We performed a proof-of-concept study using the Xpert MTB/RIF test for the detection of Mycobacterium tuberculosis from fecal samples. The overall sensitivity was 85.7% (95% CI; 73.8 – 93.6%) and the specificity was 100% (95% CI; 86.2–100).
Keywords: feces, Mycobacterium tuberculosis, Xpert MTB/RIF
Abstract
Background. The Xpert Mycobacterium tuberculosis (MTB)/rifampicin (RIF) is a fully automated diagnostic test that allows for the detection of MTB including its RIF resistance. Although the test is used for the diagnosis of tuberculosis (TB) in sputum samples worldwide, studies using fecal specimens are scarce. We therefore evaluated the efficacy of the Xpert MTB/RIF test for detection of MTB in fecal specimens obtained from adult pulmonary TB patients, confirmed by culture and/or molecular diagnostic methods.
Methods. We conducted a retrospective case-control study to provide proof-of-concept regarding the efficacy of the Xpert MTB/RIF test using fecal samples for diagnosing pulmonary TB via detection of MTB in adult patients (≥20 years) at the Fukujuji Hospital in Tokyo, Japan.
Results. Fecal specimens were obtained from 56 active pulmonary TB patients (including 48 sputum smear-positive and 8 sputum smear-negative patients), 10 non-TB patients (including 4 Myocobacterium avium complex infections), and 27 healthy individuals who were exposed to active pulmonary TB patients. The sensitivity of the fecal Xpert MTB/RIF was 100% (81.7%–100%) for detection of MTB in specimens from sputum smear-positive (1+ to 3+) patients, 81.0% (58.1%–94.6%) in specimens from sputum smear scanty positive patients, and 50.0% (15.7%–84.3%) in specimens from sputum smear-negative patients. Meanwhile, each of the fecal specimens from the non-TB group was negative for MTB (specificity 100%; 95% confidence interval, 86.2–100).
Conclusions. The fecal Xpert MTB/RIF test could detect MTB in a large proportion of smear-positive pulmonary TB patients, without frequent false-positive results at a TB referral hospital in Japan.
The Xpert Mycobacterium tuberculosis (MTB)/rifampicin (RIF) (Cepheid, Sunnyvale, CA) is a fully automated diagnostic test that allows for the detection of MTB and its RIF resistance, using real-time polymerase chain reaction (PCR) [1–5]. Indeed, Boehme et al [1] reported that the Xpert MTB/RIF test using sputum samples correctly identified bacterial infection in 98.2% of sputum smear-positive and 72.5% of sputum smear-negative samples obtained from culture-positive tuberculosis (TB) patients. Furthermore, this group found that the Xpert MTB/RIF accurately identified 97.6% of the RIF-resistant MTB and 98.1% of the RIF-susceptible MTB [1].
A total of 20 495 patients (16.1 per 100 000 of the general population) were diagnosed with some form of TB in Japan in 2013. Of these, 57.4% were ≥70 years of age [6]. Indeed, the advanced age of the TB patient population is a critical issue affecting TB control in Japan. In particular, obtaining sputum samples from certain older individuals that are unable to expectorate effectively is not an easy task. In these cases, a gastric aspirate (GA) is collected for laboratory examination; however, this is an invasive procedure associated with a lower level of sensitivity than the analysis of sputum samples [7]. Although analyses of fecal specimens may assist in the bacteriological diagnosis of TB, few studies have examined the sensitivity or specificity of the Xpert MTB/RIF test when using fecal samples [8–10]. Nicol et al [8] reported that the fecal Xpert/RIF test detected MTB in only 47% (8 of 17) of the samples from children with culture-confirmed pulmonary TB. Meanwhile, Walters et al [9] reported that Xpert analysis of feces and GA accurately detected MTB in 75% (3 of 4) of children exhibiting intrathoracic TB and positive GA cultures.
