Introduction
Reactive perforating collagenosis (RPC) is the most common type of acquired perforating dermatoses (APD), [1] and is characterized by umbilicated papules and plaques with central crusted ulceration. Pruritus is the most common symptom of RPC, and the lesions are most commonly found on the extensor surfaces of the extremities. This entity is histopathologically characterized by invagination of the epidermis and transepidermal elimination of collagen bundles. The pathogenesis of RPC is still somewhat unclear, but it has been reported that trauma resulting from scratching may induce damage to the epidermis or dermal collagen [1,2]. Moreover, RPC is commonly found in association with chronic renal failure and diabetes mellitus.
Dermoscopy is a noninvasive technique that has been used in the diagnosis of APD [3], especially for perforating folliculitis (PF). However, to our knowledge, the dermoscopic features of RPC have not been previously described in the literature. Here, we report the dermoscopic features and their histological correlations in RPC as a means to improve the diagnosis of this condition.
Case presentation
A 46-year-old Thai woman presented with a one-month history of multiple pruritic ulcerated papules and plaques with yellowish crust on the upper and lower extremities (Figure 1). Differential diagnoses included prurigo nodularis, perforating granuloma annulare, factitious disorder, and other acquired reactive perforating dermatosis. Dermoscopy showed a yellowish-brown structureless area in the center, a whitish rim and pink-white structureless area, and hairpin vessels observed at the periphery (Figure 2). She had several underlying diseases, including diabetes mellitus and chronic renal failure. She was on hemodialysis three times a week. A perforating disorder seemed to be the most probable because of these clinical and dermoscopic findings. Prurigo nodularis and perforating granuloma annulare would show non-ulcerating red papules and nodules as main features and factitious disorder would show more linearly or irregularly shaped lesions. However, dermoscopic features of these conditions were not previously reported.
Figure 1A.

Multiple ulcerated papules and plaques with yellowish crust on the upper and lower extremities. [Copyright: ©2015 Kittisak et al.]
Figure 2.
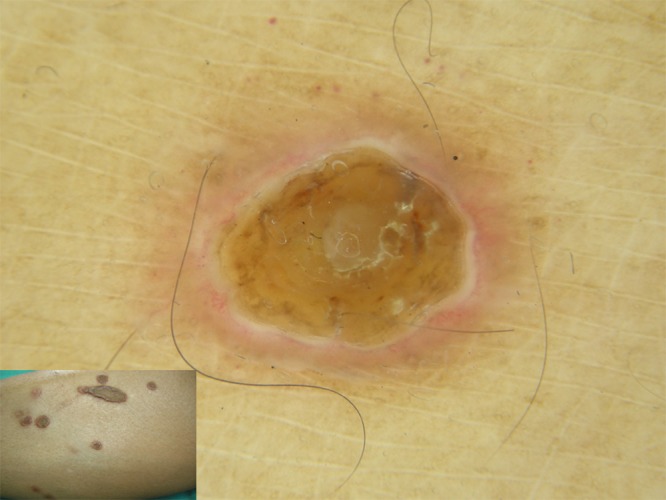
Dermoscopy showing a yellowish-brown structureless area in the center and a white rim with an erythematous halo at the periphery of the lesion. [Copyright: ©2015 Kittisak et al.]
A biopsy from an ulcerated papule on the thigh showed perforation through the epidermis forming a tunnel filled with cellular debris, covered with scale-crust. Thick collagen fibers were observed perforating through this tunnel. The area of perforation was not connected to the hair follicles. The dermis showed proliferation of small blood vessels and mild perivascular inflammatory cell infiltrate comprising lymphocytes and a few neutrophils (Figure 3). Masson-trichrome staining showed transepidermal elimination of the collagen bundles. Based on these clinical and histological features, a diagnosis of RPC was established.
Figure 3.
(A) Cup–shaped ulceration with basophilic crust (hematoxylin and eosin, ×4). (C) Perforation through the epidermis forming a tunnel filled with cellular debris, covered with scale-crust. Thick collagen fibers were perforating through this tunnel (hematoxylin and eosin, ×10). (B, D) Transepidermal elimination of the collagen bundles (Masson-trichrome stain, ×4 and ×10). [Copyright: ©2015 Kittisak et al.]
