Short abstract
Our bodies depend on an exquisitely sensitive and refined temperature control system to maintain a state of health and homeostasis. The exceptionally broad range of physical activities that humans engage in and the diverse array of environmental conditions we face require remarkable strategies and mechanisms for regulating internal and external heat transfer processes. On the occasions for which the body suffers trauma, therapeutic temperature modulation is often the approach of choice for reversing injury and inflammation and launching a cascade of healing. The focus of human thermoregulation is maintenance of the body core temperature within a tight range of values, even as internal rates of energy generation may vary over an order of magnitude, environmental convection, and radiation heat loads may undergo large changes in the absence of any significant personal control, surface insulation may be added or removed, all occurring while the body's internal thermostat follows a diurnal circadian cycle that may be altered by illness and anesthetic agents. An advanced level of understanding of the complex physiological function and control of the human body may be combined with skill in heat transfer analysis and design to develop life-saving and injury-healing medical devices. This paper will describe some of the challenges and conquests the author has experienced related to the practice of heat transfer for maintenance of health and enhancement of healing processes.
Keywords: thermoregulation, circadian cycle, bioheat transfer, glabrous skin, arteriovenous anastomoses, skin blood flow, core temperature, cryotherapy, therapeutic hypothermia, thermal stimulation
Introduction
Humans are able to survive exposure to a remarkably broad range of environmental thermal stressors and to alterations in the distribution and magnitude of internal energy generation, all while maintaining a nearly constant core temperature necessary for health and well being. The thermoregulatory system consists of thermal sensors and diverse actuators located throughout the body. A continual stream of input signals is integrated and processed in a multicomponent distributed controller that regulates the functions of blood flow magnitude and allocation throughout the body in coordination with various metabolic activities, all while balancing other essential control needs such as sustaining adequate blood pressure levels and meeting the nutritive requirements of cells.
Maintenance of homeostasis depends directly on effective thermoregulation, and as a consequence it has been the subject of widespread research for many, many years. There is a compelling rationale for measuring, modeling, and simulating of the thermoregulatory behavior in humans, including enhancing the ability to understand foundational physiological principles and processes, to design systems and processes that interface with or enhance thermoregulatory function, and to identify performance limitations and conditions of danger both for stressful environments and for devices to provide thermal therapies or physical performance amplification. Much of this work has been conducted over many decades by brilliant and innovative physiologists in parallel, and often in concert, with engineers who have driven the understanding and utilization of bioheat transfer.
In addition to thermoregulatory considerations at the whole body level, local temperature sensing and manipulation have important consequences for health and healing. Endogenous thermal sensors, particularly in the skin, play a key role in providing feedback signals that drive the response of the thermoregulatory system as well as local and peripheral skin blood flow. The application of heating or cooling is widely used therapeutically. On the one hand, the temperature manipulation may be used to direct and enhance a healing process. Alternatively, the temperature may be controlled so as to cause necrosis of malignant tissues. In all cases, the regulation of temperature with respect to its spatial and temporal distributions is important in governing the process outcome.
Most of the foregoing phenomena have a long history of study, including investigation by many of the pioneering physiologists. In more recent years, the author has continued this line of inquiry using engineering and heat transfer technologies, plus modern instrumentation. The general field of bioheat transfer is very broad and is impossible to cover comprehensively in this short publication. This paper will provide only a brief summary of the current state of background understanding relating to the author's involvement in these areas of research and their practical impact as an illustration of the opportunities for significant contributions by heat transfer engineers in medicine and physiology.
Human Thermoregulation in Health and Daily Activities
The human thermoregulatory system operates across a broad spectrum of physical and physiological conditions to maintain the body core temperature within a very small window of variation on the order of 0.3 °C. It is vitally (life sustaining) important to health and well being, as well as personal comfort and functionality that the core temperature be held proximal to its set point. Excursions outside of this small thermal window may have serious consequences, including illness or even death.
The function of the thermoregulatory system occurs through a network of neural sensor and effector pathways that detect temperatures at a multitude of central and peripheral locations and integrate those inputs into a balanced pattern of energy generation and heat transfer responses [1]. A wealth of insightful studies over the past century have shown that the feedback control function of thermoregulation is highly nonlinear and that the operational mechanisms are altered remarkably over the wide range of physiological states that may be encountered [2–4]. Further complicating the understanding is the emerging fact that the feedback depends on dynamic as well as static state properties [5].
It is well-recognized that the pre-optic anterior hypothalamus (POAH) in the brain is the primary central control site for thermoregulation [6–8]. Both local temperature and signals from around the body are weighted and integrated to determine outputs for controlling effector mechanisms such as blood flow distribution, sweating, and shivering [9,10]. Although the POAH is intimately involved in the control, it has also been recognized that there are parallel peripheral (to the brain) thermoregulatory control tissues, primarily along the spinal cord [3,11–13]. The existence of peripheral thermoregulatory control tissue has been confirmed via experiments involving the chronic implantation of thermodes surrounding the spine in various mammalian species, including sheep [14,15], dogs [11–13], goats [16], and oxen [17]. Of course, such testing is impossible to conduct in humans. Nonetheless, there has been extensive testing to attempt to identify whether there are peripheral sites from which blood flow to the skin in distal regions would respond to thermal stimulation [18–21], but with only limited success. None of these studies identified the spine as a site of peripheral thermoregulatory control tissue.
In its simplest portrayal, the function of thermoregulation is to hold the central body temperature to within a small tolerance of a set point value that for adults is taken to be 37 °C. However, it is also well known that the target does not remain constant, resulting a circadian thermal cycle in which the core temperature varies by approximately 1 °C over a 24-hr-period [22–25]. This cyclic core temperature variation is achieved by modulation in the magnitudes and balance between rates of internal energy generation and heat exchange with the environment. Multiple parallel processes are regulated in a highly sophisticated pattern of coordination.
Parallel mechanisms exist by which heat may be transported between the body core and surface. The heat conduction path remains nearly constant irrespective of physiological status and environmental conditions, being dependent only on anatomical structure and composition. On a relative basis, it is ineffective as a means of transport owing to the long conduction distance and low conductivity of tissues. Heat flow in conjunction with respiration is slightly variable, changing primarily with the breathing rate as air moves inward and outward between the lungs that are at core temperature and the environment. The respiratory rate is quite consistent for normal conditions, although it may increase by a factor of up to two under stressful conditions. The thermophysical properties of air limit the amount of energy that can be transported.
By far, the most important and effective mechanism of energy flow between the core and surface is via the convection of blood. Its liquid phase renders a relatively large heat capacity, and the ability of the cardiovascular system to alter the temporal and spatial distribution of blood over great spatial and temporal ranges [26] provides a tremendous efficiency for increasing or decreasing the flow of heat between the core and the surface to meet changing thermoregulatory requirements [9,10,27,28]. One very important aspect of the cardiovascular function in thermoregulation is that blood flow is not distributed uniformly across the skin surface. Under conditions for which heat rejection to the environment is a priority, blood flow is biased to glabrous (hairless) skin of the hands, feet, and face, where arteriovenous anastomoses (AVAs) are located. When vasodilated, the AVAs may have a diameter, an order of magnitude larger than that of the parallel capillaries, and thereby present a flow resistance that is reduced by a factor of 104 from the alternate flow pathway. As a consequence, the AVAs are capable of carrying a substantially higher rate of blood flow than is the terminal capillary network through which blood is perfused to hairy skin [9,29,30], and therefore have a greater participation in convective heat transfer.
Grahn and Heller have noted that under conditions of AVA vasodilation, with the vessels further distended by the application of mild negative pressure, the locally high perfusion of blood in glabrous skin can be recruited with surface heat exchangers to more effectively move energy into [31] and out of the body core [32–34]. Given the long awareness of the existence and heat transfer function of the AVAs [35,36], it is puzzling that most devices intended for manipulating the body core temperature are designed to apply a controlled temperature only to nonglabrous skin, where the capacity for convective heat transfer with blood is substantially limited. These other options include a wide range of medical heating and cooling devices, plus the space suit liquid cooling garment [37].
The primary limiting factor on the negative pressure heat transfer technology as applied to the AVAs by Grahn and Heller is that it is not possible to proactively overcome the neurologically mediated state of vasoconstriction by mechanical means. Thus, if a vasoconstrictive tone is being maintained by the body, external application of a negative pressure will not cause distention of the AVAs to produce the high blood flow rate requisite to effective convective heat transfer between the skin and core. Therefore, the efficacious application of this type of heat transfer device is limited to conditions for which the AVAs are already naturally vasodilated such as occurs in response to heat stress or the introduction of an anesthetic agent. Their measurements show glabrous skin heat fluxes can be achieved in excess of 0.2 W/cm2 [34], which is four to five times larger than that is possible on nonglabrous skin surfaces under matching thermal conditions. Thus, adaptation of surface heat transfer procedures to target glabrous skin areas represents a significant opportunity for improving therapeutic device performance and medical outcomes.
Alteration of Thermoregulation in Disease and for Medical Procedures
The normal functioning of the thermoregulatory system may be altered by certain disease states and by the application of medical devices or procedures designed to achieve a specified therapeutic outcome. In some cases, the effect is produced by altering the set point of the physiological controller and/or the processes it invokes. Alternatively, a device may simply overwhelm the physiological function and impose a revised thermal state on the body.
A well known example of this situation is when a febrile state occurs. As the set point is raised, thermal inputs from the body indicate that the temperature is too low. The result is an autonomous response to raise the core temperature via an increase of metabolic heat production by shivering until the core temperature reaches the new set point. The skin also becomes vasoconstricted during this process. Conversely, when a febrile state breaks, the core temperature is then interpreted as being too high, inducing processes to deliver heat to the environment by large scale cutaneous vasodilation and sweating.
