Abstract
Purpose
To measure intravitreal low-density lipoprotein receptor-related protein 6 (LRP6) and vascular endothelial growth factor (VEGF) levels in the eyes of patients with proliferative diabetic retinopathy (PDR) and to observe their correlation with PDR activity.
Methods
Fifty-five eyes of 55 patients were enrolled consecutively. Vitreous samples from 30 eyes with PDR and 25 eyes with nondiabetic macular disease were collected. Active PDR was present in 16 patients and quiescent PDR in 14 patients according to retinal neovascularization. LRP6 and VEGF concentrations in samples were determined using enzyme-linked immunosorbent assay (ELISA).
Results
ELISA revealed significant increases in the vitreous levels of VEGF in eyes affected with PDR compared to the controls (p<0.001). The mean concentrations of LRP6 were also higher in the vitreous samples from patients with PDR compared to the nondiabetic controls: 39.85 ng/ml and 15.48 ng/ml, respectively (p=0.002). In addition, the vitreous levels of LRP6 and VEGF were significantly higher in active PDR than in quiescent PDR (p=0.022 and p=0.015, respectively). Furthermore, a significant positive correlation was found between intravitreal levels of LRP6 and VEGF in patients with PDR (r=0.567, p=0.001). However, comparison of patients with PDR with controls revealed that the plasma levels of LRP6 were not significantly different between the two groups (p=0.636).
Conclusions
LRP6 and VEGF levels in the vitreous body from patients with PDR were increased and correlated mutually. LRP6 may be a good diagnostic biomarker and a new therapeutic target for PDR.
Introduction
Angiogenesis, the formation of new vessels from a preexisting vascular network, is regulated by two counterbalancing systems, including proangiogenic factors and antiangiogenic factors [1]. Among these cytokines, vascular endothelial growth factor (VEGF) is the most powerful player, which can activate VEGF receptor 2 (VEGF-R2), stimulate endothelial cell proliferation, and result in pathological angiogenesis [2,3]. Previous studies have shown that the disrupted balance between the levels of VEGF and other factors may contribute to angiogenesis, one of the hallmarks in the pathogenesis of diabetic retinopathy [4,5].
Proliferative diabetic retinopathy (PDR), the advanced stage of diabetic retinopathy, is a major cause of visual impairment globally, characterized by retinal angiogenesis and formation of fibrovascular tissue [6]. To date, the exact mechanism for PDR remains unclear. Increasing evidence has revealed that the Wnt signaling pathway may be involved in the development of diabetic retinopathy [7,8]. Wnts are a group of secreted, cysteine-rich glycoproteins, which may participate in multiple biologic and pathological processes, including cell angiogenesis and inflammation [9,10]. Upon binding of certain Wnts to the Frizzled and low-density lipoprotein receptor (LDLR)-related proteins (LRP) 5/6 coreceptors, glycogen synthase kinase-3β is inactivated, preventing phosphorylation of β-catenin and resulting in its accumulation [11]. β-catenin then translocates into the nucleus, interacts with T-cell factor/lymphoid enhancer factor (TCF/LEF), and regulates the expression of target genes including VEGF [12,13].
A previous study found components of Wnt signaling, including Frizzled4 and LRP5, were significantly overexpressed in pathological neovascularization of oxygen-induced proliferative retinopathy (OIR) in an animal model [14]. More recently, it was detected that Dickkopf (DKK)1, a Wnt antagonist, was also decreased in the plasma of patients with diabetic retinopathy, which suggests the Wnt signaling pathway is associated with the pathogenesis of diabetic retinopathy [15]. LRP6, a member of the LDLR family, has been demonstrated to be an indispensable coreceptor for the canonical Wnt pathway. By binding with LRP6, a monoclonal antibody was confirmed to inhibit Wnt signaling, which prevented vascular leakage and inflammation in a streptozotocin (STZ)-induced diabetic animal model [16]. These previous findings led us to hypothesize that LRP6 may be upregulated in PDR and the protein’s levels in vitreous may be associated with retinal angiogenesis. In this regard, we aimed to examine the concentrations of LRP6 in vitreous fluid and plasma in the present study. We also analyzed the relationship between intravitreal levels of LRP6 and VEGF.
Methods
Study subjects
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review committee of Beijing Tongren Hospital, Capital Medical University. All procedures were performed in accordance with the ARVO Statement on human subjects. Informed consent for all examinations and procedures was obtained from each subject. A total of 55 participants, who met the inclusion and exclusion criteria, were enrolled (30 subjects in PDR group and 25 subjects in control group, respectively). All cases were referred to the ophthalmology clinic of Beijing Tongren Eye Center.
Inclusion criteria were patients with PDR who received pars plana vitrectomy (PPV) for long-standing (>3 months) or recurrent vitreous/preretinal hemorrhage or tractional retinal detachment involving or threatening the macula. The control group consisted of patients without diabetes who were candidates for the treatment of macular hole (MH) or idiopathic epiretinal membrane (ERM). Exclusion criteria included photocoagulation in the preceding 3 months or previous vitreoretinal surgery within the past 2 years, recent vitreous hemorrhage (<3 months before vitrectomy), and past history of ocular inflammation, rubeosis iridis, and rhegmatogenous retinal detachment.
All patients underwent a careful examination with slit-lamp biomicroscopy using a 90-D fundus non-contact lens (Volk Super Field NC, Mentor, OH). Standardized digital fundus photography and fluorescence angiography (Tokyo Optical Co. Ltd, Tokyo, Japan) were performed preoperatively to confirm the fundus findings. Fasting blood glucose and hemoglobin A1c (HbA1c) data were obtained from the clinical laboratory. Hypertension was defined as resting arterial blood pressure no less than 140/90 mmHg or a history of antihypertensive drug therapy. A detailed questionnaire was used to collect information about previous history.
Retinopathy was graded intraoperatively in all eyes by two ophthalmologists. In summary, active diabetic retinopathy was defined as eyes with PDR showing perfused preretinal capillaries in the retina or optic disc. Quiescent diabetic retinopathy was defined as eyes with only nonperfused gliotic vessels or fibrosis [17].
Sample collection
At the beginning of the vitrectomy, 0.5–1.0 ml of the undiluted vitreous sample was obtained by aspiration into a 2 ml sterile syringe attached to the vitreous cutter (Accurus Ophthalmic Surgical System; Alcon Surgical Laboratory Inc., Irvine, CA) before the infusion line was opened. The samples were then transferred in sterilized microcentrifuge tubes (2 ml), placed immediately on ice, and centrifuged at 2,500 ×g for 5 min at 4 °C. Supernatants without sediment were divided into aliquots and frozen immediately at −80 °C until assayed. Blood samples were collected into tubes containing EDTA. Plasma was obtained from the blood samples by centrifugation at 2,500 ×g for 10 min at 4 °C and stored at −80 °C until assayed.
Measurement of LRP6 and VEGF Levels
The vitreous or plasma samples were diluted, and then LRP6 and VEGF levels were measured with enzyme-linked immunosorbent assay (ELISA) using the human LRP6 assay kit (Human LRP6, Donglin Sci&Tech Development Co. Ltd., Wuxi, China) and the Quantikine human VEGF assay kit (Quantikine human vascular endothelial growth factor, R&D Systems, Inc., Minneapolis, MN). Each assay was performed in triplicate, according to the manufacturer’s instructions. Briefly, 100 µl each of dilutions of standard, blank, and samples were added into the wells of a 96-well plate coated with a monoclonal antibody. After incubation for 2 h at 37 °C, the liquid of each well was removed, and an enzyme-labeled antibody was added. Following further incubation for 2 h at 37 °C, the plate was washed, and the substrate solution was added. After 30-min incubation, the reaction was completed by adding the stop solution, and the optical density was read at 450 nm using a spectrophotometer (SpectraMax Gemini UVmax; Molecular Devices, Sunnyvale, CA) with a wavelength correction of 540 nm. A standard curve was plotted from the results measured with the standard solution (5–1,000 pg/ml for VEGF, 0.156–10 ng/ml for LRP6). The VEGF or LRP6 concentration in each sample was determined from the standard curve. The minimum detectable concentration for the VEGF ELISA kit is 5.0 pg/ml, with the intra-assay coefficient of variation (CV) 4.5% and the inter-assay CV 7.0%. The detection limit for the LRP6 ELISA assays was 0.059 ng/ml, with the intra-assay CV 6.0% and the inter-assay CV 6.5%.
Statistical analysis
Statistical analysis was performed using SPSS software (version 19.0; SPSS, Chicago, IL). Measurement data were presented as mean ±SD (standard deviation) or median [minimum to maximum range]. The Mann–Whitney U test or independent Student t test was used to analyze the differences between two groups after the data were checked for normal distribution. The chi-square or Fisher exact test was used to compare noncontinuous variables. The Spearman-rank correlation coefficient test was performed to analyze the associations between the intravitreal LRP6 levels and the other measured parameters. A value of p<0.05 was considered statistically significant.
Results
The patients with diabetes included 12 men and 18 women, who were 54.30±10.06 years. Active PDR was present in 16 patients and quiescent PDR in 14 patients. The control group included 11 men and 14 women whose age ranged from 37 to 73 years with a mean of 56.88±9.35 years.
Concentrations of LRP6 and VEGF in vitreous samples of all patients
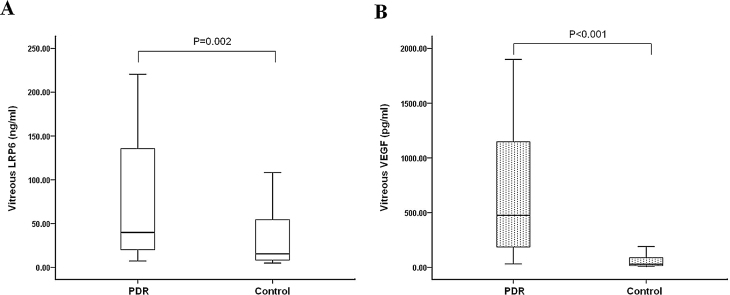
Demographic, clinical, and laboratory data for the PDR group and the control group are shown in Table 1. There were no statistically significant differences in age and gender between the two groups (p>0.05). The statistical analysis revealed a significant difference in blood glucose levels between patients with PDR and the controls (p<0.001). The intravitreal LRP6 levels were significantly higher in the PDR group (39.85 ng/ml [7.27–220.48]) than in the control group (15.48 ng/ml [4.88–108.20], p=0.002; Figure 1A). In addition, the VEGF concentrations in the vitreous fluid of the PDR eyes were also higher, compared with those in the control group (474.62 pg/ml [31.64–1899.34] versus 29.29 pg/ml [10.20–190.05]; p<0.001; Figure 1B).
Table 1. Demographic, clinical and laboratory data for proliferative diabetic retinopathy (PDR) patients and controls.
| Variable | PDR (n=30) | Controls (n=25) | P value |
|---|---|---|---|
| Age (years) |
54.30 ± 10.06 |
56.88 ± 9.35 |
0.333 |
| Gender/male (%) |
12(40.00) |
11(44.00) |
0.765 |
| Glucose (mmol/l) |
7.30 ± 1.48 |
5.34 ± 0.53 |
<0.001 |
| Hypertension (%) |
12(40.00) |
8(32.00) |
0.539 |
| HbA1c (%) |
7.45 ± 0.79 |
- |
- |
| Duration of diabetes (years) | 12.80 ± 5.94 | - | - |
HbAlc=glycosylated hemoglobin
Figure 1.
The box-and-whisker plot represents the median and minimum to maximum range of vitreous LRP6 or VEGF levels. Vitreous samples were measured among 30 subjects with PDR and 25 subjects as control. The levels of (A) lipoprotein receptor-related protein 6 (LRP6) and (B) vascular endothelial growth factor (VEGF) were significantly increased in eyes with proliferative diabetic retinopathy (PDR) compared to controls (LRP6: p=0.002 and VEGF: p<0.001, respectively).
Concentrations of LRP6 and VEGF in vitreous samples of patients with active PDR and quiescent PDR
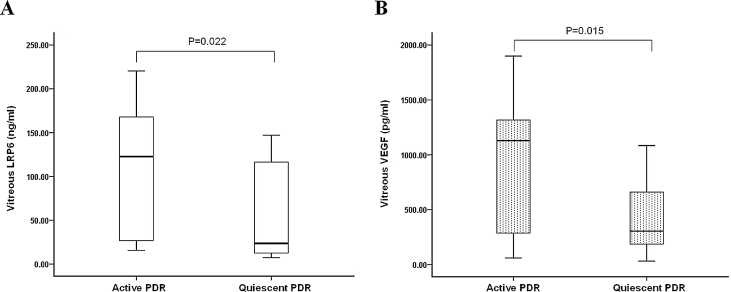
We compared the baseline data of the subgroups between patients with active PDR and those with quiescent PDR (Table 2). No differences were observed for age, gender, duration of diabetes, levels of blood glucose, and HbA1c between the two groups (p>0.05). We found significantly higher intravitreal concentrations of LRP6 and VEGF in the active PDR group compared to the quiescent PDR group (LRP6: 122.69 ng/ml [15.69–220.48] versus 23.60 ng/ml [7.27–147.08]; p=0.022 and VEGF: 1129.70 pg/ml [60.01–1899.34] versus 304.56 pg/ml [31.64–1084.02]; p=0.015; Figure 2A, B).
Table 2. Demographic, clinical and laboratory data for patients with active and quiescent proliferative diabetic retinopathy (PDR).
| Variable | Active PDR (n=16) | Quiescent PDR (n=14) | P value |
|---|---|---|---|
| Age (years) |
54.38 ± 10.73 |
54.21 ± 9.63 |
0.966 |
| Gender/male (%) |
5(31.30) |
7(50.00) |
0.457 |
| Glucose (mmol/l) |
7.27 ± 1.61 |
7.33 ± 1.38 |
0.915 |
| Hypertension (%) |
7(43.80) |
5(35.70) |
0.722 |
| HbA1c (%) |
7.58 ± 0.76 |
7.30 ± 0.82 |
0.349 |
| Duration of diabetes (years) | 13.44 ± 5.83 | 12.07 ± 6.21 | 0.539 |
HbAlc=glycosylated hemoglobin
Figure 2.
LRP6 and VEGF concentrations were measured in the vitreous fluid of 16 patients with active PDR and 14 patients with quiescent PDR. The box-and-whisker plot represents the median and minimum to maximum range of vitreous LRP6 or VEGF levels. There was a statistically significant difference between the two groups for (A) lipoprotein receptor-related protein 6 (LRP6; p=0.022) and (B) vascular endothelial growth factor (VEGF; p=0.015).
Concentrations of LRP6 in plasma samples of all patients
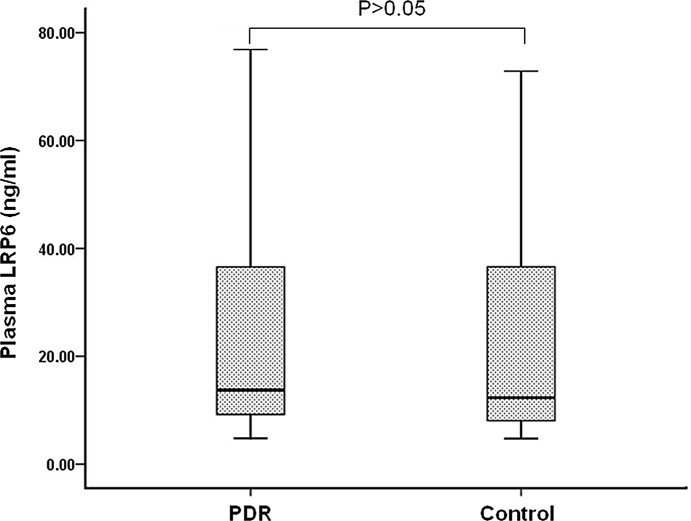
LRP6 levels were detected in all plasma samples from the patients with PDR, as well as from the control group without diabetes (Figure 3). Comparing the patients with PDR with the controls revealed that the LRP6 plasma levels were not significantly different between the two groups (PDR: 13.74 ng/ml [4.80–76.86]; controls: 12.32 ng/ml [4.75–72.85]; p=0.636).
Figure 3.
Detectable LRP6 levels in all plasma samples from 30 subjects with PDR and from 25 subjects as control. There was no significant difference between the two groups (p=0.636). The box-and-whisker plot represents the median and minimum to maximum range of plasma LRP6 levels.
Correlation between LRP6 and VEGF levels in vitreous fluid
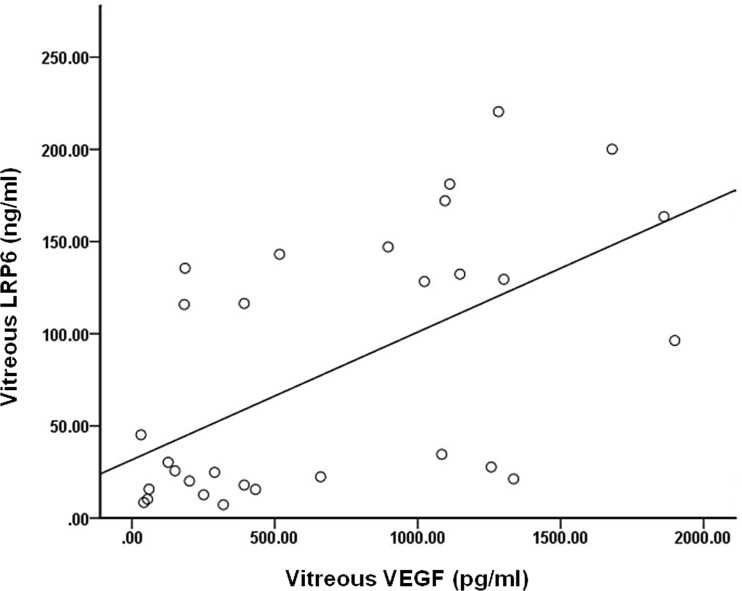
Association-based analysis was performed on the LRP6 and VEGF levels in the vitreous. In the 30 eyes of the patients with PDR, the intravitreal LRP6 levels revealed a significantly positive correlation with the VEGF levels (r=0.567, p=0.001; Figure 4). However, no significant relationships between the vitreous concentrations of LRP6 and VEGF were detected in the 25 eyes of the nondiabetic controls (r=0.125, p=0.553).
Figure 4.
Correlation between levels of LRP6 and VEGF in vitreous fluid. Intravitreal lipoprotein receptor-related protein 6 (LRP6) levels revealed a significant correlation with vascular endothelial growth factor (VEGF) in patients with proliferative diabetic retinopathy (PDR) (r=0.567, p=0.001).
Correlation between intravitreal LRP6 levels and various clinical and laboratory parameters
No significant correlation was observed between the intravitreal LRP6 concentrations and age (r=0.164, p=0.386), gender (r=0.024, p=0.901), frequency of hypertension (r=0.118, p=0.533), blood glucose (r=0.067, p=0.726), and HbA1c (r=0.196, p=0.300).
Discussion
Despite recent rapid progress in the use of anti-VEGF therapies for treating diabetic retinopathy, the long-term effect of this therapy remains unclear. In addition, some patients with PDR do not respond well to anti-VEGF therapy. Accordingly, these provide a rationale for the development of new therapeutics for diabetic retinopathy. Increasing experimental evidence has indicated that Wnt signaling is involved in the development of diabetic retinopathy in animal models [7,8], but to our knowledge, limited data are available in humans. The present study is the first to examine the intravitreal levels of LRP6 in patients with PDR to evaluate the role of Wnt signaling in diabetic retinopathy. This study provided evidence of significantly increased concentrations of LRP6 and VEGF in the vitreous fluid from patients with PDR compared to nondiabetic controls. We also demonstrated that intravitreal LRP6 levels were significantly higher in PDR eyes with active neovascularization than in the quiescent PDR group. Furthermore, a significantly positive correlation between the LRP6 and VEGF levels in the vitreous fluid was detected in patients with PDR. However, the LRP6 levels in plasma were not significantly different between the PDR group and the control group. Our findings suggested that LRP6 might be associated with the development and progression of diabetic retinopathy.
The canonical Wnt signaling pathway was suggested to participate in the process of inflammation and angiogenesis in ocular diseases [18,19]. It has been shown that Wnt receptors are expressed in pathological neovascular tufts and the receptors’ mRNA levels are significantly increased in neovessels during OIR [14]. Another previous study also demonstrated that upregulation of Wnt signaling might play a pathogenic role in diabetic retinopathy and the activation occurred mainly in the inner retina. LRP6, another well-known receptor for the Wnt pathway, is also expressed in multiple retinal layers [7]. In this study, we detected significantly higher intravitreal levels of LRP6 in patients with PDR compared with those in controls, providing further evidence to support the role of Wnt signaling in diabetic retinopathy. Intravitreal injection of SERPINA3K, an LRP6 antagonist and an endogenous inhibitor of Wnt signaling [20], was shown to inhibit retinal inflammation and attenuate retinal neovascularization in the OIR model [21]. Similarly, by binding LRP6, DKK1 and pigment epithelium-derived factor (PEDF) were proved to suppress Wnt signaling, and thus ameliorate angiogenesis and inflammation in OIR or diabetic animals [7,22]. Another endogenous antagonist of LRP6, kallistatin, was also demonstrated to inhibit Wnt signaling and exert antiangiogenic and anti-inflammatory activities in diabetic retinopathy [23]. In addition, we compared the intravitreal concentrations of LRP6 in the active PDR group and the quiescent PDR group. A significant difference was found between the two forms of PDR, with higher levels in active PDR, indicating the expression of LRP6 may be associated with the activity of PDR. Taken together, these observations indicated that LRP6 activity might be responsible, at least in part, for the pathological angiogenesis in PDR.
Neovascularization of the retina is one pathogenic feature of PDR and has good correlation with intravitreal levels of VEGF [24,25]. Our results are consistent with these previous data. The intravitreal concentrations of VEGF in the PDR group were significantly increased compared to the controls, revealing the involvement of VEGF in disease pathogenesis. