Abstract
For several years we used rayon or Dacron swabs with liquid transport media for collection and transport of throat swab specimens for testing with the Gen-Probe Group A Strep Direct Test (GASDT). A report of favorable results with a Dacron swab without any transport media for GASDT by another laboratory prompted us to compare detection of group A streptococci (GAS) with and without transport media (referred to as “wet” and “dry” swabs, respectively). Phase one of this study used swabs seeded with GAS. Initially, six recent clinical isolates of GAS were inoculated onto wet and dry swabs and stored at room temperature (RT). After 1, 2, and 3 days of storage, colony counting and GASDT were performed with the swabs. The results, expressed as the mean percentage of the results at zero time, were as follows: for GASDT with wet swabs at 1, 2, and 3 days, 62, 51, and 56%, respectively; for GASDT with dry swabs at 1, 2, and 3 days, 105, 80, and 85%, respectively; for colony counts with wet swabs at 1, 2, and 3 days, 52, 26, and 13%, respectively; for colony counts with dry swabs at 1, 2, and 3 days, 10, 0, and 0%, respectively. An additional six strains of GAS were tested in a similar manner, except that extracts of pharyngeal flora (PF) were added to the inocula. The results obtained with extracts of PF were comparable to those obtained with GAS alone. We also compared the performance of GASDT with wet and dry swabs stored at RT and 4°C. Ten strains of GAS were inoculated onto wet and dry swabs, and GASDT was performed each day for 9 days. The GASDT results for swabs on day 9, expressed as the mean percentage of the results obtained at zero time, were as follows: dry swab and 4°C, 59%; wet swab and 4°C, 31%; dry swab and RT, 33%; and wet swab and RT, 19%. In phase two of this study we conducted a clinical evaluation to determine whether the differences observed with seeded specimens would also be evident with patient specimens. We used a single dry Dacron swab paired with a single rayon Bacti-Swab with liquid Stuart transport medium for the clinical evaluation. Specimens were collected from 1,005 outpatients, plated onto a Strep Selective Agar plate, and then tested within 30 min by GASDT. If culture of GAS from the same swab is used to define a true-positive test result, the sensitivities and specificities were as follows: GASDT with wet swabs, 86.2 and 98.5%, respectively; GASDT with dry swabs, 90.7 and 98.1%, respectively. However, the use of culture as the “gold standard” may understate the actual performance characteristics of GASDT, particularly for the dry swabs. In conclusion, for GASDT the use of swabs without transport media may be preferable to the use of swabs with transport media.
We have used the Gen-Probe Group A Strep Direct Test (GASDT) in our laboratory for about 8 years. We have previously evaluated the performance of GASDT using both rayon and Dacron swabs with liquid transport media (1, 3). A report of favorable results of GASDT with a swab without any transport media by another laboratory (S. Wood, H. Takahashi, and J. Fusco, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1567, 1999) prompted us to evaluate the performance of GASDT in our laboratory using swabs with no transport media.
We conducted a two-phase study. In phase one we used seeded swabs with and without transport media to assess the quantitative detection of group A streptococci (GAS), as determined by the results of GASDT and from colony counts. Additional studies were carried out to determine the effect of storage temperature prior to testing on the results of GASDT for seeded swabs with and without transport media stored at different temperatures. In phase two, we conducted a clinical evaluation to determine the performance of GASDT with throat swab specimens collected and transported with and without transport media.
MATERIALS AND METHODS
GASDT.
GASDT uses a chemiluminescence-labeled, single-stranded DNA probe that is complementary to GAS rRNA. The chemiluminescent output is measured quantitatively in relative light units (RLU). A cutoff of 4,500 RLU has been established by the manufacturer to differentiate between negative and positive test results for swabs specifically approved for use with GASDT.
Phase one: experiments with swabs seeded with GAS.