Significant upward and downward excursions from the core temperature set point may occur under environmental conditions that overwhelm the capacity of the thermoregulatory system to compensate. The results are thermally related illnesses, such as heat stroke [38] and deep hypothermia [39], which in the extreme may be fatal. For these cases, special external intervention is required to guide the body back to a healthy state. The most effective therapy is sometimes counterintuitive. For example, in recovering a person from heat illness (stroke), the conventional clinical wisdom is to immerse the patient into a water bath as cold as possible so as to create the largest possible temperature differential between the core and the environment [40]. However, the extremely cold surface temperature will elicit a strong local vasoconstriction response [41], with the consequences that the main heat transport route between the core and the environment will be minimized, thereby trapping heat within the body interior and extending the period of dangerous hyperthermic exposure [42]. Also, the low skin temperature may elicit a shivering response which will accelerate the internal generation of energy that will exacerbate the hyperthermic state. An alternative procedure is to limit the temperature of a water immersion bath to temperature levels so as to avoid vasoconstriction and preserve the convective circulation of blood between the core and skin [42]. Even with a smaller overall temperature difference, the net rate of heat transport from the core may be enhanced.
The theme of taking advantage of the body's primary natural process for effectively moving heat between the core and surface via blood flow has been followed by the author in developing methods and devices for induction of therapeutic hypothermia (TH). In recent times, there has been an emerging realization that TH may provide great benefit in mortality and morbidity that is often associated with medical events that cause major organ ischemia, including stroke, cardiac arrest, and traumatic brain injury [43–45]. Although there are many approaches to lowering the body core temperature, the most widespread involves covering large portions of the body surface with cooling blankets [46]. This and other methods embody a brute force tactic of simply overwhelming the thermoregulatory defense against a substantial lowering of the core temperature [47]. A prominent response that must be suppressed is shivering, for which pharmacologic agents are administered [48]. Selected agents may be used to reduce the shivering threshold to 33.5 °C for long term TH episodes [47]. As is the case for most other noninvasive surface heat transfer devices intended for manipulating the body core temperature, cooling blankets for inducing TH are usually applied to torso, legs, and arms, to the specific exclusion of the areas of glabrous skin where convective exchange with blood is most effectively accomplished in the AVAs.
The foremost challenge in realizing the potential physiological and therapeutic benefits of heat transfer applied at the glabrous skin is to be able to create a high flow rate of blood through the AVAs on demand. Given that the key role in thermoregulation played by AVA heat transfer in glabrous skin has been realized for approximately a century, there is a long history of attempts to thermally stimulate blood flow to the appendages [18–21]. More recently, peripheral thermoregulatory control tissue has been identified along the spinal cord in various mammalian species, as noted earlier [11–17]. In these studies, a tube through which heated water could be circulated was implanted chronically through the vertebral canal of the spine to control the local temperature. Heating the spine caused a thermoregulatory response to reject heat to the environment, even though the hypothalamus was not above the set point temperature. In ox, core temperature drops of 5 °C and larger were achieved with thermal stimulation of the spine. Although such experiments are impossible to conduct in humans, the implication is that a similar thermal stimulus could produce the same type of result. Of note, since the central goal of TH is to drop the brain temperature, and, additionally, that lower hypothalamus temperatures will invoke thermoregulatory conservation to fight off the core temperature dropping, an independent and parallel control site along the spine could be an effective option for inducing upregulation of AVA perfusion so that blood may be cooled as it flows through glabrous skin prior to circulating back to the core. This specific process is termed glabrous skin heat transfer (GSHT) and can result in much greater energy fluxes between the skin and body core than are possible through nonglabrous skin areas. GSHT can be particularly effective when it is mediated via thermal communication with an environment that is liquid phase (e.g., water) rather than vapor phase (e.g., air).
The procedure of applying low grade heating (at a surface temperature not exceeding 42 °C) at specific small areas aligned with the spine has been dubbed selective thermal stimulation (STS). In humans, STS may be used to cause an increase in glabrous skin blood flow (GSBF) in conjunction with devices for effecting GSHT to enable core temperature management on demand. Figure 1 is a simple graphical representation of the concept of coordinated STS and GSHT applied to a human.
Fig. 1.

A pictorial representation of the skin areas involved in STS along the cervical spine and GSHT at the palms and plantar surfaces. An important feature is that STS and GSHT occupy only minor fractions of the total body surface area, providing minimal interference with other simultaneous medical procedures.
Hundreds of human trials have been conducted in the author's laboratory over several years with full UT IRB approval to explore the efficacy of STS in manipulating GSBF. Primary instrumentation consists of thermocouples applied to the skin at the site of STS and at representative glabrous and nonglabrous skin locations and laser Doppler blood flow probes also at glabrous and nonglabrous locations. A typical trial is illustrated in Fig. 2.
Fig. 2.
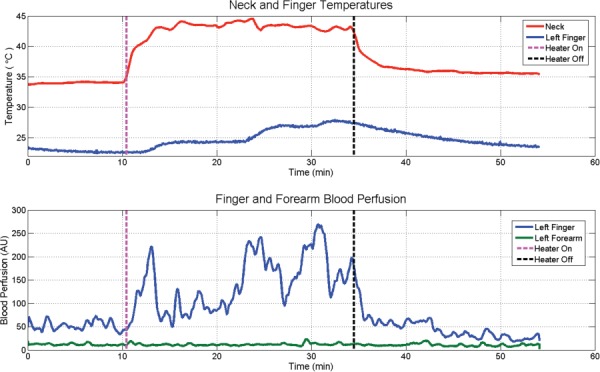
Temperatures on the skin overlying the cervical spine and the fourth finger pad and concurrent blood perfusion values in the middle index finger (glabrous skin) and the matching medial aspect of the forearm (nonglabrous skin) during a neck heating trial to stimulate GSBF. Temperatures are in the upper pane and blood flow in the lower pane.
Cutaneous blood perfusion was monitored with laser Doppler flow probes placed on the distal pad of the left ring finger (over glabrous skin) and on the medial aspect of the left forearm (over nonglabrous skin). Thermocouples were placed on the distal pad of the middle finger on the left hand and on the skin at the C4 vertebra. Following an initial benchmark period of 10 min, an electric heating pad was applied over the cervical spine for 25 min and then physically removed. Blood flow is expressed in arbitrary units as output from the instrumentation, since only relative changes in magnitude were of interest in this study.
This trial was conducted with STS effected by a direct application of a simple electric heating pad along the cervical spine. More precise and rapid heating is accomplished alternatively via a special heater with closed loop feedback control. Nonetheless, for this trial there is an apparent direct correlation between the upregulation and down regulation of GSBF in conjunction with the application and removal of STS. Note that the non-GSBF is totally unaffected by the STS. The STS effect appears to be initiated approximately when the cervical skin temperature rises to the range of 39–40 °C. The surface temperature of the finger pad follows the level of blood perfusion measured in the adjacent finger. Higher blood flows result in an increase in surface temperature, presumably as a consequence of increased convection with warm blood perfused through the vasculature, adding heat to the skin that then diffuses to the cooling surface region. This test subject was initially in a strongly vasoconstricted state that was holding stable, as manifested in the finger surface temperature of about 23 °C. In response to STS of the cervical skin, there were three episodes of glabrous blood upregulation, each of which produced an increase in finger pad temperature. Also, owing to the initial state of relatively deep glabrous vasoconstriction, there was a substantial potential for upregulation of the flow, which eventually was five fold in magnitude. When the STS was withdrawn there was a nearly immediate drop in GSBF to levels even lower than during the baseline period. The total differential between maximum and minimum GSBF with and without STS was approximately 10X.
A series of trials was conducted with seven subjects dressed in shorts and a tee shirt in a room with air temperature at 22 °C. All trials were conducted with subjects resting in a prone position with no environmental stimuli. Application of instrumentation required nearly an hour, allowing acclimation to the room thermal environment, following which data acquisition was started. Trials started with 15 min of baseline data after which STS was activated at a temperature in the range of 40–42 °C for 30 min. The perfusion is expressed in terms of cutaneous vascular conductance (CVC), which is calculated as the ratio of the voltage output from the laser Doppler probe divided by the mean arterial pressure as measured periodically with an automated pneumatic arm cuff. The CVC was averaged over the final 5 min of the baseline period and over the 5-min-interval for which it reached the largest differential from baseline. Control trials were conducted in the same manner except that the neck heater was never activated. The CVC values at the start of STS and at the point of largest divergence from baseline are plotted in Fig. 3 for the individual trials.
Fig. 3.
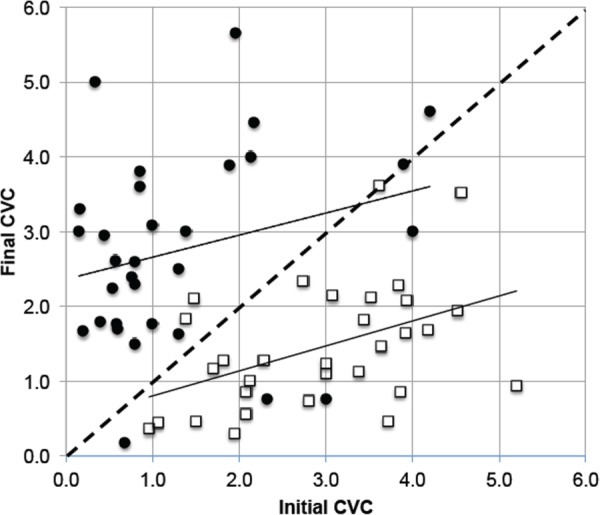
Initial and stimulated finger pad CVC data as a function of application of neck heating stimulation for subjects exposed to room air at 22 °C. Open squares are for controls with no stimulation. Filled circles are for stimulation. Straight lines are linear least squares fits to the two data sets.
A two sample t test analysis shows that there is a probability greater than 99% that the data sets are different, i.e., that the cervical STS does result in an upregulation in GSBF. That being the case, a two-step method for inducing TH in which first, mild STS is applied along the cervical spine to cause an increase in GSBF, and second, cooling heat exchangers are applied on the palmar and plantar skin surfaces to withdraw heat from blood flowing through the AVAs prior to its recirculation back to the body core. The heat exchanger surfaces should be maintained at temperatures above about 20 °C so as to avoid local cold-induced vasoconstriction (CIVC) [49].
