Abstract
Infantile perianal pyramidal protrusion, it is a rare benign cutaneous condition described in relatively recent times. It is considered to be under-reported in the pediatric literature because it is often mistaken for other conditions. The unawareness of this lesion may be responsible for an excessive concern both in physician and in parents, which leads to overly aggressive and unnecessary treatments. Thus its recognition has many implications regarding proper management and treatment.
We report a typical presentation of IPPP in which the diagnosis was based on the use of non-invasive diagnostic tools and in particular of dermoscopy and ultrasonography.
Keywords: dermoscopy, perianal, protrusion
Introduction
The term infantile perianal pyramidal protrusion (IPPP) was first proposed by Kayashima et al in 1996 to describe a rare benign condition characterized by a pyramidal protrusion on the midline of the perineum, anterior to the anus [1]. Since its initial description, 108 cases have been reported in the literature. The condition is not believed to be as rare, but it is often erroneously interpreted as a vascular or anatomical anomaly or sometimes even an outcome of sexual abuse [2].
Here we report a case of IPPP in baby girl studied by dermoscopy and ultrasonography.
Case report
A healthy 9-month-old Caucasian girl presented with a two-month history of edematous perianal protrusion. No anatomical defect was present at birth. Medical history included intermittent constipation that was untreated. There was no suspicion of sexual abuse and no history of maternal cervical dysplasia or condyloma. Family history was positive for ulcerative colitis (father). Physical examination revealed a solitary, edematous, light-red, tongue-like, sessile projection with diameters of 3×5 mm, located on the midline, just anterior to the anus. It had soft elastic consistency to palpation (Figure 1). There was no inguinal lymphadenopathy, pain or other symptoms.
Figure 1.

Clinical appearance: a solitary pyramidal light-red skin protrusion, located anterior to the anus. [Copyright: ©2015 Lamberti et al.]
Dermoscopic evaluation showed patchy structureless white areas and a vascular pattern composed of red globular and dotted vessels (Figure 2). Ultrasound examination of the lesion with a high frequency linear probe (18 MHz, Esaote Mylab 70XVG device, Genoa, Italy), gray scale and power Doppler analysis revealed a thick hypoechoic area of skin under which increased power Doppler signal indicated hypervascularization (Figure 3). Vascular and anatomical anomalies were therefore excluded. We diagnosed infantile perianal pyramidal protrusion and no treatment was suggested, but only dietary modifications and laxatives to facilitate intestinal transit. Two months later the lesion had completely regressed.
Figure 2.
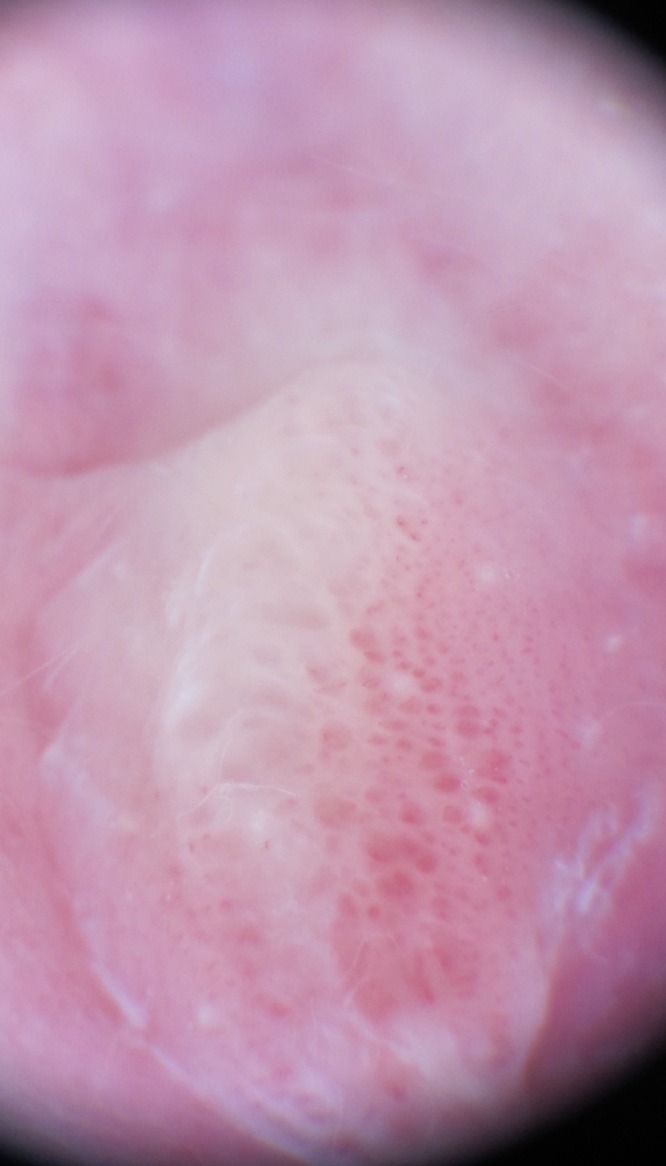
Dermoscopic image: patchy structureless whitish areas and a vascular pattern composed of red globular and dotted vessels. [Copyright: ©2015 Lamberti et al.]
