Abstract
Background:
Onychomycosis is the most common nail disease, representing 50% of cases affecting the nail apparatus. The diagnosis is made by clinical examination along with the KOH exam of the nail and culture of the sample. However, not all dermatologists have access to a mycology lab.
Objective:
To determine the correlation between KOH examination and dermoscopic patterns in patients with clinical diagnosis of onychomycosis.
Patients/Methods:
A descriptive, open, observational, prospective, cross-sectional study of 178 patients with clinical suspicion of onychomycosis was conducted. All patients underwent clinical examination, dermoscopy with a DermLite PHOTO dermatoscope (3Gen, San Juan Capistrano, CA, USA), KOH assessment and culture analysis. The most frequent dermoscopic patterns were identified and their correlation with the clinical subtype of onychomycosis was analyzed.
Results:
The study included 178 patients with clinical suspicion of onychomycosis. Of these, 155 (87.1%) had positive direct KOH examination for onychomycosis. Eighty-seven patients (56.13%) presented with clinical onychomycosis pattern of total dystrophic onychomycosis (TDO), 67 (43.23%) with distal lateral subungual onychomycosis (DLSO), 1 (0.65%) with trachyonychia). Dermoscopic patterns of onychomycosis showed the following frequencies: the spiked pattern was present in 22 patients (14.19%), longitudinal striae pattern in 51 patients (32.9%) and linear edge pattern in 21 patients (13.55%). We identified a pattern described as “distal irregular termination” in 41 patients with TDO and 26 with DLSO.
Conclusions:
This is the fist study conducted in a Mexican population that uses dermoscopy as a diagnostic tool along with the KOH examination for the diagnosis of onychomycosis. Dermoscopy may be used as an important diagnostic tool when evaluating nail disease. However, it should not be used as the only diagnostic criteria for onychomycosis.
Keywords: dermoscopy, onychomycosis, nails, patterns, clinical, KOH
Introduction
Onychomycosis is one of the most common nail disorders, accounting for nearly 50% of them. Within skin diseases it has a prevalence of 0.44% with a worldwide prevalence ranging from 2 to 50% [1]. It mainly affects adults between 30 and 60 years of age. The vast majority of cases are caused by dermatophytes as T. rubrum (71–87%) or T. mentagrophytes var interdigitale (9–22%). Candida species are isolated from cultures much less frequently (10–20%) [1].
The differential diagnosis of onychomycosis includes inflammatory diseases like psoriasis, lupus erythematosus, lichen planus, lichen striatum, alopecia areata, pityriasis rubra pilaris and also systemic diseases (amyloidosis, diabetes, porphyria, dysthyroidism) [2]. Infectious diseases that may mimic onychomycosis include viral warts and chronic paronychia. Other important differential diagnoses are cosmetic trauma (manicure or pedicure), secondary trauma (tight shoes or friction) and nail apparatus tumors (squamous cell carcinoma, melanoma or digital fibromas) [2].
Accurate diagnosis is important since the treatment of onychomycosis can be long-standing, expensive and may be accompanied by severe adverse effects [2]. Currently, the diagnosis is made by clinical suspicion along with KOH examination followed by culture of the samples [3,4]. However, the sensitivity and specificity of the potassium hydroxide examination and culture depend on the center where the study is conducted and can be as low as 15% [3,5].
When clinical suspicion of onychomycosis is high despite having a negative KOH examination and a negative culture, a punch biopsy or shaving the distal portion of the nail can be performed [3], followed by microscopic observation using stains such as PAS (periodic acid Schiff) or Gomori-Grocott. The procedure is simple and easy to perform, but invasive and expensive compared to direct examination.
Dermoscopy is a noninvasive tool that is widely used for the diagnosis of melanocytic, non melanocytic lesions, inflammatory and infectious diseases [6,7]. Currently there are specific algorithms for the analysis of patterns of melanocytic and non-melanocytic lesions, yet, there is no algorithm described and validated for the assessment onychomycosis [1,8].