In this study, we evaluated the efficacy of the Xpert MTB/RIF test for the detection of MTB in fecal specimens obtained from adult pulmonary TB patients confirmed by culture or molecular diagnostic methods. In addition, the results of these analyses were compared with those obtained by sputum smear, fecal smear, and culturing methods. The goal of this study was to provide proof-of-concept regarding the potential use of the Xpert MTB/RIF test for the detection of MTB from fecal samples as a possible method for TB detection in incommunicable patients or in patients of advanced age.
METHODS
This retrospective case-control study of bacteriologically confirmed active pulmonary TB and other definite non-TB patients/people was conducted at the Fukujuji Hospital, a referral hospital in Tokyo, Japan that specializes in TB and other respiratory diseases. Patients referred to our hospital with suspected TB disease or infection, based on clinical history and symptoms, are routinely screened by sputum smear, culture, molecular diagnostic, and chest radiograph analyses. Diagnosis of pulmonary TB or non-TB disease is then made by pulmonologists based on the resulting clinical and laboratory data. In this study, fecal specimens were collected (convenience sampling) from eligible adult patients (≥20 years of age) who were diagnosed with either active pulmonary TB, definite non-TB diseases, or healthy TB contacts that were admitted to the hospital or examined in the outpatient department between September 2013 and July 2014. Thus, sputum samples were examined prior to the collection of fecal specimens. The enrollment criteria for active pulmonary TB cases, non-TB cases, and healthy TB contacts were as follows: active pulmonary TB cases were bacteriologically confirmed by sputum culture and/or molecular diagnostics, and the patients were treated with anti-TB drugs for no more than 7 days; non-TB cases were classified based on the obtained negative results for sputum smear, culture (at least 3 times), chest radiograph analyses, and computed tomography (CT), which were read by pulmonologists; the healthy TB contacts, who were referred to the hospital for TB contact screening, displayed no clinical symptoms and a normal chest radiograph (read by pulmonologists), and tested negative for TB by the QuantiFERON-TB Gold In-Tube (Cellestis; QIAGEN, Venlo, Netherlands) test. Patients whose diagnoses were not clearly mentioned in the medical record as well as those lacking appropriate rationale for their diagnoses were excluded from this study. It is noteworthy that the Xpert MTB/RIF analyses were performed independently from the other tests. As a result, the researchers that performed the Xpert MTB/RIF tests and analyzed the results were blinded to specific patient information, including clinical diagnoses and the results of other microbiological tests. The study protocol was approved by the Ethics Committees of the Fukujuji Hospital, and informed consent was obtained from all patients.
Laboratory Protocols
Sputum analyses, including smear microscopy, culturing on 2% Ogawa medium (Kyokuto, Tokyo, Japan) and in mycobacterial growth indicator tubes ([MGIT] Becton Dickinson and Company, Franklin Lakes, NJ), and transcription-reverse transcription concerted reactions (TRC) (TRCRapid M.TB; Tosoh Bioscience, Tokyo, Japan), were performed on samples from all patients, except for those in the healthy TB contact group, upon admission to the hospital. Sputum smears and culture were routinely performed at least 3 times. After confirmation of MTB growth, organisms were subjected to drug susceptibility testing using the Welpack S system (Japan BCG Laboratory, Tokyo, Japan).
One fecal specimen was collected from each enrolled patients after confirmation of his/her diagnosis. Approximately 10 mL distilled water was added to 2 cm3 of the fecal specimen and homogenized by vortex mixing. This mixture was incubated for 15 minutes at room temperature to allow the particulate matter to settle. The supernatants were then collected and centrifuged at 3000 × g for 20 minutes. The resulting sediments were decontaminated by incubating with 10 mL of 3% NALC-NaOH solution for 15 minutes at room temperature, and the samples were then mixed with 40 mL of phosphate buffer ([PB] pH 6.8) and centrifuged at 3000 × g for 20 minutes. The sediments were then resuspended in 3 mL PB and used (0.1 mL of each sample) to inoculate 2% Ogawa medium and MGITs and for the preparation of smears. The remainder of each suspension was stored at −80°C for 1 to 3 months until the Xpert MTB/RIF tests were performed.