Conclusions
The pathogenesis of RPC is still unknown but it has been hypothesized that inherited susceptibility, diabetic vasculopathy, and hypoxic conditions may be the predisposing factors [4]. Although recent reports suggest that allopurinol might be a good therapeutic option for RPC in some patients, treatment of RPC is difficult and still no standard therapy is available [4–6]. The present case was treated with antihistamine and emollients and relieved the symptom.
In the present case report, the dermoscopy-pathology correlations were evaluated in RPC (Figure 4). The homogeneous yellowish-brown structureless area observed at the center of the lesion was found to correspond to the scale-crust; the whitish rim to the epidermal invagination, the thickness of which varied in some areas; and the pink-white halo seemed to correspond to a combination of small blood vessels surrounding the lesion. Further, the hairpin vessels at the periphery corresponded to the irregular vessels in the papillary dermis at the periphery of the lesion.
Figure 4.

The homogeneous yellowish-brown structureless area at the center of the lesion corresponding to the scale-crust; the whitish ring-shaped rim to the epidermal invagination (EI), the thickness of which varied; and the pink-white halo seemed to correspond to a combination of small blood vessels surrounding the lesion. [Copyright: ©2015 Kittisak et al.]
Ramirez-Fort et al. first described the dermoscopic feature of APD in 2013, and confirmed the diagnosis of PF by clinical and histopathological examinations [3]. The dermoscopic features described included bright white clods and a structureless gray area surrounded by brown reticular lines. Although RPC is included in APD, the clinical, dermoscopic, and histological features are substantially different from those of PF. We speculate that the differences between APD and PF reported by Ramirez-Fort and those between RPC and PF reported herein are mainly owing to the different sizes of the lesion and due to the differences in ethnicity between the reported cases.
In conclusion, the dermoscopic features of RPC include central yellowish-brown structureless areas and a whitish rim with a pinkish white halo, as well as some hairpin vessels at the periphery. These features seem to contribute to the diagnosis of RPC.
Figure 1B.
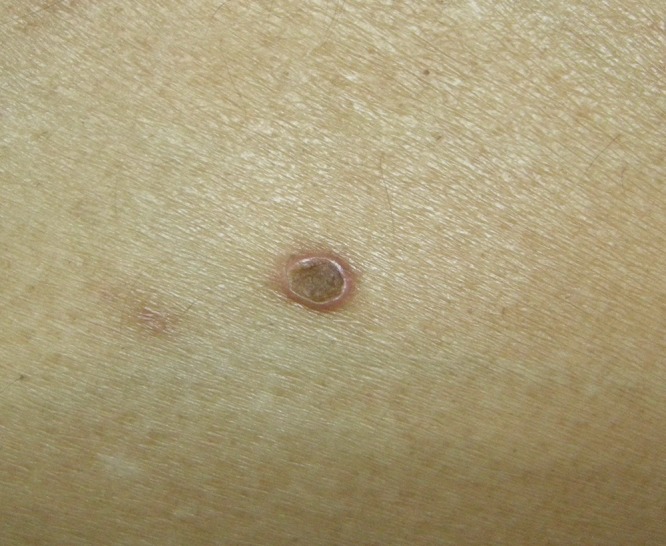
A close up photo of the lesion. [Copyright: ©2015 Kittisak et al.]
Footnotes
Funding: None.
Competing interests: The authors declare no conflict of interest.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26(2):162–71. doi: 10.5021/ad.2014.26.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11(8):723–9. doi: 10.1111/ddg.12131. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez-Fort MK, Khan F, Rosendahl CO, et al. Acquired perforating dermatosis: a clinical and dermoscopic correlation. Dermatol Online J. 2013;19(7):18958. [PubMed] [Google Scholar]
- 4.Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88(1):94–7. doi: 10.1590/S0365-05962013000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krüger K, Tebbe B, Krengel S, et al. Acquired reactive perforating dermatosis. Successful treatment with allopurinol in 2 cases. Hautarzt. 1999;50(2):115–20. doi: 10.1007/s001050050874. [DOI] [PubMed] [Google Scholar]
- 6.Karpouzis A, Giatromanolaki A, Sivridis E, Kouskoukis C. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37(7):585–92. doi: 10.1111/j.1346-8138.2010.00918.x. [DOI] [PubMed] [Google Scholar]