Piraccini et al identified and described specific dermoscopic signs for onychomycosis to distinguish it from traumatic onycholysis [9]. A retrospective analysis of 57 digital dermoscopic images with clinical diagnosis of onycholysis diagnosis was performed. Dermoscopic findings were compared with mycological findings and 3 dermoscopic patterns were described, 2 for onychomycosis (jagged proximal edge with spikes of the onycholytic area and longitudinal striae) were identified and 1 for traumatic onycholysis (linear edges without peaks in the area of onycholysis). Thus, these authors proposed a new diagnostic algorithm based on the presence of spiked edges [9].
Recently, Nakamura et al [1], performed the dermoscopic study of 500 cases of nail disorders (onychomycosis, psoriasis, lichen planus and nail brittleness), identifying the most frequent dermoscopic characteristics of each one. In the case of onychomycosis they identified four patterns (chromonychia, onycholysis, opacity and longitudinal stripes), similar to those previously described by Piraccini [9].
The objective of our study was to determine the correlation between the KOH examination and dermoscopic patterns in patients with clinical diagnosis of onychomycosis.
Materials and methods
We included in our study patients with clinical and mycological diagnosis of onychomycosis that were studied in the Dermatology and Mycology Departments of the General Hospital “Dr. Manuel Gea González” in Mexico City. Patients with previous treatment for onychomycosis were excluded.
Diagnosis of onychomycosis was made with KOH examination and the presence of fungal elements (hyphae, spores or yeasts) was considered positive for the diagnosis. Part of the sample was also cultured in all patients.
Macroscopic images of the affected nails were obtained with Canon PowerShot G1x® camera and digital dermoscopic images were obtained with DermLite PHOTO dermatoscope, with 20× and 40× magnifications.
A single observer analyzed each of dermoscopy images in order to identify the most common patterns and compare them with the patterns described by Piraccini [10], describing the subtype of onychomycosis and the dermoscopic pattern.
Data were analyzed with the SPSS Statistics Desktop (V21.0.0) program, X2 test was applied. Percentages, measures of central tendency and dispersion were performed. The correlation between positive KOH examination and culture, KOH examination and dermoscopic pattern, and clinical subtype of onychomycosis and dermoscopic pattern were determined.
The study was approved by the Research and Ethics Committee of the General Hospital “Dr. Manuel Gea González” in Mexico City, Mexico.
Results