All sputum smears, sputum cultures, fecal smears, and fecal cultures, as well as the pretreatment of fecal samples for Xpert MTB/RIF tests were conducted at the Fukujuji Hospital laboratory. Meanwhile, the Xpert MTB/RIF tests were conducted at the laboratory of the Research Institute of Tuberculosis, according to the manufacturer protocols for analysis of sputum samples (Cepheid). In brief, 1 mL of each specimen was mixed with 2 mL of the sample reagent and incubated for 15 minutes at room temperature. Mixtures were then transferred to an Xpert MTB/RIF cartridge and analyzed using a GeneXpert (Cepheid) system. In the case of invalid results, the same treated specimens were reanalyzed by the MTB/RIF test; the final reports were then drawn up based on these results. Acid-fast bacilli were quantified by smear microscopy, according to the standards prescribed by the World Health Organization.
Statistical Analysis
Sensitivity and specificity were calculated up to a confidence interval of 95% (95% CI), using positive sputum culture or molecular diagnostics as the reference standards for TB-positive cases, whereas non-TB cases and healthy TB contacts were used as reference standards for TB-negative cases. The mean shortest cycle threshold (Ct) values for samples with different sputum smear grades were compared using unpaired t tests. Probability values < .05 were considered to be statistically significant. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing) [11].
RESULTS
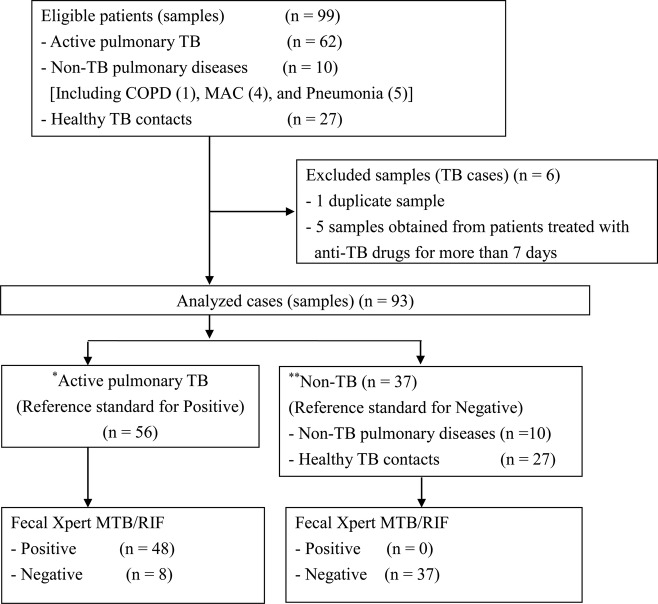
Of the 99 specimens obtained for this study, 6 were excluded from our analyses. Although 1 of these 6 was a duplicate sample, the remaining 5 were obtained from patients treated with anti-TB drugs for more than 7 days and who therefore did not fit the inclusion criteria. The 93 fecal specimens examined in this study were obtained from 56 patients with active pulmonary TB (48 smear-positive and 8 smear-negative cases), 10 patients with non-TB illnesses (1 patient with chronic obstructive pulmonary disease, 4 with Mycobacterium avium complex infections, 1 with mycoplasma pneumonia, 2 with pneumococcal pneumonia, and 2 with aspiration pneumonia), and 27 healthy TB contacts (Figure 1). The median age of the participants was calculated to be 59.6 years of age (interquartile range, 45.0–75.0), the male/female ratio was 51:42 (55% male), and none of the patients was infected with human immunodeficiency virus (HIV). Only one patient, who was prescribed prednisolone and salazosulfapyridine for treatment of rheumatoid arthritis, was on immunosuppressive medication.
