Abstract
Bacteria often thrive in natural environments through a sessile mode of growth, known as the biofilm. Biofilms are well-structured communities and their formation is tightly regulated. However, the mechanisms by which interspecies interactions alter the formation of biofilms have not yet been elucidated in detail. We herein demonstrated that a quorum-sensing signal in Pseudomonas aeruginosa (the Pseudomonas quinolone signal; PQS) inhibited biofilm formation by Streptococcus mutans. Although the PQS did not affect cell growth, biofilm formation was markedly inhibited. Our results revealed a unique role for this multifunctional PQS and also indicated its application in the development of prophylactic agents against caries-causing S. mutans.
Keywords: biofilm, quorum sensing, Streptococcus mutans
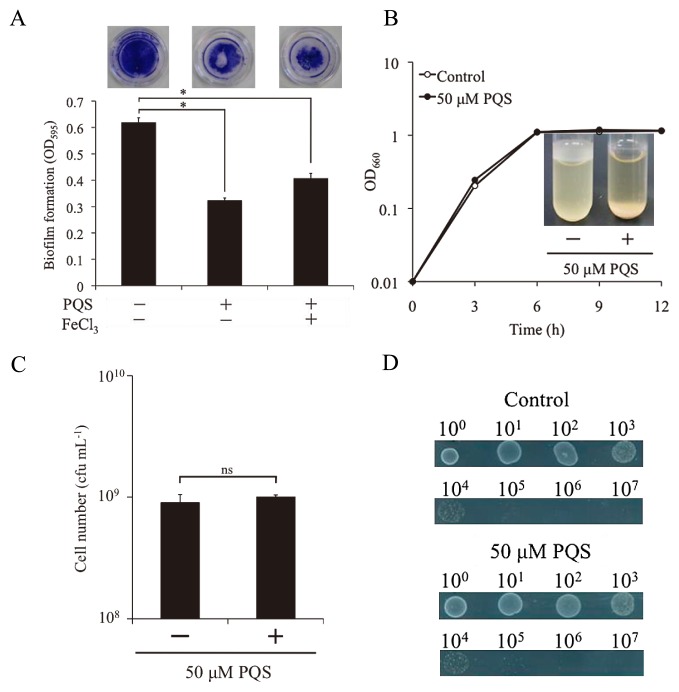
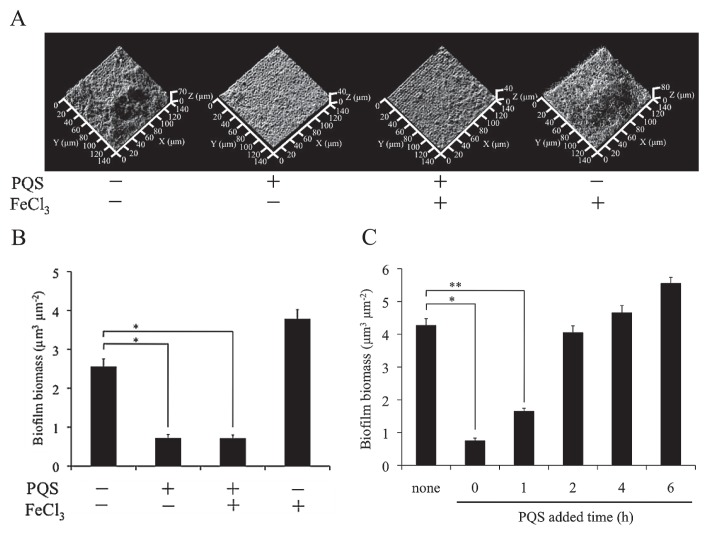
A biofilm is an aggregate of cells that is attached to a surface and enclosed in an extracellular matrix. One of the major problems associated with biofilms is that they can cause chronic infections. A previous study estimated that 65% of all bacterial infections involve biofilms (11). It has also been estimated that more than 500 bacterial species exist in the human oral cavity (4, 10). These diverse bacteria have been suggested to communicate with each other in the oral cavity and establish an oral multispecies biofilm (5). Bacterial signaling molecules were recently shown to have several effects on other bacterial species, including antibiotic activity and an influence on membrane vesicle production (12, 18, 19). Therefore, complex interactions may occur between oral bacteria and other bacteria through signaling molecules. Pseudomonas aeruginosa has been identified as one of the indigenous bacteria in the oral cavity (16). Furthermore, the signaling molecule of P. aeruginosa, the Pseudomonas quinolone signal (PQS), was previously shown to affect other bacterial species (14, 18, 19). However, the effects of the PQS on biofilm formation by other bacterial species have not yet been clarified. By examining the interaction between P. aeruginosa and Streptococcus mutans, we herein demonstrated that the PQS inhibited biofilm formation by S. mutans (Fig. 1A). The growth of S. mutans in liquid and solid media was not affected by the addition of the PQS (Fig. 1B–D). These results suggested that biofilm formation by S. mutans was inhibited by the PQS because the ability of the bacteria to attach to the surface was reduced (Fig. 1B). Microscopic observations also revealed that autoagglutination was not induced by the addition of the PQS (data not shown). Therefore, we tested the effects of the PQS on the formation of biofilms on hydroxyapatite (HA) disk surfaces. Hydroxyapatite is a major component of human teeth and is a useful substratum that experimentally imitates natural oral conditions for biofilm formation assays. The exact nature of the interaction between biofilms and HA needs to be clarified in order to obtain a better understanding of biofilm formation in natural settings. We previously reported that this new approach for observing biofilms allowed for the simultaneous visualization of biofilms and its attached substratum (3, 21). In the present study, we also demonstrated the usability of our observation technique for biofilm-measuring assays. Using the biofilm formation assay, we showed that the formation of biofilms by S. mutans on HA disks with the addition of the PQS was markedly less than that on glass surfaces (Fig. 2A and B). In S. mutans, gene expression levels have been shown to differ in a manner that is dependent on the type of attached surface (17). This finding suggests that the PQS may affect specific genes that play important roles in biofilm formation on HA disks. Moreover, the effects of the PQS on other bacteria were previously attributed to the iron-chelating activity of PQS molecules (8). Therefore, the influence of the chelating activity of the PQS was examined by the addition of iron (III) chloride. The addition of iron (III) chloride did not affect the biofilm inhibitory effects of the PQS (Fig. 1A, 2A and 2B). These results indicated that the inhibition of biofilm formation by the PQS was not caused by its iron-chelating activity; therefore, generation of the PQS by P. aeruginosa may be favorable for survival in the oral cavity. S. mutans