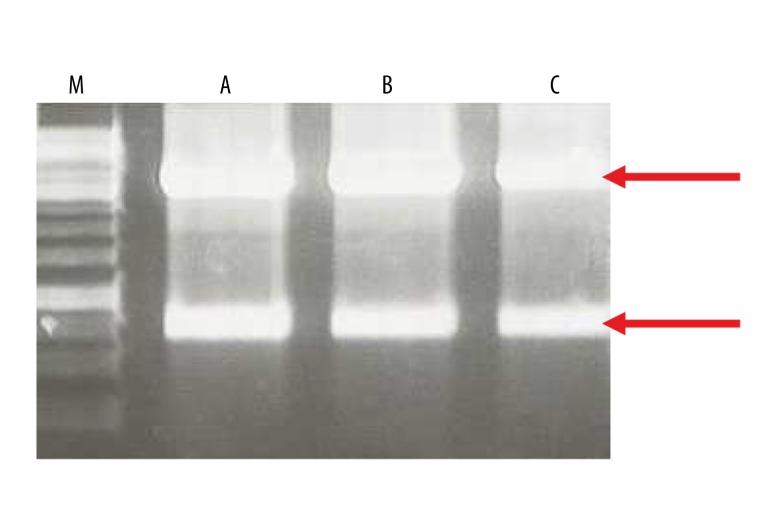
The generated synthetic genes were cloned separately into L. lactis-replicating pIL253:PptcB plasmid. Obtained recombinant vectors were introduced independently into electrocompetent cells by using an efficient transformation technique commonly used for L. lactis [40,41]. Clones containing synthetic genes were identified in the culture population by analyzing the obtained transformants using ‘colony PCR’ technique for the presence of inserts corresponding in length to the expected DNA fragments (Figure 1). PCR performed directly on grown cells using specific primers, allowed isolating colonies specifically carrying recombinant vectors. Digestion patterns of obtained from recombinant plasmid DNA isolated from these colonies were in conformity with the predicted restriction pattern generated using bioinformatics program (Figure 2). Such result, in parallel with DNA sequencing, provided proof that these plasmids do not undergo any rearrangements and are stably maintained in L. lactis, suggesting at the same time that the encoded myelin peptides are not toxic to the cells.
Figure 1.
Colony PCR on selected transformant clones. (A) pIL253:PptcB:MOG35-55 (8 clones tested), (B) pIL253:PptcB:MBP85-97 (3 clones tested), (C) pIL253:PptcB:PLP139-151 (12 clones tested). Colony PCR carried out on transformant clones obtained after electroporation of L. lactis cells with recombinant constructs and selection of M17 solid medium supplemented with 0.5% glucose and erythromycin at 5 μg/ml. Specific primers used (ptcBfor/pILrev) were homologous to the vector sequence flanking the potentially cloned fragment. Expected product size: ~550 bp (marked with red arrows), 1 kb DNA Ladder (Fermentas) used as DNA size reference marker.
Figure 2.
Control digestions of recombinant plasmid DNA using HindIII (Fermentas) enzyme. (A) pIL253:PptcB:MOG35-55, (B) pIL253:PptcB:MBP85-97, (C) pIL253:PptcB:PLP139-151. Expected DNA fragments after restriction analysis for correct constructs: 3897 bp, 845 bp (marked by red arrows), 1kb DNA ladder (M) 1kb DNA Ladder (Fermentas) used as DNA size reference marker.
Transcriptional studies on the expression of synthetic MOG35-55, MBP85-97 and PLP139-151 genes in L. lactis
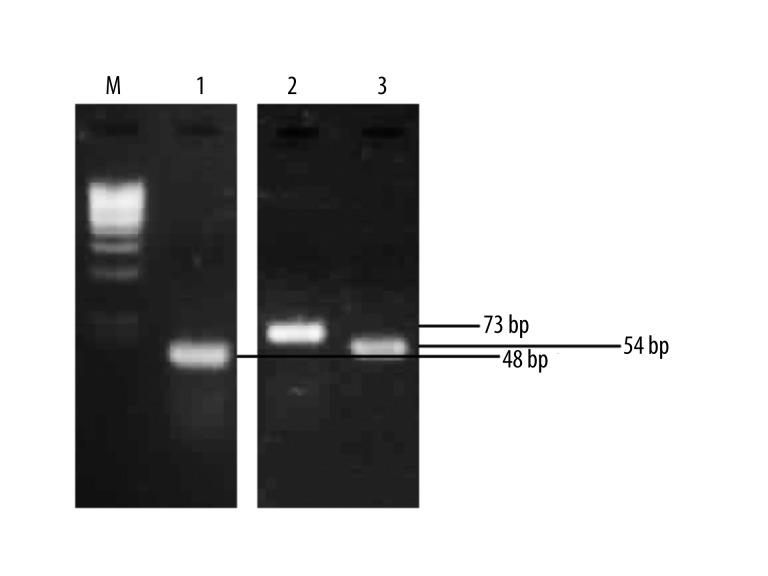
To examine whether the cloned synthetic neuropeptide genes are properly expressed in L. lactis, transcriptional studies using RT-PCR have been carried out. Total RNA was isolated from bacterial strains carrying the recombinant plasmids and, after specific treatment, was subjected to reverse transcription reaction using a specific reverse ‘SHORT’ primer complementary to the 3′ end of the strand encoding the particular synthetic gene (Table 2). Obtained cDNAs were subsequently subjected to amplification by the classical PCR technique using two ‘SHORT’ primer pairs (for/rev) homologous to each neuropeptide sequence (Table 2). In result, DNA fragments were obtained which length corresponded to the length of individual synthetic genes confirming the presence of synthetic neuropeptide gene transcripts (Figure 3).