Several important features of the data in Fig. 3 should be pointed out. The diagonal dashed-line defines the null effect outcome for which the initial and final CVC values were equal. The CVC values never are zero since the tissue always maintains a residual nutritive level of blood flow. Neither is the CVC able to increase without limit, as there is a maximum level at which the heart is able to pump blood through the local vascular network when the AVAs are at greatest vasodilation. An exception is the technique of Grahn and Heller [31] as discussed previously in which a mild negative pressure is applied to the glabrous tissue to further distend the AVAs beyond their normal maximum vasodilation.
There is a very large spread in the initial CVCs measured amongst the subjects, which is typical of a human population in a thermal neutral environment. Subjects having a low initial CVC have a larger potential for an increase in blood perfusion, as is indicated by the least squares curve fits for both data sets. The control subject data all tended to fall below the null effect line, and the thermal stimulation data above it, with a few exceptions. The control subjects had a strong tendency toward a progressive vasoconstriction in a 22 °C room over their nearly 2 hr of exposure therein. It is generally about 75 min from the start of instrumentation until the end of baseline. It is likely that this is a long enough period during which the subjects, in a prone position with minimal physical activity and having a large fraction of the skin surface uncovered, will begin to develop a deficit between the rates of internal energy generation and external heat loss. Although 22 °C air would be considered thermally neutral for normally clothed persons engaged in office type activities, it is insufficient to maintain an energy balance for the subjects, and they respond with a slow, progressive vasoconstriction. The therapeutic use of this technology has its objective the end point of a high CVC so that glabrous skin heat exchangers will be effective in transferring energy out from the core via high flow rate circulation of blood. The objective is achieved whether the CVC is initially high or reaches that state via thermal stimulation.
A small number of the data points appear to be outliers in comparison to their cohort: controls with increased CVC and interventions with decreased CVC. A comprehensive review of hundreds of STS experiments has indicated that one factor on which the response of GSBF may be dependent is whether the thermoregulatory system is in a transient state of control. For example, if a subject is in a condition of strongly becoming vasoconstricted, it is proportionately more difficult to increase the magnitude of CVC via STS. Subjectively, this condition may be realized in terms of a feeling of one's hands and feet becoming progressively colder when exposed to a cool environment. We have quantified this phenomenon in terms of the temporal gradient in CVC, or dCVC/dt, and termed it as the thermoregulatory inertia (TRI) [50]. As can be seen in Fig. 2, there is typically a temporal variation in the CVC signal in conjunction with the complex mode of regulation of blood delivery throughout the circulatory system [26]. Therefore, TRI is quantified by averaging the CVC over a 5-min-window of time. Figure 4 presents a scatter plot of TRI values before and after stimulation for the same data sets shown in Fig. 3.
Fig. 4.
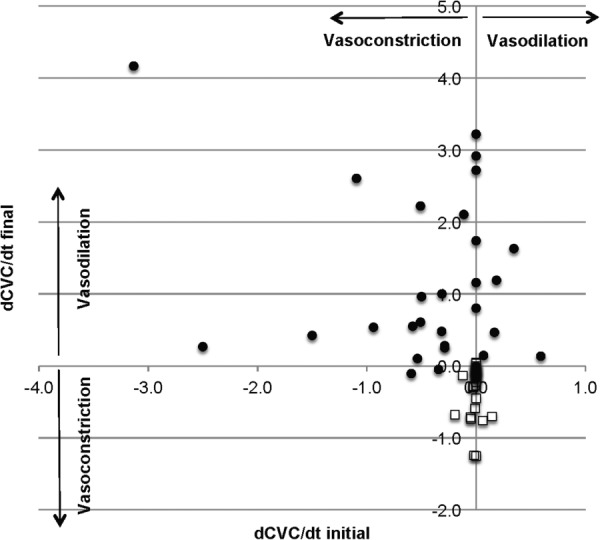
Time rate of change of finger pad CVC (TI) as a function of application of neck heating stimulation for subjects exposed to room air at 22 °C. Open squares are for controls with no stimulation. Filled circles are for stimulation.
The control data show no change toward a more positive TRI (toward vasodilation), whereas cervical STS blunted or reversed most of the initially negative TRI (toward vasoconstriction). Nearly, all STS trials resulted in a positive TRI, and many reversed a large initially negative TRI (data points in the upper-left quadrant). The clinical implications for the TRI data remain to be determined, but in terms of improving convective access to the body's core energy storage, they clearly hold positive implications. A clinical trial is currently under way with this technology. The objectives of this phase I study are to measure in a patient population in the intensive care unit the efficacy of STS for upregulating the GSBF and to measure the ability of cooling applied to glabrous skin surfaces of the hands and feet in conjunction with STS to alter the heat content of the body, and especially the core temperature.
The foregoing data are derived from preliminary experiments and are an incomplete description of the phenomenon by which cervical STS may be applied to increase GSBF. More comprehensive data, statistical analysis, and detailed evaluation are being prepared for separate dedicated publications. For example, extensive testing has been conducted at ambient temperatures substantially above and below the thermal neutral range so that subjects are initially tending toward vasoconstriction or vasodilation. Also, glabrous skin cooling and warming (the second step for TH) have been combined with STS (the first step) to manipulate the body core temperature, with ongoing testing in progress.
The two-step procedure for proactively adjusting the body core temperature for therapeutic purposes can be considered as embodying a unique elegance. On the one hand, the heat transfer devices and procedures by which the process is conducted are quite compact, easily portable, safe, totally noninvasive, well adapted for field use by first responders, and convenient and intuitive to apply without extensive training. On the other hand, the governing physiological principles and processes involve a sophisticated understanding and extrapolation of long identified thermoregulatory behavior in mammals. However, it is not necessary to own a comprehensive understanding of the physiological principles in order to successfully practice the technology. The philosophy of the technology is to coax the body to cooperate with and achieve the therapeutic objectives by induction of endogenous thermoregulatory processes rather that applying thermal boundary conditions that overpower the body's ability to thermally defend itself. It is likely that the greatest efficacy of the technology will be realized under circumstances of starting therapeutic cooling in the field as soon as possible following an ischemia-inducing event. This is the time point in the chain of treatment when withdrawing heat from the body is likely to have the greatest benefit. It could also be applied within health care facilities for simple and convenient initiation of body cooling. Plus, the technology is physically compatible with existing cooling blanket devices that do not access glabrous skin areas for heat transfer. Our initial studies with prototype devices designed to practice the two-step technology show that heat can be removed from the body at a rate of at least 100 W, and calculations indicate that 200 W should be possible. Further development and testing are required to define the upper limit for thermal interaction with this type of device, but these studies are yet to be conducted. For comparison, the basal metabolic rate (BMR), which is a function of multiple physiological properties such as body mass, height, age, and gender, generally varies over the range of 50–100 W for adults. Further analysis and publications on this technology are forthcoming.
An additional application of this technology for body temperature control is associated with the affect that general anesthesia has to disable normal thermoregulatory function, resulting in patients tending toward a state of perioperative hypothermia during surgical procedures [51–53]. There may be a remarkable drop in core temperature owing to a confluence of factors including: an initial redistribution of energy within the body from the core to the periphery (primarily muscle mass) associated with increased peripheral blood perfusion; relaxation of AVA vasoconstriction allowing large blood flow to the heat transfer vessels, where heat is lost to the environment; suppression of the core temperature at which vasoconstriction is activated; possible direct exposure of visceral organs during surgery; and the relatively cold air temperature (low 20's °C) typically maintained within the operating theater. In effect, the anesthesia reprograms the thermoregulatory controller, allowing much larger variations from the normal set point temperature than are routinely possible.
Modeling and Simulation of Thermoregulation
Maintenance of human homeostasis depends directly on effective thermoregulation. There is a compelling rationale for modeling and simulation of the thermoregulatory behavior in humans, including enhancing the ability: to understand foundational physiological principles and processes; to design strategies and processes that interface with or enhance thermoregulatory function; and to identify performance limitations and conditions of risk both for stressful environs and for devices to provide thermal therapies or physical performance amplification.
The Wissler human thermoregulation model has been under continuous development and refinement for more than 50 yr [54–55]. As local colleagues at the University of Texas, we have taken advantage of the opportunity to collaborate in various past studies [56]. The finite difference model consists of 25 anatomical elements, each of which embodies 21 radial layers, starting from the bones and moving outward, including environmental components (such as clothing). All elements have 12 angular segments that generally are asymmetric, for a total of 6300 nodes. Each node has distinct thermal properties based on its fractional tissue composition and has time-varying properties such as the heat generated by metabolism and blood perfusion. Within the body, heat transfer occurs among nodes primarily through conduction with neighbors and convection of blood flow, as the nodes are anatomically connected. Boundary conditions for the entire body are expressed in terms of conduction, convection, radiation, and evaporation. Conservation of energy and mass flow are applied to every node. Figure 5 is a simplified cartoon of the nodal connectedness of the model. The elements for the hands and feet are components added recently to represent the glabrous skin areas and mass of the hands and feet [57].
Fig. 5.
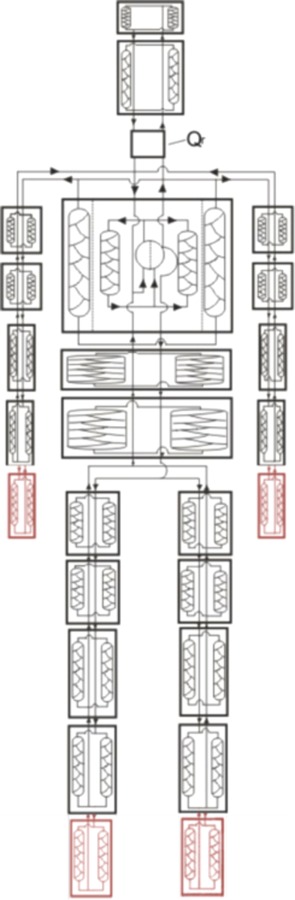
Graphical representation of the connectedness among elements for the Wissler human thermoregulation finite difference model. The classic model has four additional elements for two hands and two feet [57].
