Abstract
We report the case of a 37-year-old woman (phototype II) who presented at our outpatient clinic with a two-month history of hyperpigmented plantar macules. Medical history revealed that the patient had taken capecitabine in the past three months as adjuvant chemotherapy for recurrent breast cancer. Dermoscopic examination of the plantar macules showed parallel ridge pattern with pigmentation in the furrows without obliteration of eccrine gland apertures. Besides in acral melanoma, parallel ridge pattern can also be observed in benign plantar lesions, such as congenital or acquired acral nevi, subcorneal hemorrhage, dye-related pigmentation and drug-induced hyperpigmentation, especially in patients with phototypes III–VI. The few reported cases of capecitabine-induced hyperpigmentation have been associated with hand and foot syndrome in patients with phototypes IV–V and palmar as well as plantar involvement.
Keywords: dermoscopy, capecitabine, plantar, hyperpigmentation
Introduction
Capecitabine (Xeloda®) is an orally administered antineoplastic pro-drug, which in tumour tissue is preferentially converted to 5-fluorouracil (5-FU) by thymidine phosphorylase (TP) [1]. It is used as adjuvant chemotherapy for metastatic breast and colon cancer [2]. Common dose-limiting side-effects of capecitabine include hyperbilirubinemia, nausea, vomiting, diarrhoea, bone marrow suppression and hand and foot syndrome (HFS) [1–3]. Capecitabine-induced skin pigmentation may occur in the context of skin manifestations of HFS [4–9].
Case report
A 37-year-old woman (phototype II) presented with irregular pigmented macules in the plantar regions. The lesions were roundish in shape, 0.2–0.5 cm in diameter and light to dark brown in colour with poorly defined margins (Figure 1). They appeared nearly two months before our observation. No other body sites were involved. Dermoscopic examination showed parallel ridge pattern with preservation of eccrine gland apertures (Figure 2). Clinical history revealed that the patient had taken capecitabine (Xeloda®) in the last two months as adjuvant chemotherapy for recurrent breast cancer. Hyperpigmentation was noticed after two cycles of therapy (cycles consisted of 1250 mg/m2 twice daily for two weeks, separated by a 7-day interval). Assuming that capecitabine was responsible for the lesions, we enquired whether the patient had ever previously had signs or symptoms of HFS. The patient replied that she had never previously experienced paresthesia, burning pain, erythema, swelling, blistering, desquamation or ulceration of the feet.
Figure 1.
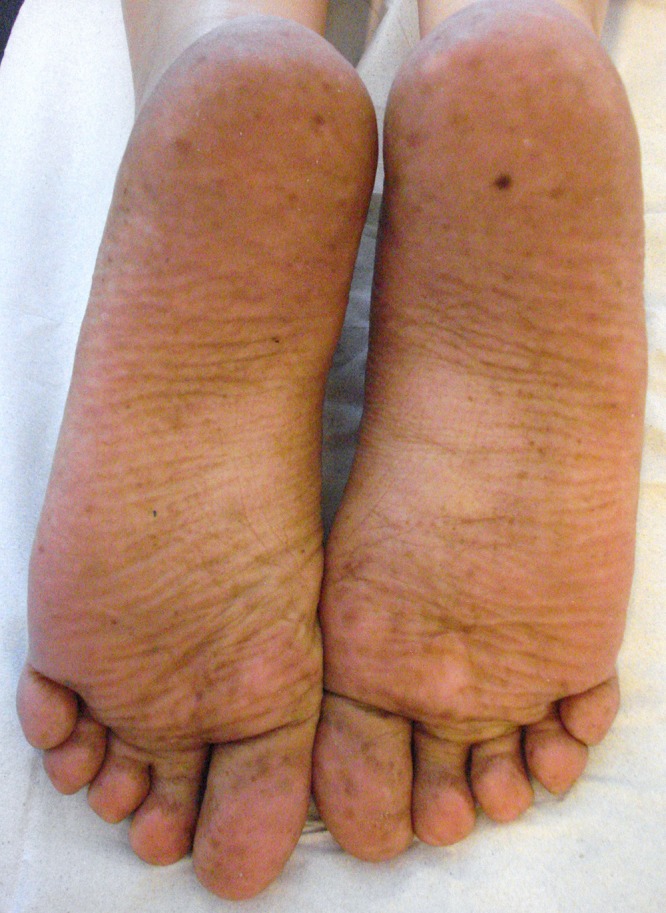
Diffuse hyperpigmentation of the feet localized on the soles at presentation time. [Copyright: ©2015 Tognetti et al.]