Figure 1.
Flowchart depiciting the breakdown of the study subjects. *Individuals with active pulmonary tuberculosis (TB), bacteriologically confirmed by sputum culture or molecular diagnostic analysis, that were not treated with anti-TB drugs for more than 7 days. **Patients were classified into the non-TB group based on negative sputum smear, culture (at least 3 times), and chest radiograph (read by pulmonologists) results. The healthy TB contacts exhibited a lack of clinical symptoms and a normal chest radiograph (read by pulmonologists), and they tested negative for the QuantiFERON-TB Gold-in-Tube test. Abbreviations: COPD, chronic obstructive pulmonary disease; MAC, Mycobacterium avium complex; MTB, Mycobacterium tuberculosis; RIF, rifampicin.
We obtained a low proportion of both initial invalid results (3.2%; 1 error and 2 invalid) and indeterminate results for the detection of RIF resistance (n = 4; 4.3%). However, a total of 93 valid Xpert MTB/RIF results, excluding 1 unclear result regarding RIF resistance, were obtained after re-examination of these samples.
Of the 56 pulmonary TB cases, 17 (including 3 individuals infected with multidrug-resistant [MDR] strains) were categorized as sputum smear 3+, 10 (including 1 MDR) were sputum smear 2+, 11 (including 1 MDR) were 1+, 10 were scanty positive, and 8 cases (including 1 MDR) were sputum smear-negative. Among the 8 sputum smear-negative cases, 6 were sputum culture-positive, whereas the remaining 2 cases were culture- and TRC-negative (determined at the Fukujuji Hospital) but PCR-positive (determined at the referring hospital). These 2 individuals were diagnosed with TB, because they exhibited symptoms, chest radiographs, and CT results, such as cavity formation in the lung, that were indicative of TB (at the Fukujuji Hospital). Furthermore, there were 54 patients that were both sputum culture- and TRC-positive at the Fukujuji hospital. Four of these individuals exhibited miliary TB, whereas 2 suffered from TB meningitis.
In total, MTB was detected in 48 of the 56 samples harvested from patients with pulmonary TB cases (sensitivity, 85.7%; 95% CI, 73.8%–93.6%) by fecal Xpert MTB/RIF. In contrast, each of the samples from patients with non-TB illnesses was fecal Xpert MTB/RIF-negative (specificity, 100%; 95% CI, 86.2–100). Furthermore, among the 54 samples from culture-confirmed pulmonary TB patients, 47 were positive for MTB by fecal Xpert MTB/RIF analysis (sensitivity, 87.0%; 95% CI, 75.1–94.6). Although each of the samples from sputum smear-positive (1+ to 3+) TB patients yielded positive results by fecal Xpert MTB/RIF testing (sensitivity, 100%; 95% CI, 81.7–100), the analysis of the sputum scanty positive and negative samples was markedly less sensitive (sensitivities of 81.0% and 50%, and 95% CIs of 58.1–94.6 and 15.7–84.3, respectively) (Table 1). In addition, although fecal Xpert MTB/RIF failed to detect MTB in 1 of the 6 fecal samples from patients infected with MDR TB strains, RIF resistance was detected in 4 of the remaining 5 samples from MDR TB patients (sensitivity, 80.0%; 95% CI, 28.4–99.5).
Table 1.