The addition of glabrous skin elements to the Wissler human thermoregulation model is anticipated to increase its domain of applicability and to enhance its accuracy in addressing processes that involve blood perfusion in the AVAs as a prime platform for environmental heat exchange. A key component of the model is the controller algorithm that must replicate the processing of the thermoregulatory system afferent and efferent signals that drive the physiological responses. Thermoregulatory mechanisms integrate both central control and diffuse afferent signals and effect the distribution of blood flow throughout the body, plus the initiation and intensity of sweating and shivering. Of particular interest, in our recent studies, is the specific control of blood flow to the AVAs that has a major role in effecting or suppressing energy transfer between the body core and the environment. The AVA control algorithm for normal conditions is shown in Fig. 6.
Fig. 6.
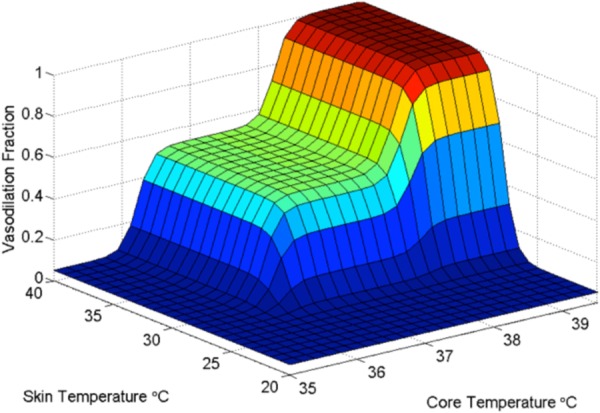
Graphical representation of the control algorithm for blood flow to AVAs as a function of core and mean skin temperatures. Vasoconstriction occurs progressively below 28 °C mean skin temperature and 36 °C core temperature. Vasodilation occurs progressively above 38 °C core temperature. Normothermic perfusion exists for the plateau of states between these thresholds [57].
The central broad plateau of temperatures for which an intermediate level of GSBF exists represents the response to thermoneutral conditions. The specific thermal limits of thermoneutrality are highly individualistic, and the temperature limits used in the simulations presented herein are simply illustrative of the phenomenon and are not to be taken as universally applicable. During disease and fever, the thresholds for the thermoneutral state are altered to higher temperatures so that the thermoregulatory system maintains the febrile state until it breaks.
The Wissler model was applied to simulate the reaction to application of the two-step cervical STS and glabrous skin cooling technology as manifested in changes in core temperature for conditions similar to the study for Figs. 3 and 4. A subject was placed in a thermoneutral room and given a neck heating stimulation. Glabrous skin water circulation heat exchangers were applied to the hands and feet to maintain a constant skin surface temperature of 22 °C. This temperature is above the threshold of approximately 21.5 °C which can cause locally enforced vasoconstriction of AVAs [49] that would thwart a therapeutic cooling process that depends on an active circulation of blood between glabrous skin and the body core. Simulations were run for incremental fractions of the cardiac output (CO) directed to the glabrous skin areas that cover the generally acknowledged available range [9,58,59]. The predicted influence on core temperature is shown in Fig. 7.
Fig. 7.
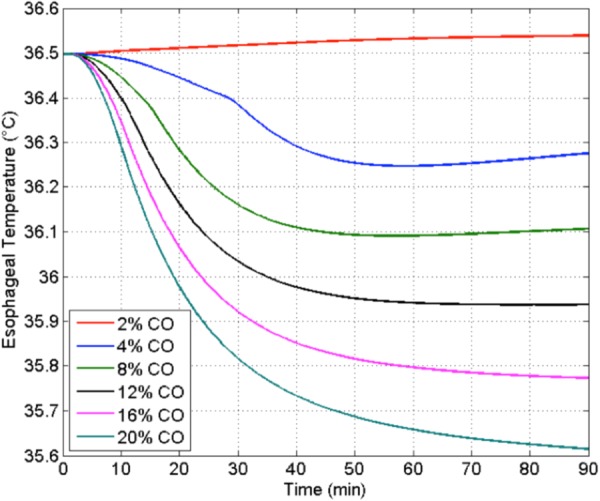
Effect on modulation of core temperature as a function of the magnitude of GSBF as a percent of CO to both hands and both feet with surface cooling at 22 °C [57]
CO is assumed to be modulated by cervical STS, with special heat exchangers applied at the palmar and plantar surfaces to extract heat from circulating GSBF.
The simulation data illustrate the very strong effect that GSHT may have on core temperature management. At low GSBF rates, the core temperature actually raises slightly during the protocol. However, as the glabrous perfusion increases to its largest value, the added convective capacity of the AVA circulation for moving heat between the body core and surface is dramatically apparent. The eventual drop in core temperature toward an asymptotic value is proportional to the blood flow rate through the AVAs. Plus, the initiation of core cooling begins sooner and more strongly with increased AVA flow. This simulation illustrates the potential for the two-step technology for TH induction. It is being applied to help guide device design.
As was discussed earlier, the administration of an anesthetic agent can have a debilitating influence on thermoregulatory function resulting in a state of perioperative hypothermia. This phenomenon has been simulated with the Wissler model, including evaluating the thermal efficacy of various devices that are used to offset the thermoregulatory deficit. Figure 8 shows how the basal level of vasodilation is elevated, the thresholds for sweating and, especially, shivering are offset further from the normal values (dashed line), and hysteresis loops exist for rising and falling temperatures [60].
Fig. 8.
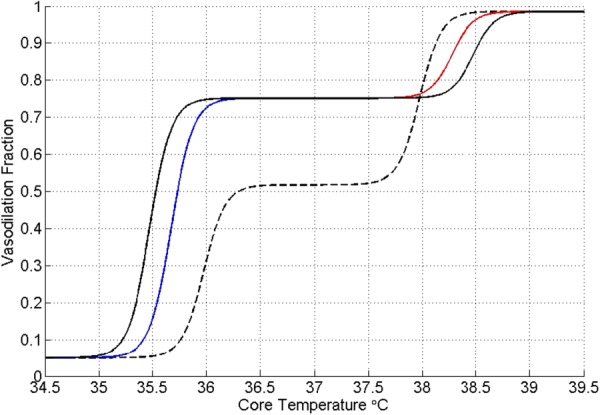
Anesthesia-modified AVA perfusion control algorithm. Dashed line depicts the normal function. Vasodilation is elevated at normothermia and persists to lower core temperatures. Hysteresis occurs in transitions to normothermia. The mean skin temperature threshold states are unchanged [60].
This model has been used to simulate the thermal efficacy of devices and methods reported in the literature for remediating the tendency toward anesthesia-induced perioperative hypothermia [60]. For example, Andrzejowski et al. [61] published data from a 68-patient clinical study for forced air body surface warming during Propofol anesthesia in comparison with forced air warming initiated 60 min prior to the start of the surgical procedure. The objective of prewarming was to store thermal energy in the body prior to the induction of anesthesia to counteract the thermal redistribution resulting from ablation of thermoregulatory vasoconstriction by anesthetic agents [61,62]. Only 43% of patients maintained temperatures above hypothermia with interoperative warming, whereas with the addition of 60 min of prewarming, 68% remained above the core temperature threshold of 36 °C. Thus, even with the extra prewarming intervention, fully 1/3 of patients fell into an undesirable thermal state referred to as inadvertent perioperative hypothermia. Plus, although prewarming led to a better outcome, the process cost was to engage device, facility, and personnel resources for an added hour, which is significant. The interoperative forced air warming at 43 °C alone and combined with 60 min of preoperative warming were simulated with the Wissler model and compared with the clinical data [61]. As an alternative, a simulation was made of only interoperative forced air warming combined with glabrous skin warming with water at a temperature of 42 °C [60]. The results of the simulations along with the clinical data are plotted in Fig. 9.
Fig. 9.
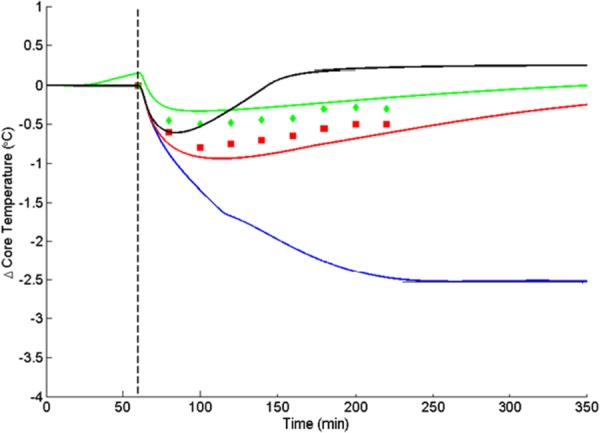
Change in core temperature in response to forced air heating at 38 °C during spinal surgery with Propofol anesthesia [61]. Data points are from the clinical study without (lower) and with (upper) preheating. Solid lines are simulations for no external heating (bottom), interoperative heating (next higher), preheating + interoperative heating (next higher), and glabrous skin + interoperative heating (top) [60].
In the absence of heating intervention, the simulations show that the core temperature drops by 2.5 °C after about 4 hr. Forced air warming reduces the temperature loss considerably, and prewarming even more. The effect of prewarming in elevating the pre-induction temperature slightly is obvious, but the core temperature still drops for both cases.
When AVA heating is combined with forced air heating there is an initial drop in temperature owing to internal energy redistribution. However, the addition of heat input via blood flow through the AVAs is able to reverse this process within 20 min. The anesthesia induced vasodilation of the heat transfer vessels in glabrous skin becomes an advantage when combined with warm water flow in heat exchange pads placed on the hands and feet. The result is a final equilibrium temperature in excess of the pre-induction value. Since the heat exchangers can be applied to the hands and feet in conjunction with the forced air warmers over the general body surface, this technique offers a strong promise for being able to obviate the incidence of unintended perioperative hypothermia. Other simulations indicate that AVA warming may be highly effective when used alone in the absence of other technologies.