A total of 178 patients with clinical suspicion of onychomycosis were initially included in the study (Table 1). Of these, 155 (87.07%) had a positive KOH examination for onychomycosis and were finally included. Ninety-nine (55.6%) were women and the mean age was 50–59 years of age, with a median time of onychomycosis evolution of 24 months.
TABLE 1.
General characteristics of 178 patients initially included in the study [Copyright: ©2015 Jesús-Silva et al.]
| TDO* | DLSO** | TRACHYONYCHIA | WSO*** | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | ||
| TOTAL NUMBER OF CASES | 94 | 52.81 | 81 | 45.51 | 1 | 0.56 | 2 | 1.12 | |
| GENDER FEMALE | 66 | 37.08 | 48 | 26.97 | 1 | 0.56 | 1 | 0.56 | 0.386 |
| EVOLUTION | 0.66 | ||||||||
| <25 months | 40 | 22.47 | 39 | 21.91 | 1 | 0.56 | 2 | 1.12 | |
| 25–48 months | 14 | 7.87 | 11 | 6.18 | 0 | 0.00 | 0 | 0.00 | |
| > 49 months | 40 | 22.47 | 31 | 17.42 | 0 | 0.00 | 0 | 0.00 | |
| POSITIVE DIRECT KOH EXAMINATION | 87 | 48.88 | 67 | 37.64 | 1 | 0.56 | 0 | 0.00 | 0.001 |
| FINDINGS IN DIRECT KOH EXAMINATION | 0.034 | ||||||||
| Fungal hyphae | 84 | 47.19 | 73 | 41.01 | 0 | 0.00 | 2 | 1.12 | |
| Yeasts | 10 | 5.62 | 8 | 4.49 | 1 | 0.56 | 0 | 0.00 | |
| DERMOSCOPIC PATTERN | |||||||||
| Spiked pattern | 24 | 13.48 | 24 | 13.48 | 0 | 0.00 | 0 | 0.00 | 0.683 |
| Longitudinal striae | 56 | 31.46 | 50 | 28.09 | 1 | 0.56 | 1 | 0.56 | 0.843 |
| Linear edge | 23 | 12.92 | 17 | 9.55 | 0 | 0.00 | 1 | 0.56 | 0.701 |
| Distal irregular termination | 42 | 23.60 | 29 | 16.29 | 0 | 0.00 | 0 | 0.00 | 0.327 |
| CULTURE | |||||||||
| Positive | 75 | 42.13 | 58 | 32.58 | 0 | 0.00 | 1 | 0.56 | 0.937 |
| Tricophyton rubrum | 10 | 5.62 | 10 | 5.62 | 0 | 0.00 | 0 | 0.00 | |
| Trichosporon sp. | 3 | 1.69 | 1 | 0.56 | 0 | 0.00 | 0 | 0.00 | |
| Candida sp. | 3 | 1.69 | 2 | 1.12 | 0 | 0.00 | 0 | 0.00 | |
| Bacteria | 8 | 4.49 | 7 | 3.93 | 0 | 0.00 | 1 | 0.56 | |
| Negative | 70 | 39.33 | 61 | 34.27 | 1 | 0.56 | 1 | 0.56 | |
Total dystrophic onychomycosis (TDO)
Distal lateral subungual onychomycosis (DLSO)
White superficial onychomycosis (WSO)
Eighty-seven patients (56.13%) were clinically classified as total dystrophic onychomycosis (TDO), 67 (43.23%) as distal lateral subungual onychomycosis (DLSO), 1 (0.65%) with trachyonychia and none had white superficial onychomycosis (WSO).
The culture was negative in 76.13% (118 samples). In 16 samples T. rubrum was identified as the causative agent (10.33%), Trichosporon sp. was identified in 4 samples (2.59%) and Candida sp. was isolated in 5 patients (3.23%).
The spiked pattern was present in 39 patients (25.16%) (Figure 1), the longitudinal striae in 94 (60.65%) (Figure 2), linear edge in 34 (21.94%) (Figure 3) and distal irregular termination in 67 patients (43.22%) (Figure 4).
Figure 1.
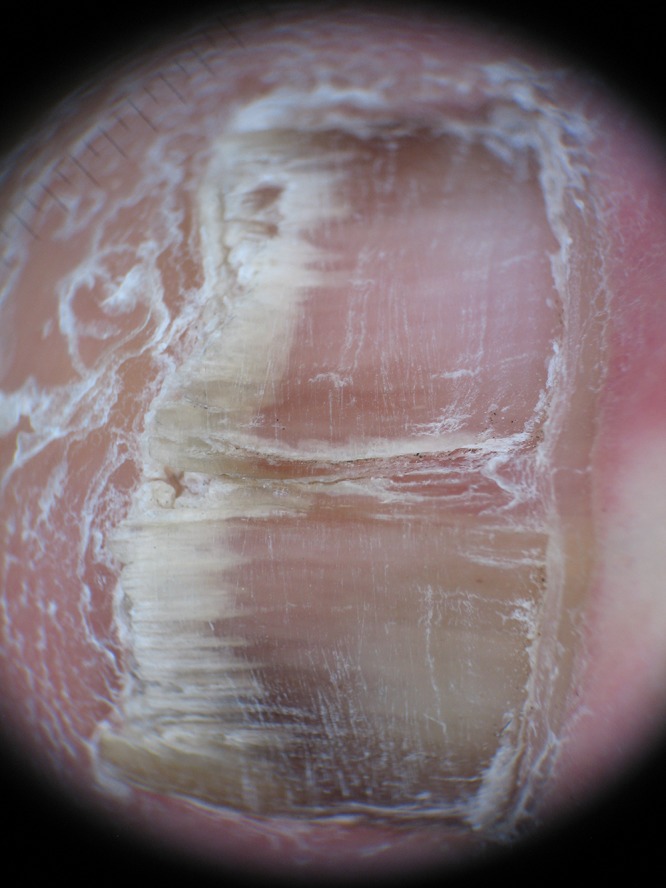
Spiked pattern, indentations at the proximal edge of the área with onycholysis. [Copyright: ©2015 Jesús-Silva et al.]