Figure 3.
Detection of synthetic myelin peptides gene expression in L. lactis by RT-PCR. (M) 100-bp DNA marker, (1) PLP139-151 gene transcript, (2) MOG35-55 gene transcript, (3) MBP85-97 gene transcript.
Preliminary determination of optimal dose of L. lactis-produced myelin peptides for oral tolerance induction
In first place, we concentrated our efforts on choosing the proper dose of recombinant L. lactis bacteria, which would elicit the desired effect, which is a crucial step in obtaining tolerance. We applied two simple parameters: (i) mean score, which is widely used to reflect the physical condition of the animals and (ii) body mass of the animals, which allows assessing the general state of the animals (it is clear that the body mass of the animal with ongoing inflammatory process will drop).
To determine the optimal amount of myelin peptides for effective induction of oral tolerance, animals with evoked EAE (n=6) were fed for 20 consecutive days with whole-cell lysates of recombinant L. lactis producing MBP85-97, PLP139-151 and MOG35-55 fragments corresponding to doses 101–108 CFU consecutive days. Based on daily evaluation, the mean score of clinical symptoms and body mass index were counted (Figure 4A, 4B). Development of the disease was initially scored during 34 days post-infection (data not shown). First clinical symptoms appeared at 11 DPI. The relapse of EAE was observed between 11 and 14 DPI. After 14 DPI the remission of symptoms occurred. Therefore, the 11–14 DPI range was determined to give the most prominent differences in the mean scores for fed vs. non-fed EAE animals and used in further assays. Longer observation periods revealed spontaneous curing of animals.
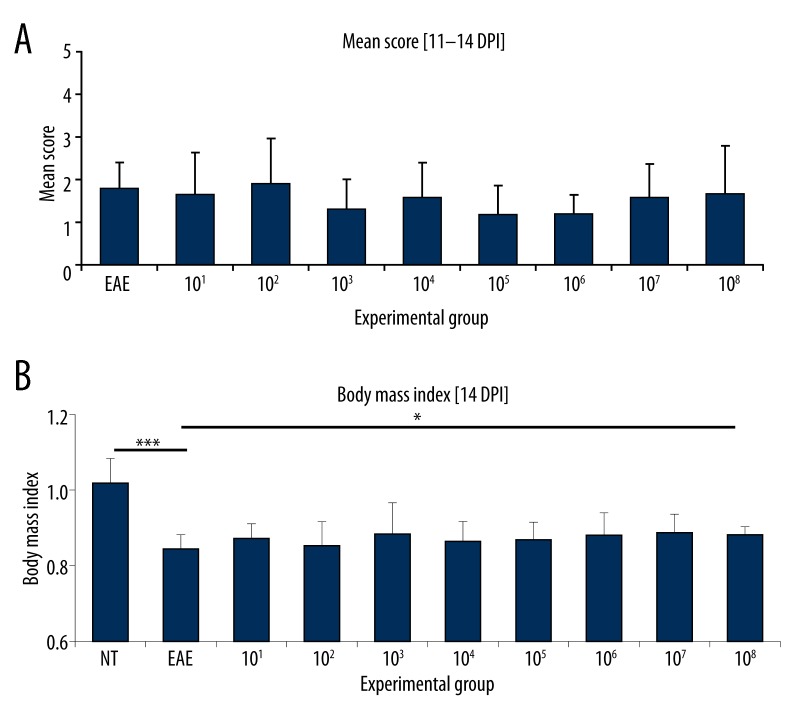
Figure 4.