Local Temperature Therapy to Aid Healing of Superficial Tissue Injuries
Localized cooling has long been commonly used following orthopedic surgery and in sports medicine to reduce swelling, pain, inflammation, metabolism, muscle spasm, and bleeding, and heating has also be applied to enhance blood perfusion and the healing process [63,64]. Cryotherapy protocols are typically carried out in conjunction with post orthopedic surgical rehabilitation via a device called a cryotherapy unit (CTU), whereas simple ice packs are frequently used in acute sports settings. A CTU generally consists of a remote source of refrigeration, such as an insulated container filled with melting ice, with a submersion pump and flow connections to a flexible bladder applied to a tissue treatment area on the skin. Temperature alone is the obvious delivery medium for thermal therapies, but alterations in blood flow response to changes in tissue temperature play a major role in the mechanisms of cryotherapy efficacy.
The surface application of cold or heat results in a local diminution or increment in blood perfusion, respectively [65]. Lower blood flow will reduce the amount of swelling and bleeding, and lower temperatures reduce pain by lowering the nerve conduction velocity [66]. Higher blood flow brings a greater supply of nutrients to injured tissues and flushes toxic metabolic byproducts from tissue. Achieving a balance between these two effects can be of direct benefit to augmenting a soft tissue healing process. Alternatively, an over-application of either cooling or heating may add to the extent of injury. Excessive heating effects are most obvious, resulting in burns at tissue temperatures exceeding 43 °C [67,68], although limited thermal stress may upregulate the production of heat shock proteins that enable damaged cells to self-repair [69]. At temperatures above 0 °C freezing is avoided, but devastating injuries may still occur owing primarily to prolonged starvation of tissue of blood flow. The term nonfreezing cold injury (NFCI) is applied to this event [70–72]. Low temperature exposure may also contribute to tissue injury because of the inability of hemoglobin to release (dissociate) oxygen at temperatures below about 12 °C [73] and the increasing rigidity of red blood cells in the range of 10–15 °C that may cause them to block the flow of blood through capillaries [74].
Given the direct dependence of the efficacy of thermal therapy on the modulation of local blood flow, we have conducted extensive human trials to quantify the domains of coupling and uncoupling of perfusion to temperature [75–77]. A wide range of CTUs have been tested with thermocouples and laser Doppler blood flow probes placed on the skin surface under the ice water perfusion bladder and at other control locations. The laser Doppler probes were a low power version that interrogates flow at an average depth of 0.5 mm and a higher power version at 2 mm [78]. Temperatures and blood flows were measured during cryotherapy protocols that included sequential cooling and warming processes. An exemplar protocol is shown in Fig. 9.
The cryotherapy protocol consists of sequential periods of active cooling by circulating ice water followed by passive warming by parasitic heat gain from underlying tissue and the room environment. The temporal gradients in temperature are greatest at the start and cessation of active cooling owing to the sudden changes in the thermal boundary conditions. In contrast, the blood perfusion behaves very differently at the start and cessation of active cooling. During cooling the perfusion gradually drops to about 20% of the baseline value. During subsequent warming, while the temperature is rising steadily, the perfusion remains profoundly depressed for an hour and a half, as which point the experiment was terminated. In the absence of external factors to restimulate the blood flow, this decoupling of perfusion from temperature during warming is typical of a cryotherapy protocol. The phenomenon is thought to be the consequence of the release of mediators that govern the vasoactive behavior of tissue, and experiments are under way in our group to identify these agents using microdialysis techniques. These results will be reported independently.
The decoupling of perfusion and temperature during tissue warming following cryotherapy cooling results in a hysteresis effect during the complete thermal cycle. The measured blood perfusion to the skin may be plotted as a function of the applied surface temperature, as shown in Fig. 11.
Fig. 11.
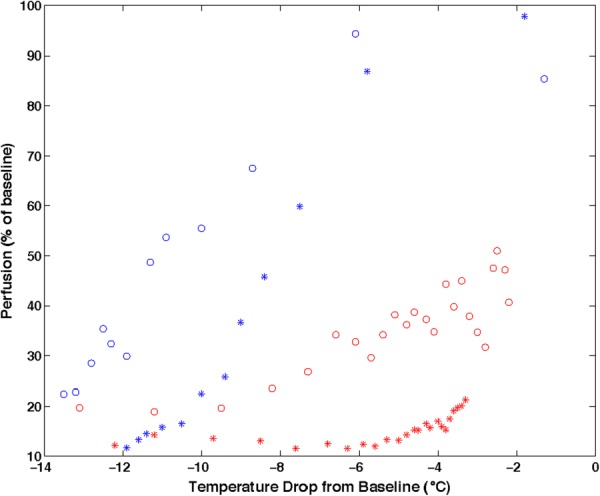
Hysteresis in local skin blood flow responding to falling and rising skin temperatures during cryotherapy for trials with two different devices and subjects (open circle and star symbols). Left data points are for cooling and right for warming. Owing to experimental time limitations, the cryotherapy cooling and heating cycles did not accommodate a complete return of perfusion to its baseline value during rewarming [76].
This hysteresis phenomenon has been observed associated with sequential cooling and heating during cryotherapy for many protocol domains. It occurs for both slow and rapid cooling and warming, i.e., across many time scales [77]. The growing body of scientific and clinical data indicate that the relationship between temperature and local blood perfusion plays an important role in determining the outcome of cryotherapy procedures. Further study and analysis of this effect are warranted.
The foregoing data points to the fact that the applied temperature may be modulated to regulate the flow of blood to injured tissue with the objective of enhancing the healing process. Periods of reduced flow and temperature may be beneficial to healing as well as periods of exposure to higher temperatures and blood flow. The combination of alternating cooling and heating has an extended history in sports medicine, being termed contrast therapy [65,79,80]. Surface cooling and warming have been practiced with packs applied manually in alternating fashion with time ratios varying over the range of 1:1–1:4. Cooling may last for only 5 min or be extended to 30 min. For sports applications, the therapeutic target is often deep muscle tissue. Thermocouples inserted to depths of 2.5–4 cm at a contrast therapy site indicate that the foregoing types of protocol have little effect on altering muscle temperatures [79,80], as is anticipated by the limitations of heat conduction in tissue.
We have adapted various CTU systems, as well as onsite thermoelectric solid state refrigeration [81], to apply alternating cooling and heat boundary conditions to skin and have measured the blood perfusion response in the underlying tissue. Figure 12 presents surface temperature and blood perfusion data from one such trial.
Fig. 12.
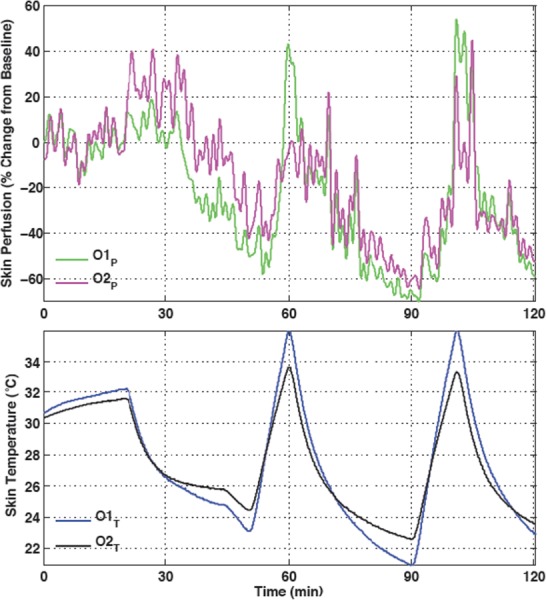
Temperature and blood perfusion histories for cyclic cooling and heating applied to the knee with a circulating ice water CTU and electric heater attached to the surface of the bladder. Following an initial period of baseline data collection, the protocol consisted of cycles of 30 min of active cooling and 10 min of active heating. The data show blood perfusions and temperatures at two locations on the skin beneath the bladder.
The blood flow is clearly responsive to both active cooling and heating, in contrast to the data in Fig. 10 for which the perfusion strongly lags the temperature during passive rewarming. Hysteresis analysis of cooling and warming cycles has demonstrated that there is always a differential between the cooling and warming behaviors of blood perfusion and surface temperature. But a hysteresis plot presents only property values and has no information about the time progression of a process. In contrast, the application of a thermal therapy clinically elicits a physiological response involving multiple rate processes that govern healing and, potentially, further injury events. Therefore, the time history of how temperature and perfusion progress during any specific treatment is of specific interest. The data in Fig. 12 illustrate graphically that manipulation of the skin surface temperature boundary condition can be used to drive blood perfusion downward and upward on demand. This capability has the potential to be highly useful and efficacious therapeutically. We are actively engaged in developing and testing devices and protocols to advance the state-of-the-art in this area.
Fig. 10.
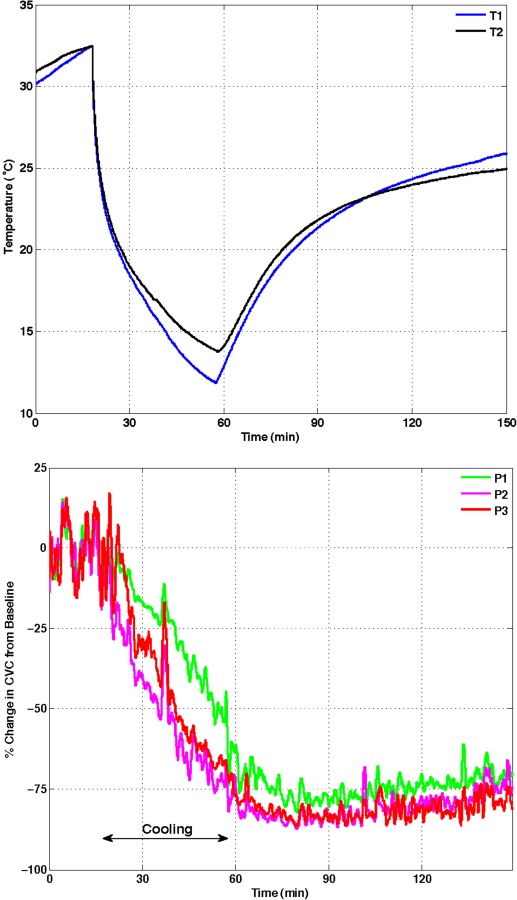
Data for a cryotherapy trial with a circulating ice water CTU applied to the shin area of the lower leg. The protocol consisted of 15 min of baseline data followed by active cooling for 40 min and passive rewarming for 90 min. The upper panel shows temperature histories measured at two locations on the skin under the water perfusion bladder. The lower panel shows two superficial (lower and upper) and one deep (middle) perfusion histories under the cooling bladder [76].