Figure 2.
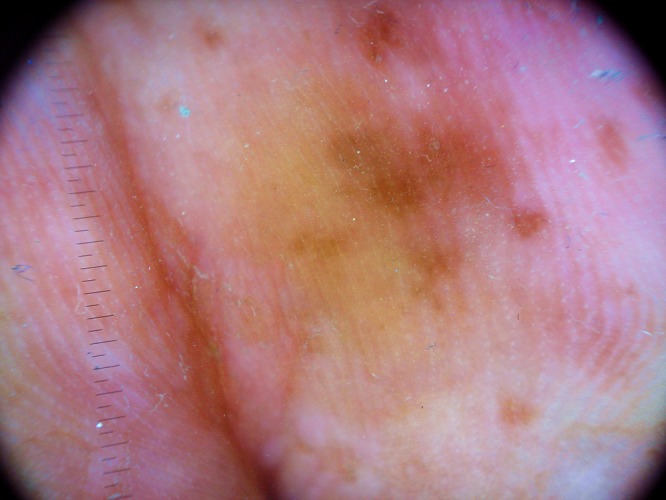
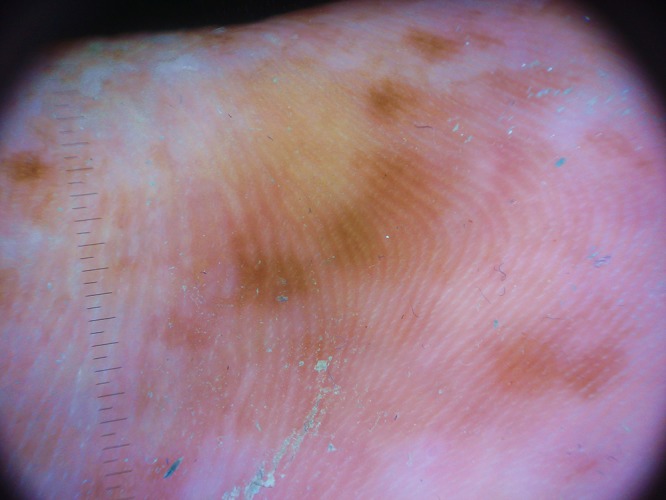
(A, B) Dermoscopic observation showing mottled pigmentation areas with parallel ridge pattern. (B) Pigmentation spares the acrosyringia aperture of the eccrine glands. [Copyright: ©2015 Tognetti et al.
Discussion
To our knowledge, very few cases of capecitabine-induced hyperpigmentation have been reported [6–13], and have mainly been in patients with phototype IV or V (Indians [8,9], Africans [5,6,10], Asians and Aborigines [8]), with only one case in a patient with phototype III [7], suggesting a racial predisposition [13]. In all eleven cases, hyperpigmentation involved palmar and plantar skin [7,8,10,11]; the oral mucosa was also involved in five cases (four Africans and one Indian) [5,6,9]. In all cases, hyperpigmentation preceded HFS6 [8–11], or was associated with palmo-plantar dysesthesia [5,7]. Indeed, many authors regard hyperpigmentation as one of the initial manifestation of HFS (grade 1 HFS) [7–9]. By contrast, we observed capecitabine-induced hyperpigmentation limited to the plantar regions in a phototype II patient without any history, sign or symptom of HFS. Hyperpigmentation is a well-known consequence of the antiproliferative drug 5-fluorouracil (a capecitabine precursor), generally occurring when therapy is administered intravenously. In such cases, hyperpigmentation usually occurs on photo-exposed skin (photomediated pathogenetic mechanism) [12]. However, our patient presented lentiginous hyperpigmentation on photoprotected areas, and there was no history of sun exposure since the start of the therapy.
Palms and soles are considered more sensitive to cytotoxic drugs due to the high proliferation rate of epidermal basal cells. In addition, there is evidence that capecitabinemetabolizing enzymes (i.e., TP and dihydropyrimidine dehydrogenase) are not only expressed in tumour tissue but also in palmar and plantar skin [3,4]. This suggests that drug metabolism was involved in our case more than racial predisposition or photo-sensitization. However, further studies are needed to assess the exact pathogenesis of hyperpigmentation induced by antiproliferative drugs manifesting in photoprotected areas in patients with phototypes I–II.