Figure 3.
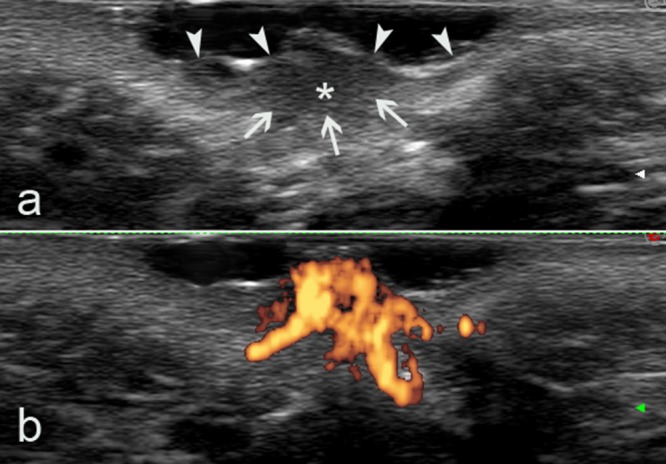
(A) Gray-scale ultrasound image clearly showing a thickened hypoechoic area (asterisk) due to inflammation. The deep margins of the lesion are not well defined (arrows), nor is the dermalsubdermal interface. Arrowheads indicate the epidermal interface. (B) Power Doppler ultrasound image showing an evident increase in PD signal in the hypoechoic area, confirming high blood flow, probably due to vessel dilation due to inflammation. [Copyright: ©2015 Lamberti et al.]
Discussion
Infantile perianal pyramidal protrusion (IPPP) typically appears as a skin protuberance, usually situated anterior to the anus. Location and shape vary widely and may be peanut-like, tongue-like or leaf-like, posterior or concomitantly anterior and posterior to the anus [3,4]. Size ranges from 5 to 30 mm2 and the overlying skin is usually pink or pale red with a smooth surface. It is distinguished from acrochordons or skin folds on the basis of clinical features. IPPP is most often observed at birth, with strong female predominance, presumably related to being more visible in females. The pathogenesis of IPPP is unclear. Three types are currently recognized: a) constitutional IPPP (congenital and/or familial), b) acquired IPPP, and c) IPPP due to lichen sclerosus et atrophicus (LSA) [3]. To better understand this classification, it is useful to report some basic anatomical concepts. The perineum is a muscle-fascial formation, which closes the bottom of the pelvic floor. It is characterized by points of weakness both median and on the side, represented by spaces existing between the muscle bundles. According to the rhomboidal shape of perineum, we can distinguish two triangular portions divided by the transverse perinei muscle. The anterior perineal triangle, or urogenital perineum, differs widely in the two sexes; the posterior triangle, or anal perineum is very similar in both males and females [5]. Constitutional IPPP develops due to a pelvic floor and perineal weak points. Congenital IPPP may be a remnant of a projecting tip of the urogenital septum. Sometimes it may be found in other members of the family [2]. In acquired IPPP, constipation seems to play a relevant role because the lesion regresses after appropriate management, though it is not clear whether regression is due to cessation of constipation or whether constipation causes development of IPPP. Diarrhea, fistulas and fissures may also be implicated in the formation of IPPP through mechanical stimulation of the perineum [6,7]. Lichen sclerosus et atrophicus associated IPPP is a skin protrusion similar to those observed in other types mentioned above but is histopathologically different. Some authors suggested that IPPP might be a peculiar form of LSA, probably an early manifestation, although in other cases it might coexist with LSA [8,9].