All of the foregoing temperature data have been obtained for measurements performed on the skin surface, which are limited to the monitoring of boundary conditions. The penetration of cold or heat waves into deeper tissue is an important component of a therapeutic protocol that dictates the progression of a healing response. Thus, in some laboratories, thermocouples may be inserted into tissue to depths of many centimeters; for example, see Refs. [79,80]. Such experiments require a higher level of commitment on the part of subjects, and we have chosen to exclude this type of instrumentation in our studies. Rather, we have applied rather simple simulation tools to estimate the temperature histories at interior locations in tissues. Owing to anatomical heterogeneity and complexity plus complicating factors such as internal distributed convection by blood perfusion, most of these models require a numerical solution method and are generally implemented via finite element analysis. In contrast, a very modest example that is nonetheless instructive is shown in Fig. 13, which depicts the internal temperature fields during cyclic surface cooling and warming. In this case, the tissue is assumed to have homogeneous properties with no blood perfusion, and the surface is subjected to step-wise temperature changes.
Fig. 13.
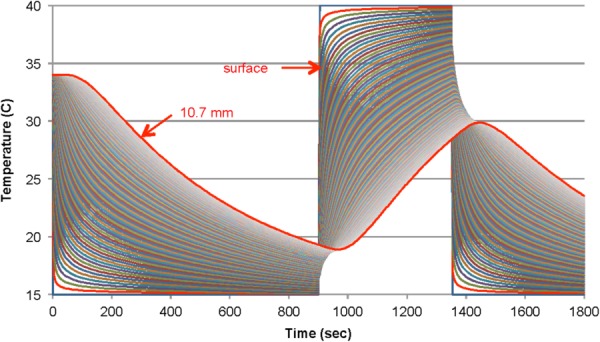
Finite difference simulation of heat penetration away from and into tissue during cyclic cooling and heating of the skin surface. Temperature histories are plotted at depth increments of 0.1 mm from the surface to 10.7 mm (outlined in red). The initial temperature is 34 °C followed by stepwise changes in the surface temperature between 15 °C and 40 °C.
Penetration of thermal effects into the tissue induced by alterations in surface temperature are obvious, along with muting of the magnitude of changing boundary conditions and a response delay that increases with depth. Simulations are useful for understanding the outcomes from specific experiments and for designing the thermal and temporal parameters of new protocols. For example, the delay in the response of blood perfusion following a change in surface temperature can be explained, at least in part, by the time constant for diffusion of heat to a depth of approximately 2 mm that can be on the order of a couple of minutes.
Finally, it is noted that frequently therapeutic CTU use is recommended or prescribed in terms of an application time and temperature. However, to assign a single numerical temperature as characterizing the thermal therapy process produced by a CTU applied to a treatment area is a misnomer and is, in the least, misleading to health care providers and patients. In actuality, depending on the spatial and temporal patterns of ice water circulation through a cooling bladder, there may be very large lateral gradients in temperature applied to the skin. Infrared thermography has been applied to measure and characterize the magnitude and distribution of cooling bladder temperature gradients for many commercial CTUs [82]. There are substantial differences in the magnitude, range, and distribution of temperatures over a bladder surface among CTU/bladder combinations. The range may be as small as 2 °C or exceed 9 °C. This phenomenon is compounded by the complex temporal coupling between locally applied temperature and the alterations to blood flow elicited in underlying tissues that are critical in determining the physiological response to cryotherapy. The use of onsite solid state refrigeration modules provides promise for more precise temperature control as well as greater protocol versatility and predictability [81].
Summary and Conclusions
Applications of heat transfer technology and analysis in biomedicine is growing at a rapid pace, presenting many opportunities for scientific advances and commercialization initiatives. This paper discusses a limited subset of bioheat transfer problems that have been most prominent in the author's recent experience and are presented as examples of topics that may be pursued. Undoubtedly, the future holds numerous avenues of study and development having high medical impact that are yet to be identified.
Acknowledgment
This research was sponsored by National Science Foundation Grants CBET 0828131, CBET 0966998, and CBET 12,50659 and National Institutes of Health Grant R01 EB015522 to the University of Texas at Austin, and the Robert and Prudie Leibrock Professorship in Engineering at the University of Texas at Austin.
Conflict of Interest Statement
Patent applications have been submitted by Dr. Diller and colleagues at the University of Texas at Austin that cover IP discussed in this paper. These patent applications variously have issued or are under review. Ownership rights to these patents reside with The University of Texas System. Some patents are licensed to companies in which Dr. Diller holds an equity interest. He has not received any financial compensation from any of these companies. Neither have any of the companies provided any direct financial support for this research. Dr. Diller has served as an expert witness for both plaintiff and defendant counsel since 2000 in numerous legal cases regarding the safety and design of existing cryotherapy devices.
The terms of these conflict of interest arrangements and their management have been reviewed and approved by the University of Texas at Austin in accordance with its policy on objectivity in research.
Glossary
Nomenclature
- AVA =
arteriovenous anastomoses
- BMR =
basal metabolic rate
- CIVC =
cold-induced vasoconstriction
- CO =
cardiac output
- CTU =
cryotherapy unit
- CVC =
cutaneous vascular conductance
- GSBF =
glabrous skin blood flow
- GSHT =
glabrous skin heat transfer
- IRB =
institutional review board
- NFCI =
nonfreezing cold injury
- POAH =
pre-optic anterior hypothalamus
- STS =
selective thermal stimulation
- TH =
therapeutic hypothermia
- TRI =
thermoregulatory inertia
Max Jakob Award paper.
References
- [1]. Hensel, H. , 1973, “Neural Processes in Thermoregulation,” Physiol. Rev., 53(4), pp. 948–1017. [DOI] [PubMed] [Google Scholar]
- [2]. Hensel, H. , 1981, Thermoreception and Temperature Regulation, Academic Press, London. [PubMed] [Google Scholar]
- [3]. Jessen, C. , 2001, Temperature Regulation in Humans and Other Mammals, Springer, Berlin: 10.1007/978-3-642-59461-8 [Google Scholar]
- [4]. Cisneros, A. B. , and Goins, B. L. , eds., 2009, Body Temperature Regulation, Nova Science Publ., New York. [Google Scholar]
- [5]. Romanovsky, A. A. , 2007, “Functional Architecture of the Thermoregulatory System,” Am. J. Physiol., 292(1), pp. R37–R46.10.1152/ajpregu.00668.2006 [DOI] [PubMed] [Google Scholar]
- [6]. Hammel, H. T. , Hardy, J. D. , and Fusco, M. M. , 1960, “Thermoregulatory Responses to Hypothalamic Cooling in Unanesthetized Dogs,” Am. J. Physiol., 198, pp. 481–486. [DOI] [PubMed] [Google Scholar]
- [7]. Hammel, H. T. , 1968, “Regulation of Internal Body Temperature,” Ann. Rev. Physiol., 30, pp. 641–710.10.1146/annurev.ph.30.030168.003233 [DOI] [PubMed] [Google Scholar]
- [8]. Nagashima, K. , 2006, “Central Mechanisms for Thermoregulation in a Hot Environment,” Ind. Health, 44(3), pp. 359–367.10.2486/indhealth.44.359 [DOI] [PubMed] [Google Scholar]
- [9]. Charkoudian, N. , 2003, “Skin Blood Flow in Adult Human Thermoregulation: How It Works, When It Does Not, and Why,” Mayo Clin. Proc. 78(5), pp. 603–612.10.4065/78.5.603 [DOI] [PubMed] [Google Scholar]
- [10]. Gonzalez-Alonso, J. , 2012, “Human Thermoregulation and the Cardiovascular System,” Exp. Physiol., 97(3), pp. 340–346.10.1113/expphysiol.2011.058701 [DOI] [PubMed] [Google Scholar]
- [11]. Jessen, C. , and Mayer, E. Th. , 1971, “Spinal Cord and Hypothalamus as Core Sensors of Temperature in the Conscious Dog. I. Equivalence of Responses,” Pflugers Arch., 324(3), pp. 189–204.10.1007/BF00586418 [DOI] [PubMed] [Google Scholar]
- [12]. Jessen, C. , and Ludwig, O. , 1971, “Spinal Cord and Hypothalamus as Core Sensors of Temperature in the Conscious Dog. II. Addition of Signals,” Pflugers Arch., 324(3), pp. 205–216.10.1007/BF00586419 [DOI] [PubMed] [Google Scholar]
- [13]. Jessen, C. , and Simon, E. , 1971, “Spinal Cord and Hypothalamus as Core Sensors of Temperature in the Conscious Dog. III. Identity of Functions,” Pflugers Arch., 324(3), pp. 217–226.10.1007/BF00586420 [DOI] [PubMed] [Google Scholar]
- [14]. Hales, J. R. S. , Fawcett, A. A. , Bennett, J. W. , and Needham, A. D. , 1978, “Thermal Control of Blood Flow Through Capillaries and Arteriovenous Anastomoses in Skin of Sheep,” Pflugers Arch., 378(1), pp. 55–63.10.1007/BF00581958 [DOI] [PubMed] [Google Scholar]