Figure 2.
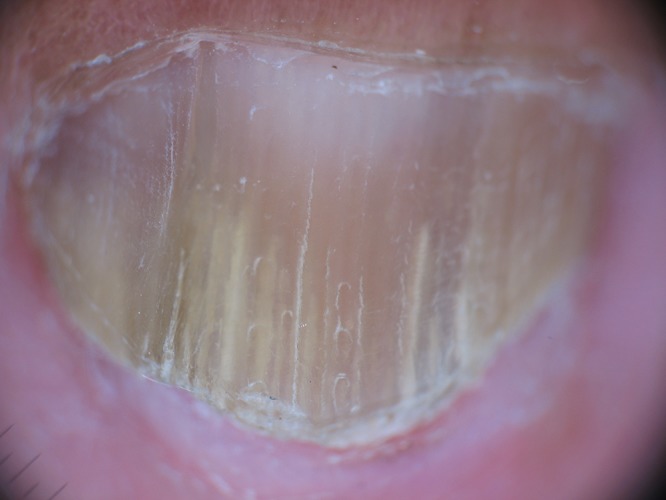
Longitudinal striae of different colors in the onycholytic nail plate. [Copyright: ©2015 Jesús-Silva et al.]
Figure 3.
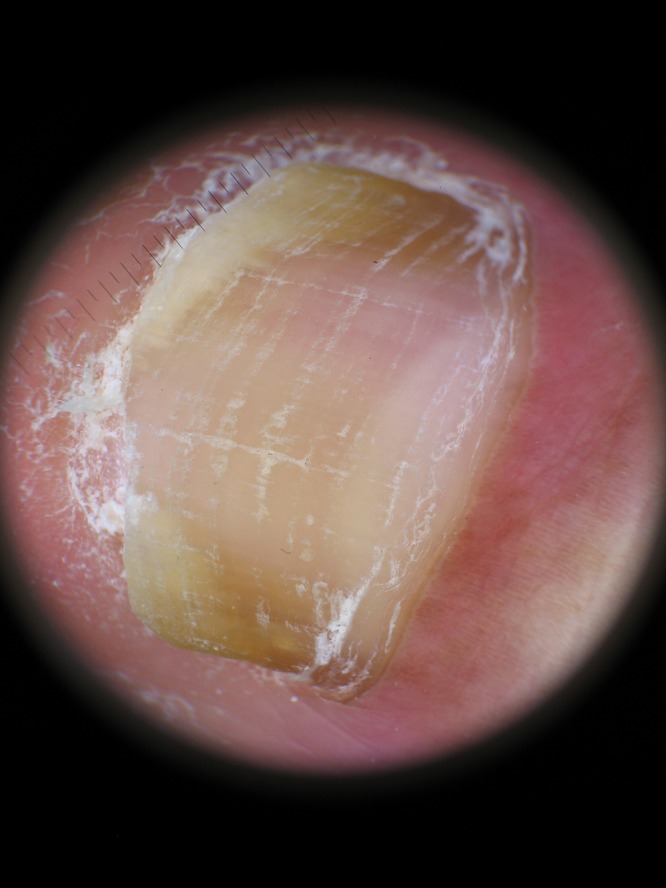
Linear edge, a smooth linear demarcation without indentations. [Copyright: ©2015 Jesús-Silva et al.]
Figure 4.