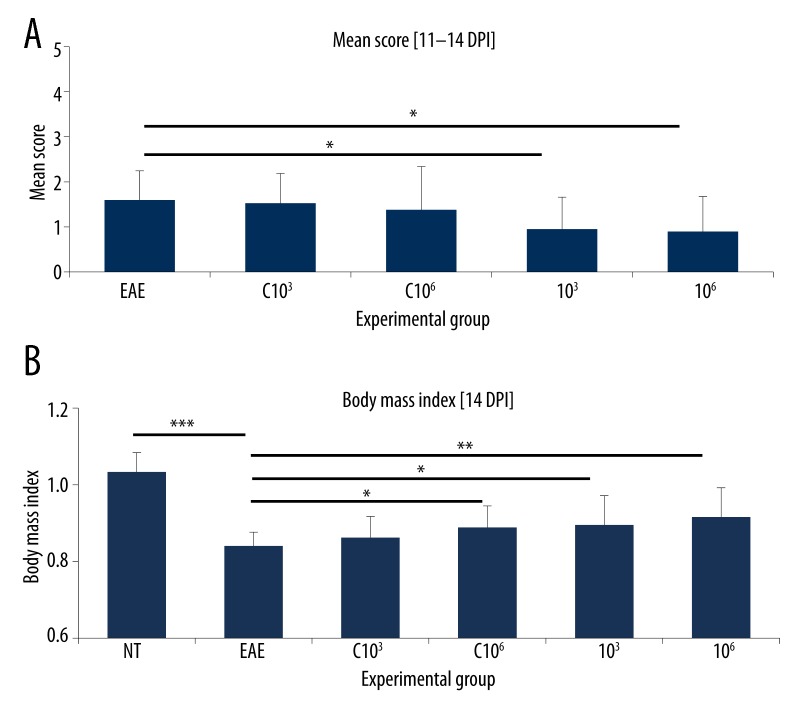
Animals with EAE fed once per day for 20 consecutive days with preparations containing mixed whole-cell lysates of recombinant L. lactis strains at doses 101–108 CFU (groups 101–108), producing MBP85-97, PLP139-151 and MOG35-55 fragments. NT – non-treated animals, EAE – non-fed animals with EAE. (A) Mean score calculated at the peak of clinical symptoms [11–14 DPI]. n=6. (B) Body mass index at 14 DPI. Body mass at 0 DPI considered as 1. n=6. * p<0.05; *** p<0.001.
Results obtained from this preliminary trial show influence of certain doses on EAE progression in rats. Feeding with bacterial lysates corresponding to doses, 103, 105 and 106 CFU exhibited the tendency to decrease the mean score, although this was not statistically relevant (Figure 4A). Significant (p<0.001) body mass reduction was observed in EAE rats in comparison to intact animals (NT) (Figure 4B). Application of bacterial preparations showed a slight trend to increase the body mass of immunized animals, but this effect was statistically relevant (p<0.05) only in respect to dose 108 CFU. However, the same dose was ineffective in declining EAE progression. Thus, for further experiments two doses, 103 and 106 CFU, were selected, which led at the same time to both, the decrease of mean score and increase of body mass vs. non-fed EAE animals.
Induction of oral tolerance by L. lactis-produced myelin peptides
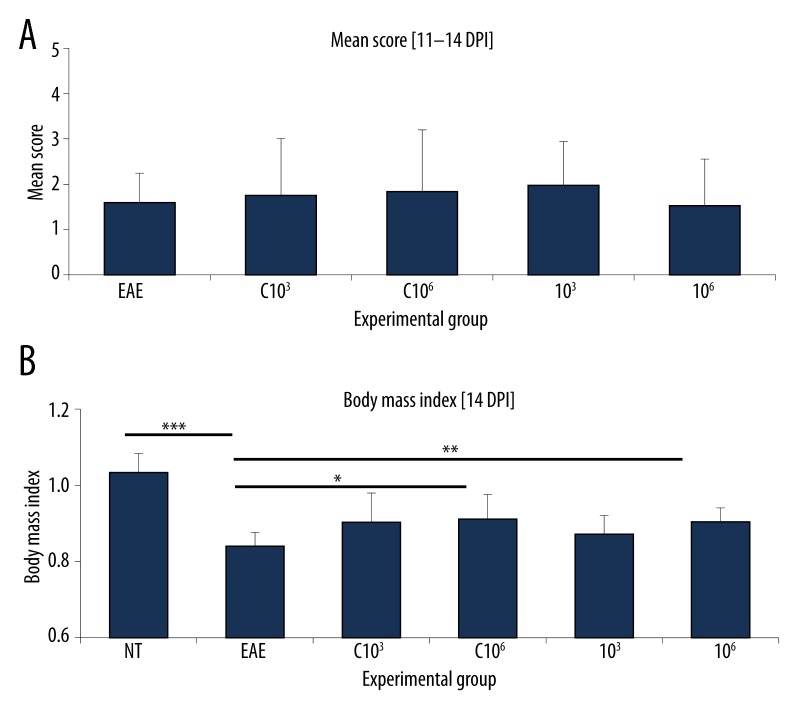
To confirm that the chosen bacterial doses (103 and 106 CFU) are efficient in inhibiting the on-set of EAE, a subsequent experiment was set-up on a larger animal group (n=12) using a 20-day feeding scheme. Results confirmed our previous observations. Both doses (103, 106) did not only significantly decrease (p<0.05) clinical symptoms (Figure 5A), but also reduced body mass drop in fed vs. non-fed EAE animals (Figure 5B). To investigate whether the observed effect is caused by myelin peptide fragments and not by bacteria themselves, whole-cell lysates of L. lactis containing the empty plasmid (pIL253:PptcB) administered at the same doses (C 103, C 106) were used as control. No relevant differences in the mean score compared to non-fed EAE animals were observed (Figure 5A). In contrast, there was a statistically significant increase in the body mass index observed for animals fed with the control preparation at dose 106 CFU in comparison to non-fed EAE animals (Figure 5B). Still, the observed effect was lower than for myelin-producing bacteria.