The Sensitivity and Specificity of the Xpert MTB/RIF, MGIT, 2% Ogawa Culture, and Smear Analyses for Detection of MTB in Fecal Specimens From TB and Non-TB Patients
| Test | Test Result | TB (n = 56) | Non-TB (n = 37) | Sensitivity | 95% CI | Specificity | 95%CI |
|---|---|---|---|---|---|---|---|
| Xpert MTB/RIF | Positive | ||||||
| Sputum Smear Result | |||||||
| Negative (n = 8) | 4 | 0 | 50.0 | 15.7–84.3 | 100a | 86.2–100a | |
| ± (n = 21) | 17 | 0 | 81.0 | 58.1–94.6 | |||
| 1–3+ (n = 27) | 27 | 0 | 100 | 81.7–100 | |||
| Negative | 8 | 37 | 85.7a | 73.8–93.6a | |||
| MGIT | Positive | 15 | 0 | 31.9 | 19.1–47.1 | 100 | 82.8–100 |
| Negative (MAC) | 32 | 29 (4) | |||||
| Contamination | 9 | 4 | |||||
| 2% Ogawa culture | Positive | 12 | 0 | 21.4 | 11.6–34.4 | 100 | 86.2–100 |
| Negative (MAC) | 44 | 33 (4) | |||||
| Contamination | 0 | 0 | |||||
| Smearb | Positive | 26 | 2 | 47.3 | 33.7–61.2 | 94.6 | 81.8–99.3 |
| Negative | 29 | 35 | |||||
Abbreviations: CI, confidence interval; MAC, Mycobacterium avium complex; MGIT, mycobacterial growth indicator tubes; MTB, Mycobacterium tuberculosis; TB, tuberculosis.
a Total sensitivity and sensitivity of the Xpert MTB/RIF test using fecal specimens.
b The fecal smear analysis was not performed on one of the samples.
The MGIT test exhibited a high rate of contamination (14.0%). In contrast, the 2% Ogawa culture method showed no contamination, despite the use of fecal specimens. The sensitivity and specificity of the MGIT and Ogawa culture method when using fecal specimens were 31.9% (95% CI, 19.1–47.1) and 100% (95% CI, 82.8–100) and 21.4% (95% CI, 11.6–34.4) and 100% (95% CI, 86.2–100), respectively (Table 1). It is noteworthy that the fecal Xpert MTB/RIF test detected MTB in 34 of the 41 MGIT-negative or contaminated specimens. Meanwhile, the total sensitivity and specificity values of the fecal smear method were 47.3% (95% CI, 33.7–61.2) and 94.6% (95% CI, 81.8–99.3), respectively (Table 1), and only 2 false-positive results (5.4%) were obtained by this method.
The Ct values from the MTB-positive samples obtained by fecal Xpert MTB/RIF analysis were grouped and compared according to their respective sputum smear results. It is noteworthy that the Ct values associated with samples derived from sputum smear 1+ to 3+ patients were significantly lower than those from samples derived from sputum-negative (P = .011) or scanty positive patients (P = .0012) (Figure 2).
Figure 2.
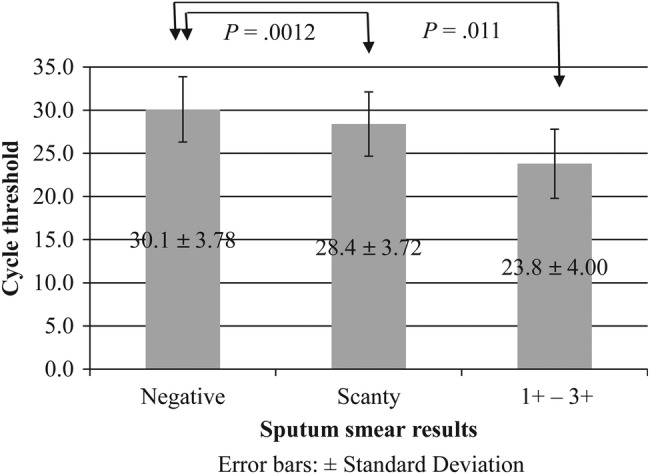
Comparison of the cycle threshold (Ct) values obtained by Xpert Mycobacterium tuberculosis (MTB)/rifampicin (RIF) analysis of fecal samples from sputum smear-positive, -scanty, and -negative patients. Legend: The Ct values obtained by Xpert MTB/RIF analysis of fecal samples from sputum-positive patients were compared with those of sputum scanty positive and sputum-negative individuals. The Ct values of the samples from sputum smear 1+ to 3+ patients were significantly lower than that those of the samples from sputum-negative (P = .011) or scanty positive patients (P = .0012).