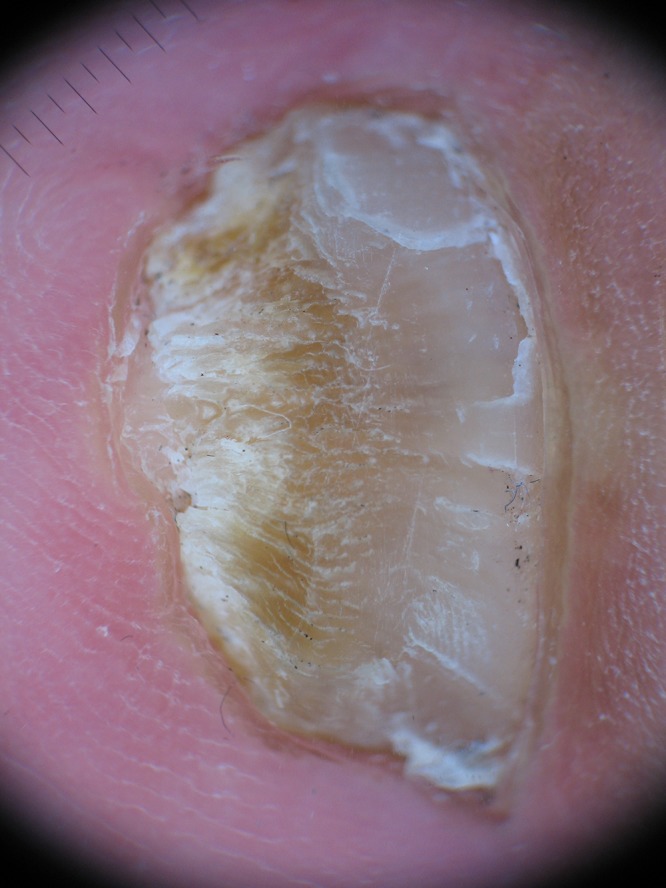
Distal irregular termination, distal pulverization characteristic of the thickening of the nail plate. [Copyright: ©2015 Jesús-Silva et al.]
The presence of onychomycosis in the KOH examination was correlated with each dermoscopic pattern. Chi square test was applied to this correlation, which was not significant in any case (Tables 1 and 2).
TABLE 2.
Characteristics of 155 patients with positive direct KOH examination and finally included on the study
| TDO* | DLSO** | TRACHYONYCHIA | P | ||||
|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | ||
| POSITIVE KOH EXAMINATION | 87 | 56.13 | 67 | 43.23 | 1 | 0.65 | |
| GENDER—FEMALE | 59 | 38.06 | 39 | 25.16 | 1 | 0.65 | 0.353 |
| EVOLUTION | 0.309 | ||||||
| <25 months | 33 | 21.29 | 31 | 20.00 | 1 | 0.65 | |
| 25–48 months | 14 | 9.03 | 11 | 7.10 | 0 | 0.00 | |
| > 49 months | 40 | 25.81 | 25 | 16.13 | 0 | 0.00 | |
| FINDINGS IN DIRECT KOH EXAMINATION | 0.027 | ||||||
| Fungal hyphae | 77 | 49.68 | 59 | 38.06 | 0 | 0.00 | |
| Yeasts | 10 | 6.45 | 8 | 5.16 | 1 | 0.65 | |
| DERMOSCOPIC PATTERN | |||||||
| Spiked pattern | 22 | 14.19 | 17 | 10.97 | 0 | 0.00 | 0.844 |
| Longitudinal striae | 51 | 32.90 | 42 | 27.10 | 1 | 0.65 | 0.633 |
| Linear edge | 21 | 13.55 | 13 | 8.39 | 0 | 0.00 | 0.678 |
| Distal irregular termination | 41 | 26.45 | 26 | 16.77 | 0 | 0.00 | 0.4 |
| CULTURE | |||||||
| Positive | 23 | 14.84 | 14 | 9.03 | 0 | 0.00 | 0.992 |
| Tricophyton rubrum | 9 | 5.81 | 7 | 4.52 | 0 | 0.00 | |
| Trichosporon sp. | 3 | 1.94 | 1 | 0.65 | 0 | 0.00 | |
| Candida sp. | 3 | 1.94 | 2 | 1.29 | 0 | 0.00 | |
| Bacteria | 8 | 5.16 | 4 | 2.58 | 0 | 0.00 | |
| Negative | 64 | 41.29 | 53 | 34.19 | 1 | 0.65 | |
Total dystrophic onychomycosis (TDO)
Distal lateral subungual onychomycosis (DLSO)
Note: White superficial onychomycosis (WSO) was not included in this analysis because no patient had a positive direct KOH examination.