uses extracellular polysaccharides to adhere to human teeth (15). Some compounds are known as biofilm inhibitors because of their inhibitory effects on the glycosyltransferase enzymes essential for the synthesis of polysaccharides (1, 6, 7, 9). However, the results of our Congo red assay revealed that the PQS did not affect the amount of extracellular polysaccharide produced, suggesting that activities associated with the synthesis of polysaccharides, such as glucosyltransferase activity, were not inhibited by the PQS (Fig. S1). We then attempted to determine which stages of biofilm formation were inhibited by adding the PQS at 0, 1, 2, 4, and 6 h after inoculation. The inhibition of biofilm formation by the PQS was only observed in the initial stage of biofilm formation (from 0 to 1 h after inoculation) (Fig. 2C). However, a microscopic visualization revealed that the attached cell biomass (1.5 h after inoculation) was not disrupted by the addition of the PQS (data not shown). These results suggested that the PQS inhibited the initial stage of biofilm development, but not initial attachment. In the present study, we showed that PQS inhibited biofilm formation by S. mutans and that it also inhibited biofilm formation without inhibiting cellular growth. These results implied that the development of a biofilm-specific inhibitor without the emergence of resistant strains was possible. Although the PQS is considered to be a quorum-sensing (QS) signal, recent findings have indicated that PQS is multifunctional (14, 18, 19). Our study adds further information for the unique effects of this signal on S. mutans biofilm formation and will contribute to a better understanding of interspecies interactions through chemical signals. S. mutans is known to cause serious infectious diseases, such as infective endocarditis and aspiration pneumonia (13, 20). Therefore, preventing the formation of biofilms by S. mutans is very important for systemic health care. A deeper understanding of the biofilm inhibition mechanism employed by the PQS may contribute to the development of new agents to control biofilm formation by S. mutans.
Fig. 1.
Effects of PQS on biofilm formation, growth, and cellular viability in S. mutans NCIMB702062. (A) Effects of PQS on biofilm formation on glass-based dishes in the presence or absence of iron. Biofilms were stained with crystal violet and quantified by measuring OD595. Biofilms were incubated in tryptic soy broth (TSB medium (Becton Dickinson, Sparks, MD, USA) at 37°C for 12 h. PQS and FeCl3 were added to the cultures at final concentrations of 50 μM, respectively. *P<0.01 (t-test) (B) Growth curve of S. mutans with or without 50 μM PQS. Each tube was incubated in TSB medium at 37°C in a static culture. Pictures of tubes were taken at 12 h. (C) Viable cell counts after a 12-h incubation in liquid TSB medium at 37°C in a static culture. After being incubated, cells were harvested and diluted with saline. A cell suspension was plated on TSB agar and cultured at 37°C for 24 h. Three independent experiments were carried out, and data represented are means ± standard deviations of triplicate assays. ns, the difference was not significant. (D) Growth of S. mutans on TSB medium (1.5% agar) with or without 50 μM PQS. Cells were incubated at 37°C for 24 h. The data shown are representative of three independent experiments.
Fig. 2.
Effects of the PQS on biofilm formation. (A) Observation of the biofilm structure on HA surfaces. The visualization was performed by confocal reflection microscopy. At least five pictures were taken per sample and representative pictures were shown. (B) Quantification of the microscopic image shown in (A). Quantification was performed using the COMSTAT program (2). *P<0.01 (t-test) (C) Effects of the time-of-addition of the PQS on the inhibition of biofilm formation by S. mutans. The PQS was added at the time indicated on the X-axis, and the biofilm was then allowed to form for a total of 12 h. Biofilms were incubated in TSB medium at 37°C for 12 h. The PQS was added to the cultures at a final concentration of 50 μM, and, in panels A and B, FeCl3 was added at a final concentration of 150 μM. respectively. The averages are based on three or more independent determinations, and the standard errors are indicated. *P<0.01, **P<0.05 (t-test). The data shown are representative of three independent experiments.