Phase one of this study was performed in three parts. In part one, we selected six recent, random clinical isolates of GAS, prepared a standard 0.5 McFarland dilution of each isolate in sterile saline, performed a 1:100 dilution, and inoculated 0.1 ml of each strain onto multiple Culturette rayon swabs (Becton Dickinson Microbiology Systems, Cockeysville, Md.). For one-half of the transport devices, the swabs were placed into the holders, and an ampoule of Stuart transport medium was crushed. For the remaining transport devices, the swabs were placed into the holders after the ampoules of transport medium and the pledgets had been removed. For the purposes of this study, the swabs without transport media are referred to as “dry” swabs, while the swabs with transport media are referred to as “wet” swabs. All swabs were then stored at room temperature (RT). Colony counts were determined at zero time and after 1, 2, and 3 days. To obtain colony counts, the swabs were vortexed in 1 ml of sterile saline, serial dilutions were performed, and subcultures were performed (in duplicate) for the swabs with and without transport media. In addition, GASDT was performed (in duplicate) with different swabs at the same time intervals.
In part two, we repeated the protocol from part one, with one significant variation. In part two, the inocula were prepared with pharyngeal flora (PF) mixed with GAS. To prepare suspensions of PF, duplicate throat swab specimens were collected from four volunteer microbiology technologists. One swab from each individual was tested by GASDT to verify that each was negative for GAS. The second swab from each individual was extracted by vortexing in saline. The extracts were pooled together, and dilutions were performed to determine the proper concentration which, when plated, would produce 3+ to 4+ growth of PF on a blood agar plate. Suspensions of the GAS isolates equivalent to a 0.5 McFarland standard were diluted 1:100 in the tubes containing PF. From that point forward, the protocol from part one was repeated with GASDT and colony counting, performed at zero time and after 1, 2, and 3 days, with swabs with and without transport media.
In part three, seeded swabs were used to assess the quantitative detection of GAS by GASDT for wet and dry swabs held at different temperatures prior to testing. We selected 10 recent, random clinical isolates of GAS, prepared a standard 0.5 McFarland dilution of each isolate in sterile saline, performed a 1:100 dilution, and inoculated 0.1 ml of each strain onto multiple Culturette rayon swabs (Becton Dickinson Microbiology Systems). For one-half of the transport devices, the swabs were placed into the holders, and the ampoule of Stuart transport medium was crushed. For the remaining transport devices, the swabs were placed into the holders after the ampoules of transport media and the pledgets had been removed. One-half of the dry swabs were stored at RT, while the other half were stored at 4°C. The wet swabs were stored in a similar manner at RT and 4°C. At zero time and each day thereafter for 9 days, GASDT was performed with each of the wet and dry swabs inoculated with each of the 10 different strains of GAS and held at the two different temperatures.
Phase two: clinical evaluation comparing detection of GAS from swabs with and without transport media from throat swab specimens.
In the clinical evaluation, a single dry Dacron swab (catalog no. 159C; Copan Diagnostics, Corona, Calif.) was paired with a single rayon Bacti-Swab (catalog no. 12100; Remel, Lenexa, Kans.) with liquid Stuart transport medium. Clinicians were instructed to use the two swabs together when collecting throat swab specimens from patients in the clinic. All swabs used in this phase of the study were sterilized with ethylene oxide. Specimens were transported to the Geisinger Medical Center Microbiology Laboratory at ambient temperature and were stored at RT prior to batch testing. Three or four times daily, each paired swab was plated onto a Strep Selective Agar plate (Remel) and then tested by GASDT within 30 min of plating. Cultures were incubated in the presence of increased CO2 concentrations at 35°C. GAS were identified by standard microbiology techniques. All cultures negative for GAS after one night of incubation were reincubated for an additional 24 h.
Statistical analysis.
Comparative detection of GAS by GASDT and colony counting was evaluated by the Wilcoxon signed rank test. Detection of GAS by GASDT for swabs held at different temperatures was assessed by a repeated-measures analysis of variance. The sign test was used to analyze the results of the clinical evaluation. Statistical significance was achieved when P values were ≤0.05.
RESULTS
Phase one: experiments with swabs seeded with GAS.