Figure 5.
Animals with EAE fed once per day for 20 consecutive days with preparations containing mixed whole-cell lysates of recombinant L. lactis cells in doses 103 or 106 CFU, containing the empty plasmid pIL253:PptcB (groups C 103 and C 106) and producing MBP85-97, PLP139-151 and MOG35-55 fragments (groups 103 and 106). NT – non-treated animals, EAE – non-fed animals with EAE. (A) Mean score calculated at the peak of clinical symptoms [11–14 DPI]. n=12. * p<0.05. (B) Body mass index at 14 DPI. Body mass at 0 DPI considered as 1. n=12. * p<0.05; ** p<0.01; *** p<0.001.
To determine whether the applied feeding scheme is optimal, we further examined the effect of oral administration of whole-cell lysates at 103 and 106 CFU/doses on EAE rats fed 4 times within one week. Such feeding scheme in combination with the applied doses was not sufficient in decreasing the clinical symptoms in fed vs. non-fed EAE animals (Figure 6A). Body mass index was higher only for animals fed with preparations corresponding to 106 CFU/dose (Figure 6B). Also for this group a slight decrease of clinical symptoms was observed (Figure 6A).
Figure 6.
Animals with EAE fed once per day for 4 days within a week with preparations containing mixed whole-cell lysates of recombinant L. lactis strains in doses 103 or 106 CFU, containing the empty plasmid pIL253:PptcB (groups C 103 and C 106) and producing MBP85-97, PLP139-151 and MOG35-55 fragments (groups 103 and 106). NT – non-treated animals, EAE – non-fed animals with EAE. (A) Mean score calculated at the peak of clinical symptoms [11-14 DPI]. n=9. (B) Body mass index at 14 DPI. Body mass at 0 DPI considered as 1. n=9. * p<0.05; ** p<0.01; *** p<0.001.
We also did not note any dissimilarities in the mean score between fed and non-fed animals when lysates of L. lactis containing the empty plasmid were applied in 4-day feeding regimen (Figure 6A). Similarly as in the 20-fold feeding scheme, a substantial variation of body mass index was noted for animals fed with the control preparation at dose 106 CFU in comparison to non-fed EAE animals (Figure 6B).
Discussion
One of the alternative means of treating MS is induction of oral tolerance against natural myelin proteins, such as MBP, MOG, PLP, which according to the current knowledge are engaged in MS pathophysiology and its animal model – EAE [12–14]. Studies show that administration of specific myelin peptides or whole mammalian spinal cord hydrolysates (e.g., from pig, rat, mouse) prevents or partially inhibits EAE progression [15,16,18–21]. However, free antigen delivery for therapeutic purposes has several drawbacks, including laborious procedures in achieving adequate protein quantity and purity. Implementation in clinical therapy of novel, efficient immunomodulation strategies based on autoantigen presentation, in first place, aims at: (i) production of sufficient amounts of autoantigens, and (ii) simple and stable delivery of antigens to mucosal surfaces. Taking this into account, various antigen-presenting systems have been developed, including antigen production in transgenic plants or antigen administration enclosed in microsphere beads, fusion of antigens with mucosa-binding molecules (e.g., cholera toxin B subunit) and other [43–46]. Treatment of autoimmune diseases by using live microbial vectors as antigen carriers has also been proven by multiple studies as a promising and effective approach for induction of specific tolerance [36,47,48].
Lactococcus lactis is a food-grade commensal bacterium, which due to its long and safe use in human and animal food production has been assigned the GRAS (generally recognized as safe) status by the American Food and Drug Administration. Recent years have demonstrated that production of heterologous proteins in L. lactis cells for vaccine development is efficient and economically substantial [33,49–51]. A great advantage of using Lactococcus in medical applications is that it is non-pathogenic and, as a Gram-positive bacteria, does not produce endotoxic lipopolysaccharides, and finally, does not induce inflammatory responses in healthy individuals.
Oral administration of L. lactis expressing antigens or allergens was previously shown to be proficient in induction of mucosal immune responses [52–54]. Moreover, L. lactis cells were determined to exhibit an adjuvant effect when administered as live antigen delivery vectors or a peptidoglycan matrix component [55,56]. These facts inclined us to investigate the potential application of Lactococcus lactis bacteria as microbial factories for production and delivery of selected myelin peptide fragments to mucosal surface. Such approach could be especially useful in obtaining sufficient amounts of myelin proteins which normally are present at low levels, such as MOG, which constitutes only 0.05% of myelin proteins of the central nervous system [57].