Supplementary Information
Acknowledgements
We would like to thank Arne Heydorn for kindly providing us with the COMSTAT program. This research was financially supported in part by the Japan Science and Technology Agency, CREST and ALCA, and a Grant-in-Aid for Scientific Research to N. Nomura from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Duarte S, Gregoire S, Singh AP, Vorsa N, Schaich K, Bowen WH, Koo H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol Lett. 2006;257:50–56. doi: 10.1111/j.1574-6968.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 2.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 3.Inaba T, Ichihara T, Yawata Y, Toyofuku M, Uchiyama H, Nomura N. Three-dimensional visualization of mixed species biofilm formation together with its substratum. Microbiol Immunol. 2013;57:589–593. doi: 10.1111/1348-0421.12064. [DOI] [PubMed] [Google Scholar]
- 4.Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 5.Kolenbrander PE, Palmer RJ, Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nature Rev Microbiol. 2010;8:471–480. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- 6.Koo H, Hayashibara MF, Schobel BD, Curry JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemoth. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 7.Koo H, Duarte S, Murata RM, Scott-Anne K, Gregoire S, Watson GE, Singh AP, Vorsa N. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010;44:116–126. doi: 10.1159/000296306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mashburn LM, Jett AM, Akins DR, Whiteley M. Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J Bacteriol. 2005;187:554–566. doi: 10.1128/JB.187.2.554-566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata RM, Branco-de-Almeida LS, Franco EM, Yatsuda R, dos Santos MH, de Alencar SM, Koo H, Rosalen PL. Inhibition of Streptococcus mutans biofilm accumulation and development of dental caries in vivo by 7-epiclusianone and fluoride. Biofouling. 2010;26:865–872. doi: 10.1080/08927014.2010.527435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potera C. Forging a link between biofilms and disease. Science. 1999;283:1837–1839. doi: 10.1126/science.283.5409.1837. [DOI] [PubMed] [Google Scholar]
- 12.Reen FJ, Mooij MJ, Holcombe LJ, McSweeney CM, McGlacken GP, Morrissey JP, O’Gara F. The Pseudomonas quinolone signal (PQS), and its precursor HHQ, modulate interspecies and interkingdom behaviour. FEMS Microbiol Ecol. 2011;77:413–428. doi: 10.1111/j.1574-6941.2011.01121.x. [DOI] [PubMed] [Google Scholar]
- 13.Sarin J, Balasubramaniam R, Corcoran AM, Laudenbach JM, Stoopler ET. Reducing the risk of aspiration pneumonia among elderly patients in long-term care facilities through oral health interventions. J Am Med Dir Assoc. 2008;9:128–135. doi: 10.1016/j.jamda.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Schertzer JW, Boulette ML, Whiteley M. More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol. 2009;17:189–195. doi: 10.1016/j.tim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Schilling KM, Bowen WH. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992;60:284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senpuku H, Sogame A, Inoshita E, Tsuha Y, Miyazaki H, Hanada N. Systemic diseases in association with microbial species in oral biofilm from elderly requiring care. Gerontology. 2003;49:301–309. doi: 10.1159/000071711. [DOI] [PubMed] [Google Scholar]
- 17.Shemesh M, Tam A, Aharoni R, Steinberg D. Genetic adaptation of Streptococcus mutans during biofilm formation on different types of surfaces. BMC Microbiol. 2010;10:51. doi: 10.1186/1471-2180-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tashiro Y, Ichikawa S, Nakajima-Kambe T, Uchiyama H, Nomura N. Pseudomonas quinolone signal affects membrane vesicle production in not only gram-negative but also gram-positive bacteria. Microbes Environ. 2010;25:120–125. doi: 10.1264/jsme2.me09182. [DOI] [PubMed] [Google Scholar]
- 19.Toyofuku M, Nakajima-Kambe T, Uchiyama H, Nomura N. The effect of a cell-to-cell communication molecule, Pseudomonas quinolone signal (PQS), produced by P. aeruginosa on other bacterial species. Microbes Environ. 2010;25:1–7. doi: 10.1264/jsme2.me09156. [DOI] [PubMed] [Google Scholar]
- 20.Ullman RF, Miller SJ, Strampfer MJ, Cunha BA. Streptococcus mutans endocarditis: report of three cases and review of the literature. Heart Lung. 1988;17:209–212. [PubMed] [Google Scholar]
- 21.Yawata Y, Toda K, Setoyama E, Fukuda J, Suzuki H, Uchiyama H, Nomura N. Monitoring biofilm development in a microfluidic device using modified confocal reflection microscopy. J Biosci Bioeng. 2010;110:377–380. doi: 10.1016/j.jbiosc.2010.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.