- [15]. Hales, J. R. S. , and Iriki, M. , 1975, “Integrated Changes in Regional Circulatory Activity Evoked by Spinal Cord and Peripheral Thermoreceptor Stimulation,” Brain Res., 87(2–3), pp. 267–275.10.1016/0006-8993(75)90424-2 [DOI] [PubMed] [Google Scholar]
- [16]. Jessen, C. , Felde, D. , Volk, P. , and Kuhnen, G. , 1990, “Effects of Spinal Cord Temperature on the Generation and Transmission of Temperature Signals in the Goat,” Pflugers Arch., 416(4), pp. 428–433.10.1007/BF00370750 [DOI] [PubMed] [Google Scholar]
- [17]. Jessen, C. , McLean, J. A. , Calvert, D. T. , and Findlay, J. D. , 1972, “Balanced and Unbalanced Temperature Signals Generated in Spinal Cord of the Ox,” Am. J. Physiol., 222(6), pp. 1343–1347. [DOI] [PubMed] [Google Scholar]
- [18]. Lewis, T. , and Pickering, G. W. , 1931, “Vasodilation in the Limbs in Response to Warming the Body; With Evidence for Sympathetic Vasodilator Nerves in Man,” Heart, 15, pp. 33–51. [Google Scholar]
- [19]. Gibbon, J. H., Jr. , and Landis, E. M. , 1932, “Vasodilation in the Lower Extremities in Response to Immersing the Forearms in Warm Water,” J. Clin. Invest., 11(5), pp. 1019–1036.10.1172/JCI100456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Freeman, N. E. , 1935, “The Effect of Temperature on the Rate of Blood Flow in the Normal and in the Sympathectomized Hand,” Am. J. Physiol., 113, pp. 384–398. [Google Scholar]
- [21]. Bader, M. E. , and Macht, M. B. , 1948, “Indirect Peripheral Vasodilation Produced by the Warming of Various Body Areas,” J. Appl. Physiol., 1(3), pp. 215–226. [DOI] [PubMed] [Google Scholar]
- [22]. Mackowiak, P. , Wasserman, S. , and Levine, M. , 1992, “A Critical Appraisal of 98.6°F, the Upper Limit of the Normal Body Temperature, and Other Legacies of Carl Reinhold August Wunderlich,” J. Am. Med. Assoc., 268(12), pp. 1578–1580.10.1001/jama.1992.03490120092034 [PubMed] [Google Scholar]
- [23]. Krauchi, K. , Knoblauch, V. , Wirz-Justice, A. , and Cajochen, D. , 2006, “Challenging the Sleep Homeostat Does Not Influence the Thermoregulatory System in Men: Evidence From a Nap vs. Sleep-Deprivation Study,” Am. J. Physiol., 290(4), pp. R1052–R1061.10.1152/ajpregu/00381.2005 [DOI] [PubMed] [Google Scholar]
- [24]. Weinert, D. , and Waterhouse, J. , 2007, “The Circadian Rhythm of Core Temperature: Effects of Physical Activity and Aging,” Physiol. Behav., 90(2–3), pp. 246–256.10.1016/j.physbeh.2006.09.003 [DOI] [PubMed] [Google Scholar]
- [25]. Reinberg, A. , and Smolensky, M. , 1990, “Chronobiology and Thermoregulation,” Thermoregulation: Physiology and Biochemistry, Schonbaum E., and Lomax P., eds., Pergamon Press, New York, pp. 61–100. [Google Scholar]
- [26]. Rowell, L. B. , 1986, Human Circulation Regulation During Physical Stress, Oxford, New York. [Google Scholar]
- [27]. Werner, J. , Mekjavic, I. B. , and Taylor, N. A. S. , 2008, “Concepts in Physiological Regulation: A Thermoregulatory Perspective,” Physiological Bases of Human Performance During Work and Exercise, Taylor N. A. S., and Groeller H., ed., Churchill Livingstone Elsevier, Amsterdam, pp. 359–377. [Google Scholar]
- [28]. Grahn, D. , and Heller, H. C. , 2004, “The Physiology of Mammalian Temperature Homeostasis,” ITACCS Critical Care Monograph, pp. 1–21.
- [29]. Wilson, T. E. , Zhange, G. , Levine, B. D. , and Crandall, C. G. , 2005, “Dynamic Autoregulation of Cutaneous Circulation: Differential Control in Glabrous Versus Nonglabrous Skin,” Am. J. Physiol. Heart Circ. Physiol., 289(1), pp. H385–H391.10.1152/ajpheart.00622.2004 [DOI] [PubMed] [Google Scholar]
- [30]. Caldwell, J. N. , Matsuda-Nakamura, M. , and Taylor, N. A. S. , 2014, “Three-Dimensional Interaction of Mean Body and Local Skin Temperatures in the Control of Hand and Foot Blood Flows,” Eur. J. Appl. Physiol., 114(8), pp. 1679–1689.10.1007/s00421-014-2894-x [DOI] [PubMed] [Google Scholar]
- [31]. Grahn, D. , Brock-Utne, J. G. , Watenpaugh, D. E. , and Heller, H. C. , 1998, “Recovery From Mild Hypothermia Can Be Accelerated by Mechanically Distending Blood Vessels in the Hand,” J. Appl. Physiol., 85(5), pp. 1643–1648. [DOI] [PubMed] [Google Scholar]
- [32]. Grahn, D. A. , Murray, J. vLS. , and Heller, H. C. , 2008, “Cooling Via One Hand Improves Physical Performance in Heat-Sensitive Individuals With Multiple Slerosis: A Preliminary Study,” BMC Neurol., 8(14), pp. 1–8.10.1186/1471-2377-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Grahn, D. A. , Dillon, J. L. , and Heller, H. C. , 2009, “Heat Loss Through the Glabrous Skin Surfaces of Heavily Insulated, Heat-Stressed Individuals,” ASME J. Biomech. Eng., 131(7), p. 071005.10.1115/1.3156812 [DOI] [PubMed] [Google Scholar]
- [34]. Heller, H. C. , and Grahn, D. A. , 2012, “Enhancing Thermal Exchange in Humans and Practical Applications,” Disruptive Sci. Technol., 1(1), pp. 11–19.10.1089/dst.2012.0004 [Google Scholar]
- [35]. Clark, E. R. , 1938, “Arterio-Venous Anastomoses,” Physiol. Rev. 18, pp. 229–247. [Google Scholar]
- [36]. Hales, J. R. S. , and Molyneux, G. S. , 1988, “Control of Cutaneous Arteriovenous Anastomoses,” Vasodilatation: Vascular Smooth Muscle, Peptides, Autonomic Nerves, and Endothelium, Vahoutte P. M., ed., Raven Press, New York, pp. 321–332. [Google Scholar]
- [37]. Nyberg, K. L. , Diller, K. R. , and Wissler, E. H. , 2000, “Automatic Control of Thermal Neutrality for Space Suit Applications Using a Liquid Cooling Garment,” Aviat. Space Environ. Med., 71(9), pp. 904–915. [PubMed] [Google Scholar]
- [38]. Lugo-Amador, N. M. , Rothenhaus, T. , and Moyer, P. , 2004, “Heat-Related Illness,” Emerg. Med. Clin. North Am., 22(2), pp. 315–327.10.1016/j.emc.2004.01.004 [DOI] [PubMed] [Google Scholar]
- [39]. Danzl, D. F. , and Pozos, R. S. , 1994, “Accidental Hypothermia,” N. Engl. J. Med., 331(26), pp. 1756–1760.10.1056/NEJM199412293312607 [DOI] [PubMed] [Google Scholar]
- [40]. Casa, D. J. , McDermott, B. M. , Lee, E. C. , Yeargin, S. W. , Armstrong, L. E. , and Maresh, D. M. , 2007, “Cold-Water Immersion: The Gold Standard for Exertional Heat Stroke Treatment,” Exercise Sports Sci. Rev., 35(3), pp. 146–150.10.1097/jes.0b013e3180a02bec [DOI] [PubMed] [Google Scholar]
- [41]. Johnson, J. M. , and Kellogg, D. L. , 2010, “Local Thermal Control of the Human Cutaneous Circulation,” J. Appl. Physiol., 109(40), pp. 1229–1238.10.1152/japplphysiol.00407.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Taylor, N. A. S. , Caldwell, J. N. , Van Den Heuvel, A. M. J. , and Patterson, M. J. , 2008, “To Cool, but Not Too Cool: That Is the Question—Immersion Cooling for Hyperthermia,” Med. Sci. Sports Exercise, 40(11), pp. 1962–1969.10.1249/MSS.0b013e31817eee9d [DOI] [PubMed] [Google Scholar]
- [43]. Mayer, S. A. , and Sessler, D. I. , eds., 2005, Therapeutic Hypothermia, Taylor & Francis, Boca Raton, FL. [Google Scholar]
- [44]. Tisherman, S. A. , and Sterz, F. , eds., 2005, Therapeutic Hypothermia, Springer, New York: 10.1007/b107316 [Google Scholar]
- [45]. Lundbye, J. B. , 2012, Therapeutic Hypothermia After Cardiac Arrest: Clinical Application and Management, Springer, New York: 10.1007/978-1-4471-2951-6 [Google Scholar]
- [46]. Ly, H. Q. , Denault, A. , Dupuis, J. , Vadeboncoeur, A. , Harel, F. , Arsenault, A. , Gibson, C. M. , and Bonan, R. , 2005, “A Pilot Study: The Noninvasive Surface Cooling Thermoregulatory System for Mild Hypothermia Induction in Acute Myocardial Infarction (The NICAMI Study),” Am. Heart J., 150(5), pp. e9–e13.10.1016/j.ahj.2005.02.049 [DOI] [PubMed] [Google Scholar]
- [47]. Sessler, D. I. , 2009, “Defeating Normal Thermoregulatory Defenses: Induction of Therapeutic Hypothermia,” Stroke, 40(11), pp. e614–e621.10.1161/STROKEAHA.108.520858 [DOI] [PubMed] [Google Scholar]
- [48]. Zweifler, R. M. , Voorhees, M. E. , Mahmood, M. A. , and Parnell, M. , 2004, “Magnesium Sulfate Increases the Rate of Hypothermia Via Surface Cooling and Improves Comfort,” Stroke, 35(10), pp. 2331–2334.10.1161/01.STR.0000141161.63181.f1 [DOI] [PubMed] [Google Scholar]
- [49]. Bergersen, T. K. , Eriksen, M. , and Walloe, L. , 1997, “Local Constriction of Arteriovenous Anastomoses in the Cooled Finger,” Am. J. Physiol., 273(3 Pt 2), pp. R880–R886. [DOI] [PubMed] [Google Scholar]
- [50]. Hensley, D. W. , Hemmen, L. , Diller, T. T. , Mark, A. E. , Rodriguez, J. C. , Cowan, K. R. , Khoshnevis, S. , and Diller, K. R. , “Upregulation of Glabrous Skin Blood Flow by Thermal Stimulation Along the Cervical Spine,” (in preparation).