In this study we have synthetically generated genes encoding myelin epitopes of the mammalian central nervous system and cloned them in L. lactis cells to assess their potential application in specific induction of oral tolerance. To express myelin peptide genes we used a pIL253-derived plasmid vector containing the L. lactis ptcB promoter. Our unpublished studies show that activity of the CcpA-controlled PptcB region, both plasmid- or chromosomally-located, is higher on cellobiose (activator) than on glucose due to catabolite repression [37,58, Aleksandrzak-Piekarczyk – unpublished data]. Therefore, to ensure efficient peptide production, cells were grown in medium with cellobiose. Based on two parameters, mean score and body mass, our results provide evidence that preparations of recombinant L. lactis encoding myelin peptides grown on medium with cellobiose render a tolerogenic effect when administered orally to EAE rats.
In the study, EAE was evoked using guinea pig spinal cord homogenate, successfully used in previous studies [21]. By using the mixture of all myelin antigens, we imitate MS, where a broad spectrum of autoantigens is believed to be engaged in pathology. Application of an antigen mix containing three myelin epitopes was expected to more surely guarantee EAE suppression than a single antigen. Moreover, a mix of antigens resembles more closely the natural, physiological situation of tolerance induction in the gut. Our study shows that use of the microbially-expressed myelin epitopes, MOG35-55, PLP139-151, MBP85-97, induces oral tolerance in rats. Although, there is a limited number of works that examined the tolerogenic effect of peptide mixes, most recently Juryńczyk et al. [23] showed that transdermal application of a mix of the same peptides can be a promising approach in treatment of MS patients.
Among the crucial factors in obtaining the desired effect of oral tolerance are optimal antigen dose and feeding regimen. Our own previous experiments showed that administration of pig spinal cord hydrolysate based on a 4-day regimen during 1 week successfully induced oral tolerance [21]. The second (20-day) scheme was implemented in experiments on recombinant lactobacilli producing myelin proteins [47]. In this work we aimed at determining the most favorable feeding scheme for the designed lactococcal recombinant cells. Results from conducted trials showed that feeding EAE animals according to a 20-day scheme with a mixture of whole-cell lysates of L. lactis cells producing MBP85-97, PLP139-151 or MOG35-55, at 103 or 106 CFU/dose, is sufficient in reducing clinical symptoms and decreasing further body mass drop in fed vs. non-fed EAE animals. The fact that the same effect was obtained by applying these two concentrations could be explained by the dose-dependent mechanism of oral tolerance development, which occurs via clonal anergy or deletion (high doses), or active suppression (low doses) [11,59].
In contrast, the 4-fold feeding regimen did not reduce the clinical symptoms of EAE. This may be owed to the necessity to precisely determine the dose of L. lactis administered to animals that would render the expected effect. Performing a pilot test using a range of doses (101–108 CFU/dose), similarly to the one made for the 20-day feeding scheme, might be useful in determining whether other bacterial doses than 103 and 106 CFU are more efficient in the 4-time feeding procedure. However, it seems that longer exposure of autoantigens in the gut resembles more the natural feeding scheme, implied to be more advantageous than short-term feedings [60].
Another significant factor that should to be assessed is the time-scheme for antigen administration. Oral tolerance can be induced both, before or after disease induction, depending on the conditions of study (e.g., antigen dose and disease progression). Yet, numerous studies report that better suppression is observed when the antigen is delivered before immunization [61–64]. Feeding animals after immunization was reported to render limited suppression; yet, a prolonged feeding regimen was necessary [61,65]. Therefore, in our study animals were exposed to the recombinant antigen for 10 instead of 4 days before immunization and additionally for 10 days after immunization to assure better oral tolerance induction. Intragastric administration of L. lactis containing the empty plasmid was also determined to influence the overall condition of EAE-induced rats. Although body mass gain/loss is not a direct parameter associated with oral tolerance, such effect could indicate the regulatory property of L. lactis bacteria. Literature data suggests that Lactococcus could increase tolerogenic signals induced by delivered autoantigens by influencing: (i) processing or presentation of the antigen, and/or (ii) expression of co-stimulatory molecules on dendritic cells by affecting natural immune molecules [56]. In contrast, clinical symptoms in rats fed with control bacteria lysates were not as significantly inhibited in comparison to rats fed with lysates of recombinant bacteria encoding myelin epitopes, and only slightly lower than for non-fed EAE rats. This suggests that although L. lactis cells alone can show some tolerogenic effect in the mucosa, myelin antigens are necessary in decreasing the clinical symptoms of EAE. The perspective of using L. lactis as a carrier may (only) play a boosting effect in evoking oral tolerance.
Conclusions
In summary, we synthetically generated genes encoding three selected myelin epitopes, which, cloned in L. lactis cells under the lactococcal ptcB promoter, were demonstrated to efficiently induce oral tolerance by decreasing the clinical symptoms of EAE in rats. Moreover, the conducted animal studies deliver valuable information concerning the optimal administration scheme for L. lactis-produced myelin antigens. Further studies on optimizing the dose and feeding regimen as well as peptide expression and delivery will be needed to determine whether increase of the therapeutic effect of L. lactis preparations could be obtained. Nonetheless, application of Lactococcus bacteria, which have a documented safe effect on human and animal health, for production of mammalian myelin epitopes and oral administration to induce immunotolerance seems to be an attractive means of treatment of autoimmune diseases. Data obtained in this work creates further perspectives for seeking new therapies for multiple sclerosis via the oral tolerance phenomenon.