We next correlated the dermoscopic patterns to the clinical pattern of onychomycosis. The skipped pattern was present in 39 patients and 22 (56.41%) of these showed a clinical pattern of TDO, while 17 (43.59%) showed a clinical pattern of DLSO. The longitudinal striae pattern was present in 94 patients, of these 51 (54.25%) showed a clinical pattern of TDO, 42 (44.68%) were clinically compatible with DLSO and 1 (1.06%) with trachyonychia (Table 3).
TABLE 3.
Dermoscopic patterns and their correlation with the different clinical types of onychomycosis
| Spiked pattern | Longitudinal striae | Linear edge | Distal irregular termination | |||||
|---|---|---|---|---|---|---|---|---|
| TOTAL | 39 | 94 | 34 | 67 | ||||
| # | % | # | % | # | % | # | % | |
| TDO* | 22 | 56.41 | 51 | 54.26 | 21 | 61.76 | 41 | 61.19 |
| DLSO** | 17 | 43.59 | 42 | 44.68 | 13 | 38.24 | 26 | 38.81 |
| TRACHYONYCHIA | 0 | 0.00 | 1 | 1.06 | 0 | 0.00 | 0 | 0.00 |
| P | 0.844 | 0.633 | 0.68 | 0.4 | ||||
Total dystrophic onychomycosis (TDO)
Distal lateral subungual onychomycosis (DLSO)
The linear edged pattern was evident in 34 patients, 21 clinically compatible with TDO (58.8%) and 13 with DLSO (38.2%) (Table 3).
Dermoscopically, a pattern described as “distal irregular termination” was present in 67 patients, 41 (61.76%) clinically compatible with TDO and 26 (38.8%) with DLSO (Table 3).
Discussion
Onychomycosis is a disease the dermatologist is faced with great frequency, representing approximately 50% of nail affections. The clinical picture is a critical element for establishing the diagnosis, although it may be insufficient. The mycological diagnosis is performed in our hospital with KOH examination and Chlorazol Black, accompanied by culture of the sample.
Potassium hydroxide examination is a technique that is observer dependent, but has acceptable sensitivity ranging from 75% up to 80% [10–15]. Culture is a method with a low sensitivity for dermatophytes; up to 60% false negatives may occur [7,11] and not all dermatology centers have a mycology laboratory. In our study the culture was not an inclusion parameter, but it was performed in all patients, being positive in only 25 patients. This is consistent with what has been reported in the literature and shows that the culture cannot be used as a gold standard for diagnosis.
To the best of our knowledge, this is the third study in which the dermoscopic findings are discussed in onychomycosis. The “longitudinal striae pattern” was more frequently observed in patients with TDO or DLSO. Our finding is in accordance to what was previously described by Piracinni [9]. Something similar happened with the spiked pattern, which was also frequently identified in patients with clinical diagnosis of TDO (22) and DLSO (17). The presence of “spikes” is important, it is characterized as indentations at the proximal edge of the area with onycholysis compared to the “linear edge” which is manifested as a smooth linear demarcation without indentations. According to the data of Piraccini et al, the presence of the “linear edge” is significantly associated with traumatic onycholysis [9]. Therefore, this simple dermoscopic finding may be useful when making the diagnosis of onychomycosis vs. traumatic onycholysis.
The “longitudinal striae” have been studied and identified as the manifestation of the progression of dermatophytes along the nail plate, showing changes in coloration secondary colony formation, flakes or subungual debris.
Another pattern was described, the “distal irregular termination” which corresponds to the distal pulverization characteristic of the thickening of the nail plate in total dystrophic onychomycosis. This pattern was not significantly associated with a specific clinical pattern of onychomycosis, however, it occurred more frequently in patients with the clinical diagnosis of TDO.