Histologic findings of IPPP, except in patients with LSA associated IPPP (in which were observed features suggestive of lichen sclerosus, such as patchy lichenoid infiltrates with vacuolar alteration and homogenization areas of the collagen in the papillary dermis), may show epidermal acanthosis, upper dermal edema, dilated capillaries with fibrous tissue elements infiltrated by lymphocytes and eosinophils in the dermis (especially upper), or an almost normal picture [2,9].
The differential diagnosis of IPPP includes rectal prolapse, perianal lesions of Crohn’s disease, hemangiomas and hemorrhoids or even sexual abuse [2,10,11]. In our opinion, the majority of cases can be resolved by medical history and physical examination. In doubtful cases ultrasonography (US) and dermoscopy may offer additional help in that biopsy and histological examination could be avoided [12,13]. In particular, US remains the most useful modality for imaging pediatric genital organs. Doppler US and color Doppler imaging allow rapid identification of normal vessels and abnormal vascular structures [14,15]. With sonography, hemangiomas are usually poorly defined solid masses that vary in their echogenicity and vascularization according to their phase. In the early phase, they tend to be hypoechoic and hypervascular, showing arterial and venous flow and sometimes arteriovenous shunts [15]. Vascular malformations can usually be detected as anechoic tubules (e.g., arterial or venous), pseudocystic spaces (e.g., venous or lymphatic), or hyperechoic areas (e.g., capillary) depending on the type of vessel [16]. Perianal fluid collections/abscesses can be detected as oval shaped hypo- to anechoic masses, which were demarcated off the regular tissue by a hypoechoic seam. In addition, a fistulous connection to the rectum could clearly be visualized [16,17]. In our case, the hypoechoic area associated with increased power Doppler signal (indicating increased blood flow, suggested by the dermoscopy image) is a sign of intense inflammation of presumably functional origin.
Dermoscopy is a simple, non-invasive method enabling observation in vivo of superficial morphological skin characters, impossible to observe with the naked eye. Its applications have multiplied in recent years, extending to non-pigmented skin lesions as well as infectious and inflammatory skin diseases [13]. It has therefore gained a more prominent position in routine clinical practice and now increasingly accompanies clinical examination [18,19]. In our case, dermoscopy revealed patchy structureless white areas consistent with fibrosis and a vascular pattern composed of red globular and dotted vessels correlating to the variably dilated capillaries [13,18,19]. The dermoscopic appearance, in addition to excluding the presence of vascular tumors, is different from that observed in lichen sclerosus involving perineum, which some authors believe is involved in the pathogenesis of IPPP. In particular, in the latter cases, linear vessels appear as anastomosing, branching dull red telangiectasias of different caliber and size, whereas dotted vessels appear as randomly arranged and loosely aggregated red dots. Moreover, scales and keratotic plugs are often seen in cases of lichen sclerosus which involve the perineum [9].
Dietary modification may be tried and no other treatment is required for functional IPPP. A conservative approach is indicated because IPPP usually resolves spontaneously within several weeks [6,7].