DISCUSSION
In this small retrospective case-control study with convenience sampling, we have demonstrated as a proof-of-concept that it is possible to detect TB in a large proportion of smear-positive pulmonary TB patients, without frequent false-positive results observed in a population of healthy TB contacts and patients hospitalized with other non-TB pulmonary complaints. The obtained results were similar to those of Cordova et al [12], who revealed that IS6110-PCR analysis of fecal samples showed a high sensitivity and specificity (86% and 100%, respectively), compared with the results of sputum culture, in pulmonary TB patients. Meanwhile, in previous studies, the fecal Xpert MTB/RIF method was associated with lower levels of sensitivity for the diagnosis of pulmonary TB in children [8, 9]; however, this was due to the paucibacillary nature of the pediatric specimens. Consistent with those findings, the fecal Xpert MTB/RIF test exhibited low sensitivity in our analysis of sample from patients with negative or scanty sputum smear results. Although other studies have assessed the efficacy of the Xpert MTB/RIF method using fecal specimens with other extrapulmonary samples, these studies evaluated markedly fewer (1 or 2) samples than the aforementioned studies and the current study [13, 14]. A systematic review conducted by Maynard-Smith et al [15] concluded that previous studies on diagnostic accuracy of fecal Xpert MTB/RIF compared with culture had a smaller sample size but demonstrated a high sensitivity and specificity. Our study supports these findings, but we used a significantly larger sample size. Furthermore, this study is the first study to examine the performance of the Xpert MTB/RIF test using fecal samples harvested from adult patients.
The rate of pulmonary TB detection from fecal specimens using the MGIT and 2% Ogawa culture medium methods was very low. Furthermore, routine culturing of feces for pulmonary TB detection was ineffective. This is in contrast to a previous study, in which MTB was successfully detected in 44% of fecal samples from 206 HIV-positive patients with positive sputum cultures, using the conventional solid and liquid culturing methods [16]. The higher sensitivity observed by this group (compared with our results) may be a result of the specimen pretreatment process, because the authors filtered the fecal specimens through sterile gauze.
The lower sensitivity of the fecal culture, compared with that of the Xpert MTB/RIF test, observed in this research might be attributed to the proportion of viable MTB in the fecal specimens. The organisms within the fecal specimens were exposed to digestive fluids, the substances contained within the ingested food, and to the pretreatment process, including treatment with NALC-NaOH and centrifugation. As a result, these factors could have affected the survival or culturability of the MTB cells contained within the samples. In contrast, the Xpert MTB/RIF test, which detects MTB complex-specific DNA sequences and therefore does not distinguish between viable or dead bacteria, eliminates this limitation.
CONCLUSIONS
In this study, the fecal specimens were centrifuged (twice) and stored at −80°C before use. Although it is possible that the relative high sensitivity observed here may be attributed to these processes, the efficiency of this method remains to be fully evaluated. Furthermore, additional studies will be required to simplify and improve the pretreatment of fecal specimens for Xpert MTB/RIF testing and to eliminate erroneous and invalid results. Lastly, the clinical efficacy of this method must be evaluated by examining multiple fecal samples from patients suspected of having TB.
Limitations of This Study
This is a proof-of-concept study aimed at evaluating the potential of the Xpert MTB/RIF test for diagnosing TB via the detection of MTB from fecal samples. The patients that were enrolled in this study were capable of expectorating sputum; however, the major beneficiaries of the fecal Xpert MTB/RIF test are those who cannot expectorate sputum. Therefore, further clinical studies must be conducted to evaluate the efficacy of this method for diagnosing TB, particularly in patients from whom it is not possible to obtain sputum samples.