Footnotes
Conflict of interests
The authors declare that they have no conflict of interests.
Source of support: Studies were funded by the Ministry of Science and Higher Education grant no. N302 009 32/1139. K. Kasarello, MSc, has been supported with a scholarship from the European Social Fund, Human Capital Operational Programme
References
- 1.Johnson D, Hafler DA, Fallis RJ, et al. Cell-mediated immunity to myelin-associated glycoprotein, proteolipid protein and myelin basic protein in multiple sclerosis. J Neuroimmunol. 1986;13:99–108. doi: 10.1016/0165-5728(86)90053-6. [DOI] [PubMed] [Google Scholar]
- 2.Link H, Sun JB, Wang Z, et al. Virus-reactive and autoreactive T cells are accumulated in cerebrospinal fluid in multiple sclerosis. Neuroimmunol. 1992;38:63–73. doi: 10.1016/0165-5728(92)90091-x. [DOI] [PubMed] [Google Scholar]
- 3.Kerlero de Rosbo N, Milo R, Lees MB, et al. Reactivity to myelin antigens in multiple sclerosis. J Clin Invest. 1993;92:2602–8. doi: 10.1172/JCI116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuohy VK. Peptide determinants of myelin proteolipid protein (PLP) in autoimmune demyelinating disease: a review. Neurochem Res. 1994;19:935–44. doi: 10.1007/BF00968703. [DOI] [PubMed] [Google Scholar]
- 5.Tuohy VK, Fritz RB, Ben-Nun A. Self-determinants in autoimmune demyelinating disease: changes in T- cell response specificity. Curr Opin Immunol. 1994;6:887–91. doi: 10.1016/0952-7915(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 6.Ewing C, Bernard CC. Insights into the etiology and pathogenesis of multiple sclerosis. Immunol Cell Biol. 1998;76:47–54. doi: 10.1046/j.1440-1711.1998.00718.x. [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL. An update on multiple sclerosis. J Neurol Sci. 2005;228:193–94. doi: 10.1016/j.jns.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Steinman L. Immunotherapy of multiple sclerosis: the end of the beginning. Curr Opin Immunol. 2001;13:597–600. doi: 10.1016/s0952-7915(00)00266-1. [DOI] [PubMed] [Google Scholar]
- 9.Cendrowski W. Selected issues of immunomodulating treatment in multiple sclerosis. Neurol Neurochir Pol. 2004;38:299–306. [PubMed] [Google Scholar]
- 10.Mowat AM. The regulation of immune responses to dietary antigens. Immunol Today. 1987;8:93–98. doi: 10.1016/0167-5699(87)90853-X. [DOI] [PubMed] [Google Scholar]
- 11.Weiner HL, Friedman A, Miller A, et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 12.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 13.Lees MB, Kuchroo VK, Sobel RA. Myelin proteolipid protein: its role in experimental allergic encephalomyelitis (EAE) Int Pediatr. 1991;6:84–90. [Google Scholar]
- 14.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor VB expression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951–59. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 15.Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J Immunol. 1988;140:440–45. [PubMed] [Google Scholar]
- 16.Kelly KA, Whitacre CC. Oral tolerance in EAE: reversal of tolerance by T helper cell cytokines. J Neuroimmunol. 1996;66:77–84. doi: 10.1016/0165-5728(96)00027-6. [DOI] [PubMed] [Google Scholar]
- 17.Abbott DJ, Blanchfield JL, Martinson DA, et al. Neuroantigen-specific, tolerogenic vaccines: GMCSFis a fusion partner that facilitates tolerance rather than immunity to dominant self-epitopes of myelin in murine models of experimental autoimmune encephalomyelitis (EAE) BMC Immunology. 2011;12:72. doi: 10.1186/1471-2172-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwiatkowska-Patzer B, Baranowska B, Barcikowska-Litwin M, Lipkowski AW. Suppression of experimental autoimmune encephalomyelitis in the rat by oral administration of spinal cord protein hydrolysate. In: Teelken, Korf, editors. Neurochemistry. New York: Plenum Press; 1997. pp. 137–40. [Google Scholar]
- 19.Kwiatkowska-Patzer B, Gajkowska B, Baranowska B, Lipkowski AW. Ultrastructural changes in the central and peripheral nervous system in the rat with experimental allergic encephalomyelitis. Folia Neuropathol. 1998;37:245–49. [PubMed] [Google Scholar]
- 20.Kwiatkowska-Patzer B, Baranowska B, Walski M, Lipkowski AW. Influence of spinal cord protein hydrolysate upon the blood brain barrier changes due to experimental encephalomyelitis in Lewis rats. Ultrastructural study. Folia Neuropathol. 2003;41:29–34. [PubMed] [Google Scholar]
- 21.Kwiatkowska-Patzer B, Michałkiewicz J, Kubiszewska I, et al. Spinal cord hydrolysate ameliorate immunological reaction in experimental allergic encephalomyelitis. Acta Neurobiol Exp (Wars) 2009;69:73–78. doi: 10.55782/ane-2009-1731. [DOI] [PubMed] [Google Scholar]