- [51]. Sessler, D. I. , Olofsson, C. I. , Rubinstein, E. H. , and Beebe, J. J. , 1988, “The Thermoregulatory Threshold in Humans During Halothane Anesthesia,” Anesthesiology, 68(6), pp. 836–842.10.1097/00000542-198806000-00002 [DOI] [PubMed] [Google Scholar]
- [52]. Sessler, D. I. , 2013, “The Thermoregulation Story,” Anesthesiology, 118(1), pp. 181–186.10.1097/ALN.0b013e3182784df3 [DOI] [PubMed] [Google Scholar]
- [53]. Sun, Z. , Honar, H. , Sessler, D. I. , Dalton, J. E. , Yang, D. , Panjasawatwong, K. , Deroee, A. F. , Salmasi, V. , Saager, L. , and Kurz, A. , 2015, “Intraoperative Core Temperature Patterns, Transfusion Requirement, and Hospital Duration in Patients Warmed With Forced Air,” Anesthesiology, 122(2), pp. 276–285.10.1097/ALN.0000000000000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Wissler, E. H. , 1961, “Steady-State Temperature Distribution in Man,” J. Appl. Physiol., 16(4), pp. 734–740. [DOI] [PubMed] [Google Scholar]
- [55]. Wissler, E. H. , 2012, “Whole-Body Human Thermal Modeling, an Alternative to Immersion in Cold Water and Other Unpleasant Endeavors,” ASME J. Heat Transfer, 134(3), p. 031019.10.1115/1.4005155 [Google Scholar]
- [56]. Nyberg, K. L. , Diller, K. R. , and Wissler, E. H. , 2001, “Model of Human/Liquid Cooling Garment Interaction for Space Suit Automatic Thermal Control,” ASME J. Biomech. Eng., 123(1), pp. 114–120.10.1115/1.1336147 [DOI] [PubMed] [Google Scholar]
- [57]. Hensley, D. W. , Mark, A. E. , Abella, J. R. , Netscher, G. M. , Wissler, E. H. , and Diller, K. R. , 2013, “50 Years of Computer Simulation of the Human Thermoregulatory System,” ASME J. Biomech. Eng., 135(2), p. 021005.10.1115/1.4023383 [DOI] [PubMed] [Google Scholar]
- [58]. Grahn, D. A. , Cao, V. H. , and Heller, H. C. , 2005, “Heat Extraction Through the Palm of One Hand Improves Aerobic Exercise Endurance in a Hot Environment,” J. Appl. Physiol., 99(3), pp. 972–978.10.1152/japplphysiol.00093.2005 [DOI] [PubMed] [Google Scholar]
- [59]. Taylor, N. A. S. , Machado-Moreira, C. , van den Heuvel, A. , Caldwell, J. , Taylor, W. A. , and Tipton, M. J. , 2009, “The Roles of Hands and Feet in Temperature Regulation in Hot and Cold Environments,” 13th International Conference on Environmental Ergonomics, Boston, pp. 405–409. [Google Scholar]
- [60]. Netscher, G. M. , Khoshnevis, S. , Hemmen, L. , Wissler, E. H. , and Diller, K. R. , “Computer Simulation of Thermoregulatory Alterations Caused by Anesthesia: Therapeutic Device Efficacy Analysis,” (in preparation).
- [61]. Andrzejowski, J. , Hoyle, J. , Eapen, G. , and Turnbull, D. , 2008, “Effect of Prewarming on Post-Induction Core Temperature and the Incidence of Inadvertent Perioperative Hypothermia in Patients Undergoing General Anesthesia,” Br. J. Anaesth., 101(5), pp. 627–631.10.1093/bja/aen272 [DOI] [PubMed] [Google Scholar]
- [62]. Hart, S. R. , Bordes, B. , Hart, J. , Corsino, D. , and Harmon, D. , 2011, “Unintended Perioperative Hypothermia,” Ochsner J., 11(3), pp. 259–270. [PMC free article] [PubMed] [Google Scholar]
- [63]. Konrath, G. A. , Lock, T. , Goltz, H. T. , and Scheidler, J. , 1996, “The Use of Cold Therapy After Anterior Cruciate Ligament Reconstruction: A Prospective, Randomized Study and Literature Review,” Am. J. Sports Med., 24(5), pp. 629–633.10.1177/036354659602400511 [DOI] [PubMed] [Google Scholar]
- [64]. Swenson, C. , Sward, L. , and Karlsson, J. , 1996, “Cryotherapy in Sports Medicine,” Scand. J. Med. Sci. Sports, 6(4), pp. 193–200. [DOI] [PubMed] [Google Scholar]
- [65]. Cochrane, D. J. , 2004, “Alternating Hot and Cold Water Immersion for Athlete Recovery: A Review,” Phys. Ther. Sport, 5(1), pp. 26–32.10.1016/j.ptsp.2003.10.002 [Google Scholar]
- [66]. Algafly, A. A. , and George, K. P. , 2007, “The Effect of Cryotherapy on Nerve Conduction Velocity, Pain Threshold and Pain Tolerance,” Br. J. Sports Med., 41(6), pp. 365–369.10.1136/bjsm.2006.031237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Moritz, A. R. , and Henriques, F. C. , 1947, “Studies of Thermal Iinjury. II. The Relative Importance of Time and Surface Temperature in the Causation of Cutaneous Burns,” Am. J. Pathol., 23(5), pp. 695–720. [PMC free article] [PubMed] [Google Scholar]
- [68]. Lawrence, J. C. , and Bull, J. P. , 1976, “Thermal Conditions Which Cause Skin Burns,” Eng. Med., 5(3), pp. 61–63.10.1243/EMED_JOUR_1976_005_023_02 [Google Scholar]
- [69]. Diller, K. R. , 2006, “Kinetics of Stress Protein Expression,” Ann. Rev. Biomed. Eng., 8, pp. 403–424.10.1146/annurev.bioeng.7.060804.100449 [DOI] [PubMed] [Google Scholar]
- [70]. Whayne, T. F. , and DeBakey, M. E. , 1958, Cold Injury, Ground Type, in World War II, Office of the Surgeon General, Washington, DC. [Google Scholar]
- [71]. Francis, T. J. R. , 1984, “Non Freezing Cold Injury: A Historical Review,” J. R. Nav. Med. Serv., 70(3), pp. 134–139. [PubMed] [Google Scholar]
- [72]. Irwin, M. S. , Sanders, R. , Green, C. J. , and Tereghi, G. , 1997, “Neuropathy in Non-Freezing Cold Injury (Trench Foot),” J. R. Soc. Med., 90(8), pp. 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Anson, J. A. , McCormick, J. , and Zabramski, J. M. , 1992, “Oxygen Dissociation Characteristics of Hemoglobin and Blood Substitute in Relation to Temperature,” Barrow Neurol. Inst. Q., 8(2), pp. 35–42. [Google Scholar]
- [74]. Waugh, R. , and Evans, E. A. , 1979, “Thermoeleasticity of Red Blood Cell Membrane,” Biophys. J., 26(1), pp. 115–132.10.1016/S0006-3495(79)85239-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Khoshnevis, S. , Craik, N. K. , and Diller, K. R. , “Cold-Induced Vasoconstriction May Persist Long After Cooling Ends: An Evaluation of Multiple Cryotherapy Units,” Knee Surg. Sports Traumatol. Arthroscopy (in press).10.1007/s00167-014-2911-y [DOI] [PMC free article] [PubMed]
- [76]. Khoshnevis, S. , Craik, N. K. , and Diller, K. R. , 2014, “Experimental Characterization of the Domains of Coupling and Uncoupling Between Surface Temperature and Skin Blood Flow,” Int. J. Transp. Phenom., 13(4), pp. 277–301. [Google Scholar]
- [77]. Khoshnevis, S. , Craik, N. K. , Brothers, R. M. , and Diller, K. R. , “Cryotherapy-Induced Persistent Vasoconstriction After Cutaneous Cooling: Hysteresis Between Skin Temperature and Blood Perfusion,” (in preparation). [DOI] [PMC free article] [PubMed]
- [78]. Clough, G. , Chipperfield, A. , Syrne, C. , de Mul, F. , and Gush, R. , 2009, “Evaluation of a New High Power, Wide Separation Laser Doppler Probe: Potential Measurement of Deeper Tissue Blood Flow,” Microvasc. Res., 78(2), pp. 155–161.10.1016/j.mvr.2009.05.003 [DOI] [PubMed] [Google Scholar]
- [79]. Higgins, D. , and Kaminski, T. W. , 1998, “Contrast Therapy Does Not Cause Fluctuations Inhuman Gastrocnemius Intramuscular Temperature,” J. Athletic Train., 33(4), pp. 336–340. [PMC free article] [PubMed] [Google Scholar]
- [80]. Myrer, J. W. , Measom, G. , Durrant, E. , and Fellingham, G. W. , 1997, “Cold- and Hot-Pack Contrast Therapy: Subcutaneous and Intramuscular Temperature Change,” J. Athletic Train., 32(3), pp. 238–241. [PMC free article] [PubMed] [Google Scholar]
- [81]. Mejia, N. , Dedlow, K. , Nguy, L. , Sullivan, P. , Khoshnevis, S. , and Diller, K. R. , “An On-Site Thermoelectric Cooling Device for Cryotherapy and Control of Skin Blood Flow,” ASME J. Med. Devices (in press).10.1115/1.4029508 [DOI] [PMC free article] [PubMed]
- [82]. Khoshnevis, S. , Nordhauser, J. E. , Craik, N. K. , and Diller, K. R. , 2014, “Quantitative Evaluation of the Thermal Heterogeneity on the Surface of Cryotherapy Cooling Pads,” ASME J. Biomech. Eng., 136(7), p. 074503.10.1115/1.4027270 [DOI] [PMC free article] [PubMed] [Google Scholar]