In conclusion, if physicians are aware of IPPP as a distinct cutaneous condition, unnecessary or invasive investigation procedures can be avoided.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Kayashima K, Kitoh M, Ono T. Infantile perianal pyramidal protrusion. Arch Dermatol. 1996;132(12):1481–4. [PubMed] [Google Scholar]
- 2.Zavras N, Christianakis E, Tsamoudaki S, et al. Infantile perianal pyramidal protrusion: a report of 8 new cases and a review of the literature. Case Rep Dermatol. 2012;4(3):202–6. doi: 10.1159/000342954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleet SL, Davis LS. Infantile perianal pyramidal protrusion: report of a case and review of the literature. Pediatr Dermatol. 2005;22(2):151–2. doi: 10.1111/j.1525-1470.2005.22213.x. [DOI] [PubMed] [Google Scholar]
- 4.Leung AK. Concomitant anterior and posterior infantile perianal protrusions. J Natl Med Assoc. 2010;102(2):135–6. doi: 10.1016/s0027-9684(15)30514-9. [DOI] [PubMed] [Google Scholar]
- 5.Kravarusic D, Swartz M, Freud E. Perineal hernias in children: case report and review of the literature. Afr J Paediatr Surg. 2012;9(2):172–5. doi: 10.4103/0189-6725.99411. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto T, Inoue S, Hagari Y, et al. Infantile perianal pyramidal protrusion with hard stool history. Br J Dermatol. 2004;151(1):229. doi: 10.1111/j.1365-2133.2004.06005.x. [DOI] [PubMed] [Google Scholar]
- 7.Mérigou D, Labrèze C, Lamireau T, et al. Infantile perianal pyramidal protrusion: a marker of constipation? Pediatr Dermatol. 1998;15(2):143–4. doi: 10.1046/j.1525-1470.1998.1998015143.x. [DOI] [PubMed] [Google Scholar]
- 8.Cruces MJ, De La Torre C, Losada A, et al. Infantile pyramidal protrusion as a manifestation of lichen sclerosus et atrophicus. Arch Dermatol. 1998;134(9):1118–20. doi: 10.1001/archderm.134.9.1118. [DOI] [PubMed] [Google Scholar]
- 9.Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, et al. Clinical, dermoscopic and histopathologic features of genital and extragenital lichen sclerosus. J Eur Acad Dermatol. 2013;27:1433–39. doi: 10.1111/j.1468-3083.2012.04595.x. [DOI] [PubMed] [Google Scholar]
- 10.Theiler M, Wälchli R, Weibel L. Vascular anomalies—a practical approach. J Dtsch Dermatol Ges. 2013;11(5):397–405. doi: 10.1111/ddg.12046. [DOI] [PubMed] [Google Scholar]
- 11.Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27(3):279–81. doi: 10.1111/j.1525-1470.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 12.Kleinerman R, Whang TB, Bard RL, et al. Ultrasound in dermatology: principles and applications. J Am Acad Dermatol. 2012;67(3) doi: 10.1016/j.jaad.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Lallas A, Giacomel J, Argenziano G, et al. Dermoscopy in general dermatology: practical tips for the clinician. Br J Dermatol. 2014;170(3):514–26. doi: 10.1111/bjd.12685. [DOI] [PubMed] [Google Scholar]
- 14.Jasaitiene D, Valiukeviciene S, Linkeviciute G, et al. Principles of high-frequency ultrasonography for investigation of skin pathology. J Eur Acad Dermatol Venereol. 2011;25(4):375–82. doi: 10.1111/j.1468-3083.2010.03837.x. [DOI] [PubMed] [Google Scholar]
- 15.Ziereisen F, Guissard G, Damry N, Avni FE. Sonographic imaging of the paediatric female pelvis. EurRadiol. 2005;15:1296–1309. doi: 10.1007/s00330-005-2648-6. [DOI] [PubMed] [Google Scholar]
- 16.Dubois J, Patriquin HB, Garel L, et al. Soft-tissue hemangiomas in infants and children: diagnosis using Doppler sonography. AJR. 1998;171:247–52. doi: 10.2214/ajr.171.1.9648798. [DOI] [PubMed] [Google Scholar]
- 17.Son JK, Taylor GA. Transperineal ultrasonography. Pediatr Radiol. 2014;44:193–201. doi: 10.1007/s00247-013-2789-8. [DOI] [PubMed] [Google Scholar]
- 18.Haliasos EC, Kerner M, Jaimes N, et al. Dermoscopy for the pediatric dermatologist, part ii: dermoscopy of genetic syndromes with cutaneous manifestations and pediatric vascular lesions. Pediatr Dermatol. 2013;30(2):172–81. doi: 10.1111/j.1525-1470.2012.01874.x. [DOI] [PubMed] [Google Scholar]
- 19.Oiso N, Kawada A. The dermoscopic features in infantile hemangioma. Pediatr Dermatol. 2011;28(5):591–3. doi: 10.1111/j.1525-1470.2011.01385.x. [DOI] [PubMed] [Google Scholar]