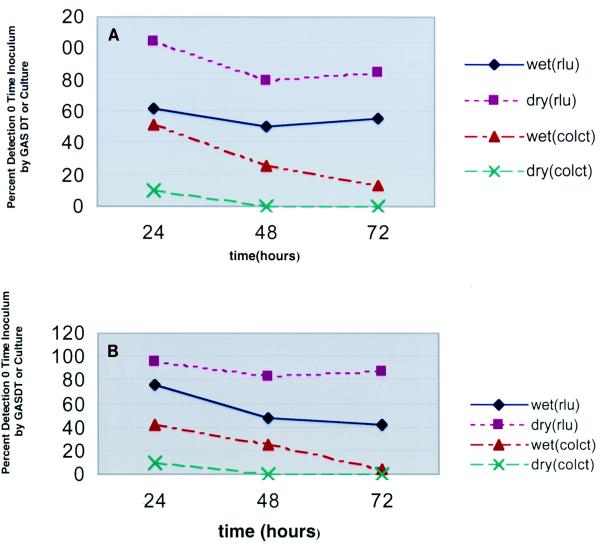
To assess the relative detection of GAS by using wet and dry swabs, we seeded specimens with GAS and measured the quantitative detection of GAS as determined by colony counting and GASDT. At 0, 1, 2, and 3 days, quantitative subcultures of the wet and dry swabs were performed (in duplicate). In addition, GASDT was performed at the same time intervals (in duplicate). The results (averaged for six strains) (Fig. 1A), expressed as a percentage of the results obtained at zero time, were as follows: for GASDT with wet swabs at 1, 2, and 3 days, 62, 51, and 56%, respectively; for GASDT with dry swabs at 1, 2, and 3 days, 105, 80, and 85%, respectively; for colony counts with wet swabs at 1, 2, and 3 days, 52, 26, and 13%, respectively; for colony counts with dry swabs at 1, 2, and 3 days, 10, 0, and 0%, respectively. The results of GASDT with dry swabs at 1 and 3 days and colony counts with wet swabs at 1, 2 and 3 days were statistically significantly different.
FIG. 1.
Mean GASDT RLU (rlu) and colony counts (colct) by hour for pure cultures of GAS (A) and cultures of GAS mixed with PF (B), expressed as a percentage of the value at zero time, for seeded swab specimens with (wet) and without (dry) transport media.
An additional six strains of GAS were tested in a similar manner, except that extracts of PF were added to the inocula. The results obtained with PF (averaged for six strains) (Fig. 1B), expressed as a percentage of the results obtained at zero time, were as follows: for GASDT (PF) with wet swabs at 1, 2, and 3 days, 76, 48, and 42%, respectively; for GASDT (PF) with dry swabs at 1, 2, and 3 days, 96, 84, and 88%, respectively; for colony counts (PF) with wet swabs at 1, 2, and 3 days, 42, 25, and 5%, respectively; for colony counts (PF) with dry swabs at 1, 2, and 3 days, 10, 0.1, and 0%, respectively. The results of GASDT with dry swabs at 1, 2, and 3 days and colony counts with wet swabs at 1, 2 and 3 days were statistically significantly different.
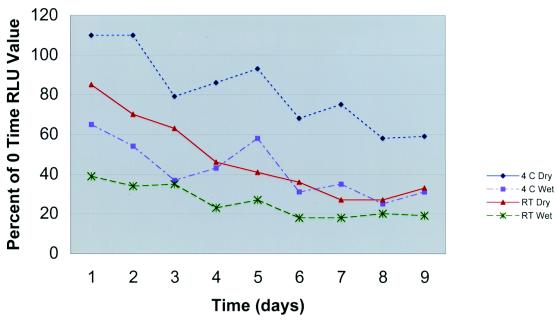
To assess the effects of the storage or holding temperature prior to performance of GASDT, we seeded swabs and measured the rates of detection of GAS. At zero time and on days 1 to 9, GASDT was performed with wet and dry swabs held at RT and 4°C. The results (averaged for 10 strains) (Fig. 2) were as follows for dry swabs at 4°C, wet swabs at 4°C, dry swabs at RT, and wet swabs at RT on each of days 1 to 9: day 1, 110, 65, 85, and 39%, respectively; day 2, 110, 54, 70, and 34%, respectively; day 3, 79, 37, 63, and 35%, respectively; day 4, 86, 43, 46, and 23%, respectively; day 5, 93, 58, 41, and 27%, respectively; day 6, 68, 21, 36, and 18%, respectively; day 7, 75, 35, 27, and 18%, respectively; day 8, 58, 25, 27, and 20%, respectively; and day 9, 59, 31, 33, and 19%, respectively. There were significant differences in the results for dry swabs at 4°C compared to those for wet swabs at 4°C, in the results for dry swabs at RT compared to those for wet swabs at RT, and in the results for dry swabs at 4°C compared to those for dry swabs at RT.