Another limitation of this study is the selection bias. Sensitivity and specificity values are affected by sampling. In this study, we enrolled 8 sputum smear-negative, 21 sputum smear scanty positive, and 27 sputum-positive (1+ to 3+) pulmonary TB patients. Of the 56 patients, 54 were also MTB culture-positive. The study site, Fukujuji Hospital, is a referral facility for local TB cases; therefore, the smear- and culture-positive rates were higher, which may have resulted in enhanced sensitivity. However, the sensitivity and specificity values associated with the Xpert MTB/RIF analysis of fecal specimens were stratified according to the sputum smear grade, which might allow for the utilization of this method as an effective diagnostic approach.
Furthermore, in this study, we did not examine the possibility of complications, such as TB colitis, that might affect the efficacy of this approach. The probable existence of such complications in active pulmonary TB cases could lead to misclassification and to increased sensitivity values for both the fecal Xpert MTB/RIF and fecal culturing methods.
Lastly, there was a limited number of patients enrolled in this study who were infected by MDR TB; therefore, care should be taken when interpreting the results obtained from these patients.
Acknowledgments
We express our sincere thanks and gratitude to all our research team and to the physicians, clinical fellows, nurses, and laboratory technicians at both Fukujuji Hospital and the Research Institute of Tuberculosis that provided their invaluable support and contributions during patient enrollment and data collection.
Financial support. This work was supported by the general research fund provided by the Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association. The Xpert MTB/RIF cartridges were a kind gift from Cepheid.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Boehme CC, Nabeta P, Hillemann D et al. . Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armand S, Vanhuls P, Delcroix G et al. . Comparison of the Xpert MTB/RIF test with an IS6110-TaqMan real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol 2011; 49:1772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marlowe EM, Novak-Weekley SM, Cumpio J et al. . Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol 2011; 49:1621–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theron G, Peter J, van Zyl-Smit R et al. . Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 2011; 184:132–40. [DOI] [PubMed] [Google Scholar]
- 5.Nicol MP, Workman L, Isaacs W et al. . Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis 2011; 11:819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuberculosis Surveillance Center: Statistics of TB 2014. Tokyo, Japan: Japan Anti-Tuberculosis Association Press, 2014. [Google Scholar]
- 7.Mitarai S, Tanoue S, Sugita C et al. . Potential use of Amplicor PCR kit in diagnosing pulmonary tuberculosis from gastric aspirate. J Microbiol Methods 2011; 47:339–44. [DOI] [PubMed] [Google Scholar]
- 8.Nicol MP, Spiers K, Workman L et al. . Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis 2013; 57:e18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walters E, Gie RP, Hesseling AC et al. . Rapid diagnosis of pediatric intrathoracic tuberculosis from stool samples using the Xpert MTB/RIF assay: a pilot study. Pediatr Infect Dis J 2012; 31:1316. [DOI] [PubMed] [Google Scholar]
- 10.Hillemann D, Rüsch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol 2011; 49:1202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanda Y. Investigation of the freely available easy-to-use software EZR for medical statistics. Bone Marrow Transplant 2013; 48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordova J, Shiloh R, Gilman RH et al. . Evaluation of molecular tools for detection and drug susceptibility testing of Mycobacterium tuberculosis in stool specimens from patients with pulmonary tuberculosis. J Clin Microbiol 2010; 48:1820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moure R, Martín R, Alcaide F. Effectiveness of an integrated real-time PCR method for detection of the Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples in an area of low tuberculosis prevalence. J Clin Microbiol 2012; 50:513–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moure R, Muñoz L, Torres M et al. . Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol 2011; 49:1137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maynard-Smith L, Larke N, Peters J, Lawn S. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis 2014; 14:3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oramasionwu GE, Heilig CM, Udomsantisuk N et al. . The utility of stool cultures for diagnosing tuberculosis in people living with the human immunodeficiency virus. Int J Tuberc Lung Dis 2013; 17:1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]