This is the first study conducted in the Mexican population that shows the usefulness of dermoscopy as a diagnostic tool coupled with mycological examination for the diagnosis of onychomycosis. We recommend future studies with homogeneous groups of different clinical subtypes of onychomycosis and including patients with suspected traumatic onycholysis or other nail diseases. The patterns that were studied have not been validated and are not universally recognized, generating a field of opportunity for the study of onychomycosis by use of dermosocopy.
The results of our study show that the diagnosis of onychomycosis cannot be performed relying on clinical grounds alone and emphasize the need to rely on tools such as KOH examination, culture and dermoscopy. Dermoscopy has proven to become an important adjunctive tool in the evaluation of nail diseases.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Nakamura RC, Costa M. Dermatoscopic findings in the most frequent onychopathies: descriptive analysis of 500 cases. Int J Dermatol. 2012;51(4):483–96. doi: 10.1111/j.1365-4632.2010.04720.x. [DOI] [PubMed] [Google Scholar]
- 2.Allevato MA. Diseases mimicking onychomycosis. Clin Dermatol. 2010;28(2):164–77. doi: 10.1016/j.clindermatol.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Richert B, Lateur N, Theunis A, et al. New tools in nail disorders. Semin Cutan Med Surg. 2009;28(1):44–8. doi: 10.1016/j.sder.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Harvey CK, Richardson A. Techniques for obtaining specimens for culture to confirm onychomycosis. J Am Podiatr Med Assoc. 2000;90(8):394–6. doi: 10.7547/87507315-90-8-394. [DOI] [PubMed] [Google Scholar]
- 5.Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620–6. doi: 10.1016/j.jaad.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Campos-do-Carmo G, Ramos-e-Silva M. Dermoscopy: basic concepts. Int J Dermatol. 2008;47(7):712–19. doi: 10.1111/j.1365-4632.2008.03556.x. [DOI] [PubMed] [Google Scholar]
- 7.Gilje O, O’Leary PA, Baldes EJ. Capillary microscopic examination in skin disease. AMA Arch Dermatol Syphilol. 1953;68(2):136–47. doi: 10.1001/archderm.1953.01540080020003. [DOI] [PubMed] [Google Scholar]
- 8.Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64(6):1135–46. doi: 10.1016/j.jaad.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Piraccini BM, Balestri R, Starance M, Rech G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27(4):509–13. doi: 10.1111/j.1468-3083.2011.04323.x. [DOI] [PubMed] [Google Scholar]
- 10.Manzano-Gayosso P, Mendez-Tovar L, Arenas R. Levaduras causantes de onicomicosis en cuatro centros dermatológicos mexicanos y su sensibilidad antifúngica a compuestos azólicos. Rev Iberoam Micol. 2011;28(1):32–5. doi: 10.1016/j.riam.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17(4):571–83. doi: 10.1016/s0190-9622(87)70239-4. [DOI] [PubMed] [Google Scholar]
- 12.Goldman L. Some investigative studies of pigmented nevi with cutaneous microscopy. J Invest Dermatol. 1951;16(6):407–27. doi: 10.1038/jid.1951.48. [DOI] [PubMed] [Google Scholar]
- 13.Bahmer FA, Fritsch P, Kreusch J, et al. Terminology in surface microscopy Consensus Meeting of the Committee on Analytical Morphology of the Arbeitsgemeinschaft Dermatologische Forschung, Hamburg, Federal Republic of Germany, Nov 17, 1989. J Am Acad Dermatol. 1990;23(6 Pt1):1159–62. [PubMed] [Google Scholar]
- 14.Kreusch J, Rassner G. Structural analysis of melanocytic pigment nevi using epiluminescence microscopy. Review and personal experiences. Hautarzt. 1990;41(1):27–33. [PubMed] [Google Scholar]
- 15.Scher R, Tavakkol A, Bact D, et al. Onychomycosis: diagnosis and definition of cure. J Am Acad Dermatol. 2007;56(6):939–44. doi: 10.1016/j.jaad.2006.12.019. [DOI] [PubMed] [Google Scholar]