- 22.Karpus WJ, Kennedy KJ, Smith WS, Miller SD. Inhibition of relapsing experimental autoimmune encephalomyelitis in SJL mice by feeding the immunodominant PLP139-151 peptide. J Neurosci Res. 1996;45:410–23. doi: 10.1002/(SICI)1097-4547(19960815)45:4<410::AID-JNR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Juryńczyk M, Walczak A, Jurewicz A, et al. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann Neurol. 2010;68:593–601. doi: 10.1002/ana.22219. [DOI] [PubMed] [Google Scholar]
- 24.Levine MM. Immunization against bacterial diseases of the intestine. J Pediatr Gastroenterol Nutr. 2000;31:336–55. doi: 10.1097/00005176-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Modlin JF. Poliomyelitis in the United States: the final chapter? JAMA. 2004;292:1749–51. doi: 10.1001/jama.292.14.1749. [DOI] [PubMed] [Google Scholar]
- 26.Libudzisz Z, Walczak P, Bardowski J, editors. Lactic acid bacteria – classification, metabolism, genetics, applications (in polish) Łódź: Technical University of Łódź; 1998. [Google Scholar]
- 27.Teusink B, Smid EJ. Modelling strategies for the industrial exploitation of lactic acid bacteria. Nat Rev Microbiol. 2006;4:46–56. doi: 10.1038/nrmicro1319. [DOI] [PubMed] [Google Scholar]
- 28.Steidler L, Rottiers P. Therapeutic drug delivery by genetically modified Lactococcus lactis. Ann NY Acad Sci. 2006;1072:176–86. doi: 10.1196/annals.1326.031. [DOI] [PubMed] [Google Scholar]
- 29.Bermúdez-Humarán LG, Kharrat P, Chatel J-M, Langella P. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microbial Cell Factories. 2011;10:S4. doi: 10.1186/1475-2859-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–55. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro LA, Azevedo V, Le Loir Y, et al. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl Environ Microbiol. 2002;68:910–16. doi: 10.1128/AEM.68.2.910-916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatel JM, Nouaille S, Adel-Patient K, et al. Characterization of a Lactococcus lactis strain that secretes a major epitope of bovine beta-lactoglobulin and evaluation of its immunogenicity in mice. Appl Environ Microbiol. 2003;69:6620–27. doi: 10.1128/AEM.69.11.6620-6627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bermúdez-Humarán LG, Langella P. Perspectives for the development of human papillomavirus vaccines and immunotherapy. Expert Rev Vaccines. 2010;9:35–44. doi: 10.1586/erv.09.145. [DOI] [PubMed] [Google Scholar]
- 34.Adel-Patient K, Ah-Leung S, Creminon C, et al. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin Exp Allergy. 2005;35:539–46. doi: 10.1111/j.1365-2222.2005.02225.x. [DOI] [PubMed] [Google Scholar]
- 35.Frossard CP, Steidler L, Eigenmann PA. Oral administration of an IL-10-secreting Lactococcus lactis strain prevents food-induced IgE sensitization. J. Allergy Clin Immunol. 2007;119:952–59. doi: 10.1016/j.jaci.2006.12.615. [DOI] [PubMed] [Google Scholar]
- 36.Huibregtse IL, Snoeck V, de Creus A, et al. Induction of ovalbumin-specific tolerance by oral administration of Lactococcus lactis secreting ovalbumin. Gastroenterology. 2007;133:517–28. doi: 10.1053/j.gastro.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 37.Aleksandrzak-Piekarczyk T, Polak J, Jezierska B, et al. Genetic characterization of the CcpA-dependent, cellobiose-specific PTS system comprising CelB, PtcB and PtcA that transports lactose in Lactococcus lactis IL1403. Int J Food Microbiol. 2011;145:186–94. doi: 10.1016/j.ijfoodmicro.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Terzaghi BE, Sandine WE. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–13. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a Laboratory Manual. 2nd ed. New York: Cold Spring Harbor Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Holo H, Nes IF. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–23. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells JM, Wilson PW, Norton PM, et al. Lactococcus lactis: high level expression of tetanus fragment C and protection against lethal challenge. Mol Microbiol. 1993;8:1155–62. doi: 10.1111/j.1365-2958.1993.tb01660.x. [DOI] [PubMed] [Google Scholar]
- 42.Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 43.Sun JB, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–99. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma S, Jevnikar AM. Autoantigens produced in plants for oral tolerance therapy of autoimmune diseases. Adv Exp Med Biol. 1999;464:179–94. doi: 10.1007/978-1-4615-4729-7_14. [DOI] [PubMed] [Google Scholar]
- 45.Matsunaga Y, Wakatsuki Y, Tabata Y, et al. Oral immunization with size-purified microsphere beads as a vehicle selectively induces systemic tolerance and sensitization. Vaccine. 2000;19:579–88. doi: 10.1016/s0264-410x(00)00120-1. [DOI] [PubMed] [Google Scholar]