FIG. 2.
Mean GASDT RLU by day for seeded swab specimens with (wet) and without (dry) transport media held at two temperatures, expressed as a percentage of the RLU at zero time.
Clinical evaluation comparing swabs with and without transport media for detection of GAS from throat swabs.
A single dry Copan Dacron swab and a single rayon Bacti-Swab with liquid Stuart transport medium were collected from each of 1,005 outpatients and tested by GASDT. The raw data are summarized in Table 1. When the results of testing are examined separately by swab type, the results for the wet swabs were as follows: 188 swabs were culture positive and GASDT positive, 775 swabs were culture negative and GASDT negative, 30 swabs were culture positive and GASDT negative, and 12 swabs were culture negative and GASDT positive. By using culture as the “gold standard,” the sensitivity and specificity of GASDT with the wet swab were 86.2 and 98.5%, respectively.
TABLE 1.
Results of comparative evaluations of 1,005 specimens by culture and GASDT with wet and dry swabs
| Resulta with wet swabs
|
Result with dry swabs
|
Total no. of specimens | ||
|---|---|---|---|---|
| GASDT | Culture | GASDT | Culture | |
| N | N | N | N | 759 |
| P | P | P | P | 178 |
| N | P | P | P | 10 |
| N | P | N | P | 9 |
| N | P | N | N | 9 |
| N | N | P | N | 9 |
| P | N | P | P | 6 |
| N | N | N | P | 6 |
| P | P | N | P | 5 |
| P | N | P | N | 4 |
| P | P | P | N | 3 |
| P | N | N | N | 2 |
| P | P | N | N | 2 |
| N | P | P | N | 2 |
| N | N | P | P | 1 |
N, negative result; P, positive result.
The results for the dry swabs were as follows: 195 swabs were culture positive and GASDT positive, 772 swabs were culture negative and GASDT negative, 20 swabs were culture positive and GASDT negative, and 18 swabs were culture negative and GASDT positive. By using culture as the gold standard, the sensitivity and specificity of GASDT for the dry swab were 90.7 and 98.1%, respectively.
Fifteen specimens were GASDT positive with one or both swabs but culture negative with both swabs (Table 1). Nine of these specimens were positive by GASDT with both swabs, two were GASDT positive with the Bacti-Swab wet swab only, and four were GASDT positive with the Copan dry swab only. The RLU for these 15 GASDT-positive and culture-negative specimens are summarized in Table 2.
TABLE 2.
Analysis of RLU for 15 GASDT-positive and culture-negative specimens
| Results for wet and dry swabs | RLU
|
|
|---|---|---|
| Wet swab | Dry swab | |
| Both swabs positive | 8,639 | 40,072 |
| 11,471 | 64,196 | |
| 11,392 | 9,453 | |
| 98,752 | 117,768 | |
| Only dry swabs positive | 1,908 | 9,835 |
| 2,206 | 6,651 | |
| 1,884 | 2,976,125 | |
| 2,232 | 14,546 | |
| 2,713 | 26,396 | |
| 2,213 | 36,666 | |
| 1,695 | 15,184 | |
| 2,688 | 4,590 | |
| 2,718 | 13,811 | |
| Only wet swabs positive | 6,794 | 1,373 |
| 7,530 | 4,403 | |
For specimens that were GASDT positive with both swabs and culture positive with both swabs, the mean RLU were 1,232,875 for the Copan dry swab and 884,510 for the wet Bacti-Swab. For specimens that were GASDT negative with both swabs and culture negative with both swabs, the mean RLU were 1,487 for the Copan dry swab and 1,854 for the wet Bacti-Swab.