Dermoscopic findings from acral pigmented lesions are sometimes confusing. In recent years, various authors have highlighted parallel ridge pattern not only in acral melanoma but also in certain benign plantar lesions, such as congenital or acquired acral nevi, subcorneal hemorrhage, dye-related pigmentation and drug-induced hyperpigmentation, especially in patients with phototypes III–VI [13–17]. In particular, Fracaroli et al emphasised the importance of obliteration of eccrine gland ducts by neoplastic proliferation of melanocytes in distinguishing malignant and benign acral pigmented lesions with parallel ridge pattern [13]. Indeed, the coexistence these two dermoscopic signs (i.e., parallel ridge pattern and acrosyringium obliteration) is considered highly indicative of acral melanoma. However, it should be borne in mind that these two signs can also be detected in benign lesions, such as plantar hemorrhage (e.g., “black heel”), dye-related pigmentation and, as in the present case, drug-induced hyperpigmentation.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27(1):23–44. doi: 10.1016/j.clinthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Mikhail SE, Sun JF, Marshall JL. Safety of capecitabine: a review. Expert Opin Drug Saf. 2010;9(5):831–41. doi: 10.1517/14740338.2010.511610. [DOI] [PubMed] [Google Scholar]
- 3.Saif MW. Capecitabine and hand-foot syndrome. Expert Opin Drug Saf. 2011;10(2):159–69. doi: 10.1517/14740338.2011.546342. [DOI] [PubMed] [Google Scholar]
- 4.Milano G, Etienne-Grimaldi MC, Mari M, et al. Candidate mechanisms for capecitabine-related hand–foot syndrome. Br J Clin Pharmacol. 2008;66(1):88–95. doi: 10.1111/j.1365-2125.2008.03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pui JC, Meehan S, Moskovits T. Capecitabine induced cutaneous hyperpigmentation: report of a case. J Drugs Dermatol. 2002;1(2):202–5. [PubMed] [Google Scholar]
- 6.Narasimhan P, Narasimhan S, Hitti IF, Rachita M. Serious hand-and-foot syndrome in black patients treated with capecitabine: report of 3 cases and review of the literature. Cutis. 2004;73(2):101–6. [PubMed] [Google Scholar]
- 7.Vázquez-Bayo C, Rodríguez-Bujaldón AL, Jiménez-Puya R, et al. Hiperpigmentación secundaria a capecitabina. Actas Dermosifiliogr. 2007;98(7):491–3. [PubMed] [Google Scholar]
- 8.Vickers MM, Easaw JC. Palmar-plantar hyperpigmentation with capecitabine in adjuvant colon cancer. J Gastrointest Cancer. 2008;39(1–4):141–3. doi: 10.1007/s12029-009-9068-9. [DOI] [PubMed] [Google Scholar]
- 9.Vasudevan B. An unusual case of capecitabine hyperpigmentation: is hyperpigmentation a part of hand-foot syndrome or a separate entity? Indian J Pharmacol. 2010;42(5):326–28. doi: 10.4103/0253-7613.70401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Tienhoven G, Wilmink JW. A woman with palmar and plantar hyperpigmentation. Ned Tijdschr Geneeskd. 2011;155(45):A4100. [PubMed] [Google Scholar]
- 11.Agharbi FZ, Meziane M, Benhemmne H. Capecitabine-induced hyperpigmentation followed by hand-foot syndrome: a new case report. Ann Dermatol Venereol. 2012;139(3):221–22. doi: 10.1016/j.annder.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103(3):231–35. doi: 10.1097/SMJ.0b013e3181ce0f5e. [DOI] [PubMed] [Google Scholar]
- 13.Scalfoni Fracaroli T, Pineiro Maceira J, Guedes Lavorato F, Barcaui C. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88(4):646–8. doi: 10.1590/abd1806-4841.20132058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147(7):809–13. doi: 10.1001/archdermatol.2011.150. [DOI] [PubMed] [Google Scholar]
- 15.Tanioka M. Benign acral lesion showing parallel ridge pattern on dermoscopy. J Dermatol. 2011;38(1):41–4. doi: 10.1111/j.1346-8138.2010.01128.x. [DOI] [PubMed] [Google Scholar]
- 16.Phan A, Dalle S, Marcilly MC, Bergues JP, Thomas L. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147(5):634. doi: 10.1001/archdermatol.2011.47. [DOI] [PubMed] [Google Scholar]
- 17.Kilinc Karaarslan I, Akalin T, Unal I, Ozdemir F. Atypical melanosis of the foot showing a dermoscopic feature of the parallel ridge pattern. J Dermatol. 2007;34(1):56–9. doi: 10.1111/j.1346-8138.2007.00217.x. [DOI] [PubMed] [Google Scholar]