- 46.Gould DJ, Chernajovsky Y. Novel delivery methods to achieve immunomodulation. Curr Opin Pharmacol. 2007;7:445–50. doi: 10.1016/j.coph.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maassen CBM, Laman JD, van Holten-Neelen C, et al. Reduced experimental autoimmune encephalomyelitis after intranasal and oral administration of recombinant lactobacilli expressing myelin antigens. Vaccine. 2003;21:4685–93. doi: 10.1016/s0264-410x(03)00522-x. [DOI] [PubMed] [Google Scholar]
- 48.Takiishi T, Korf H, Van Belle TL, et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest. 2012;122:1717–25. doi: 10.1172/JCI60530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortes-Perez NG, Bermúdez-Humarán LG, Le Loir Y, et al. Mice immunization with live lactococci displaying a surface anchored HPV-16 E7 oncoprotein. FEMS Microbiol Lett. 2003;229:37–42. doi: 10.1016/S0378-1097(03)00778-X. [DOI] [PubMed] [Google Scholar]
- 50.Bermúdez-Humarán LG, Cortes-Perez NG, Le Loir Y, et al. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol. 2004;53:427–33. doi: 10.1099/jmm.0.05472-0. [DOI] [PubMed] [Google Scholar]
- 51.Bermúdez-Humarán LG, Cortes-Perez NG, Lefèvre F, et al. A novel mucosal vaccine based on live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol. 2005;175:7297–302. doi: 10.4049/jimmunol.175.11.7297. [DOI] [PubMed] [Google Scholar]
- 52.Wu C, Yang G, Bermúdez-Humarán LG, et al. Immunomodulatory effects of IL-12 secreted by Lactococcus lactis on Th1/Th2 balance in ovalbumin (OVA)-induced asthma model mice. Int Immunopharmacol. 2006;6:610–15. doi: 10.1016/j.intimp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Cortes-Perez NG, Ah-Leung S, Bermúdez-Humarán LG, et al. Intranasal coadministration of live lactococci producing interleukin-12 and a major cow’s milk allergen inhibits allergic reaction in mice. Clin Vaccine Immunol. 2007;14:226–33. doi: 10.1128/CVI.00299-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortes-Perez NG, Ah-Leung S, Bermúdez-Humarán LG, et al. Allergy therapy by intranasal administration with recombinant Lactococcus lactis producing bovine beta-lactoglobulin. Int Arch Allergy Immunol. 2009;150:25–31. doi: 10.1159/000210377. [DOI] [PubMed] [Google Scholar]
- 55.van Roosmalen ML, Kanninga R, El Khattabi M, et al. Mucosal vaccine delivery of antigens tightly bound to an adjuvant particle made from food-grade bacteria. Methods. 2006;38:144–49. doi: 10.1016/j.ymeth.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Maillard MH, Snapper SB. Teaching tolerance with a probiotic antigen delivery system. Gastroenterology. 2007;133:706–9. doi: 10.1053/j.gastro.2007.06.055. [DOI] [PubMed] [Google Scholar]
- 57.Amiguet P, Gardinier MV, Zanetta J-P, Matthieu J-M. Purification and partial structural and functional characterization of mouse myelin/oligodendrocyte glycoprotein. J Neurochem. 1992;58:1676–82. doi: 10.1111/j.1471-4159.1992.tb10040.x. [DOI] [PubMed] [Google Scholar]
- 58.Kowalczyk M, Cocaign-Bousquet M, Loubiere P, Bardowski J. Identification and functional characterization of cellobiose and lactose transport systems in Lactococcus lactis IL1403. Archives of Microbiology. 2008;189:187–96. doi: 10.1007/s00203-007-0308-8. [DOI] [PubMed] [Google Scholar]
- 59.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faria MCA, Maron R, Ficker SM, et al. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor-β/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20:135–45. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- 61.Peng HJ, Turner MW, Strobel S. The kinetics of oral hyposensitization to a protein antigen are determined by immune status and the timing, dose and frequency of antigen administration. Immunology. 1989;67:425–30. [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshino S. Antigen-induced arthritis in rats is suppressed by the inducing antigen administered orally before, but not after immunization. Cell Immunol. 1995;163:55–58. doi: 10.1006/cimm.1995.1098. [DOI] [PubMed] [Google Scholar]
- 63.Meyer AL, Benson JM, Gienapp IE, et al. Suppression of murine chronic relapsing experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. J Immunol. 1996;157:4230–38. [PubMed] [Google Scholar]
- 64.Inada S, Kohashi O, Hamasaki Y. Humoral immune-mediated acute, antigen-induced arthritis in rats is suppressed by the inducing antigen administered orally before, but not after, immunization. Immunol Invest. 2001;30:47–56. doi: 10.1081/imm-100103690. [DOI] [PubMed] [Google Scholar]
- 65.Lamont AG, Bruce MG, Watret KC, Ferguson A. Suppression of an established DTH response to ovalbumin in mice by feeding antigen after immunization. Immunology. 1988;64:135–39. [PMC free article] [PubMed] [Google Scholar]