DISCUSSION
GASDT uses a chemiluminescent probe to detect GAS directly from pharyngeal specimens collected with swabs. Published evaluations performed in our laboratory (1, 3) and other laboratories (2, 4) have all used swabs with liquid transport media. It has been shown that the type of fiber used as well as the sterilization method used for the swabs affects the choice of swab type for the assay (1). A report by another laboratory demonstrated favorable results by GASDT with a swab without any transport media (Wood et al., 39th ICAAC). In their study, the performance of the swab without transport media was equivalent to that of a swab with transport media (wet swabs). This prompted us to conduct a thorough evaluation of the use of swabs without transport media (dry swabs) for GASDT.
Initially, we conducted in vitro experiments using a number of recent clinical isolates of GAS. We measured the quantitative detection of GAS from seeded wet and dry swabs by culture and GASDT. When RLU were measured as a percentage of the values at zero time, RLU as a measure of GAS were more stable over time for dry swabs than for wet swabs, for dry swabs held at 4°C than for dry swabs held at RT, and for wet swabs held at 4°C than for wet swabs held at RT. Lastly, RLU were more stable over time compared to GAS colony counts.
Favorable results with seeded dry swabs prompted us to conduct a clinical evaluation of paired wet and dry swabs. The mean RLU for uninoculated swabs were lower for ethylene oxide-sterilized Copan Dacron swabs than ethylene oxide-sterilized Copan rayon swabs (data not shown), which is why we chose to use Dacron swabs. With specimens from 1,005 patients, if culture is used as the gold standard, culture was more sensitive than GASDT for both the wet and the dry swabs. There were 18 and 12 false-positive GASDT results for the dry swab and wet swabs, respectively. If, however, these 30 results are considered false-negative culture results rather than false-positive GASDT results, the number of samples with true-positive results by each of the methods would be 200 by GASDT with wet swabs, 218 by culture with wet swabs, 213 by GASDT with dry swabs, and 215 by culture with dry swabs. If all of these results are accepted as true-positive test results, there is no significant difference between the sensitivity of GASDT with dry swabs and culture with dry swabs, while GASDT with wet swabs is significantly less sensitive than culture with wet swabs.
Several arguments can be advanced to support the argument that some or all of the apparent false-positive GASDT results are indeed false-negative culture results. The data with seeded specimens, both with and without added PF, demonstrated significantly higher RLU for the dry swabs than for the wet swabs at five of the six time points measured. Moreover, the actual mean RLU (data not shown) for the dry swabs were about 50% higher for the dry swabs than for the wet swabs at time zero, presumably due to the dilution effect of the transport media for the wet swabs. We postulated that there should be similarly higher RLU for the dry swabs compared to those for the wet swabs for the specimens used in the clinical study. Indeed, the results of the clinical evaluation support that contention. For the 178 specimens that were considered to have true-positive GASDT results with both swabs and by using culture as the gold standard, the mean RLU were 884,510 for the wet swabs and 1,232,875 for the dry swabs, a 39% difference. Therefore, we would have anticipated more true-positive GASDT results with the dry swabs than with the wet swabs. When a positive culture from either swab (Table 1) is used as the gold standard, 194 wet swabs and 200 dry swabs had true-positive GASDT results, which is not a significant difference. However, if all of the specimens with positive GASDT results and/or positive culture results are considered to have true-positive results, 200 wet swabs and 213 dry swabs had true-positive GASDT results, a significant difference.
The occurrence of false-negative culture results can also be demonstrated by looking at the specimens with GASDT-positive and culture-negative results with one swab but a positive culture result with the paired swab. Of the 18 dry swabs that were culture negative and GASDT positive, 5 of the paired wet swabs were culture positive. Of the 12 wet swabs that were culture negative and GASDT positive, 6 of the paired dry swabs were culture positive. This supports the contention that there can be true-positive GASDT results with swabs that are culture negative for GAS.
When the RLU for the 15 specimens that were GASDT positive and culture negative with both swabs are examined (Table 2), only 1 specimen had greater than 106 RLU. Bearing in mind that the cutoff between positive and negative results is 4,500 RLU, the values for most of the false-positive results are well above the cutoff, although they are much lower than the mean values of 1,232,875 RLU for dry swabs and 884,510 RLU for wet swabs that had true-positive GASDT results with both swabs. In our experience with GASDT, smaller values of RLU are associated with lower numbers of GAS. We would anticipate that specimens that were culture negative but GASDT positive would have lower RLU, which is exactly what was observed with these specimens.
Other than GASDT, the only other commercially available molecular analysis-based test for GAS is the LightCycler Strep-A assay, which uses a real-time PCR method to detect GAS from throat swab specimens. Uhl and colleagues (5) reported that 7 specimens were positive only by the LightCycler Strep-A assay and culture negative, while 51 specimens were positive by both methods and 4 were positive by culture only. They proposed that, on the basis of clinical signs and symptoms consistent with GAS disease, the seven specimens positive only by the LightCycler Strep-A assay were true positive.
The lack of an accepted alternative to culture as the gold standard may preclude a determination that a molecular analysis-based test has a sensitivity that is equal to or superior to that of culture. Much as occurred with testing for Chlamydia trachomatis, it may be necessary to establish a molecular standard to define a true-positive test result. A study that compared the performances of GASDT and the LightCycler Strep-A assay with that of culture might permit the establishment of such a molecular analysis-based standard.
Another interesting observation from this study that merits additional study is the performance of GASDT with specimens held or stored at RT compared to those of specimens held at 4°C. By using seeded specimens, the mean RLU were significantly higher for dry swabs held at 4°C than for dry swabs held at RT.
Lastly, the differences between GASDT and culture for the detection of GAS in the seeded specimens were striking. Admittedly, these studies were performed with seeded specimens; however, we know of no way in which a similar experiment could be performed with clinical specimens. After 3 days of storage, the RLU detected were 85 and 88% of the values at zero time for dry swabs with and without PF, respectively whereas colony counts were 13 and 5% of the values at zero time for wet swabs with and without PF, respectfully. Moreover, in separate experiments that measured RLU only, the values detected after 9 days as a percentage of those at zero time were 59% for dry swabs held at 4°C and 33% for dry swabs held at RT. These results suggest that GASDT offers significant advantages over culture for specimens that experience substantial transport times. This is an important consideration for core and reference laboratories.
In conclusion, using seeded specimens, we have demonstrated that dry swabs perform better than wet swabs in GASDT. In an evaluation that compared the performances of dry and wet swabs in GASDT for 1,005 patient specimens, the results of GASDT with dry and wet swabs were equivalent to each other when compared with the culture results. However, if all GADST-positive results are interpreted as true-positive results, the performance of GASDT with dry swabs is equivalent to that of culture and superior to that of GASDT with wet swabs. We believe that the routine use of dry swabs rather than wet swabs for GASDT could increase the sensitivity of the test while substantially lowering the cost of the transport devices.
REFERENCES
- 1.Bourbeau, P. P., and B. J. Heiter. 2003. Evaluation of Copan swabs with liquid transport media for use in the Gen-Probe Group A Strep Direct Test. J. Clin. Microbiol. 41:2686-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapin, K. C., P. Blake, and C. D. Wilson. 2002. Performance characteristics and utilization of rapid antigen test, DNA probe, and culture for detection of group A streptococci in an acute care clinic. J. Clin. Microbiol. 40:4207-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heiter, B. J., and P. P. Bourbeau. 1993. Comparison of the Gen-Probe Group A Streptococcus Direct Test with culture and a rapid streptococcal antigen detection assay for diagnosis of streptococcal pharyngitis. J. Clin. Microbiol. 31:2070-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pokorski, S. J., E. A. Vetter, P. C. Wollan, and F. R. Cockerill. 1994. Comparison of Gen-Probe Group A Streptococcus Direct Test with culture for diagnosing streptococcal pharyngitis. J. Clin. Microbiol. 32:1440-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhl, J. R., S. C. Adamson, E. A. Vetter, C. D. Schleck, W. S. Harmsen, L. K. Iverson, P. J. Santrach, N. K. Henry, and F. R. Cockerill. 2003. Comparison of LightCycler PCR, rapid antigen immunoassay, and culture for the detection of group A streptococci from throat swabs. J. Clin. Microbiol. 41:242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]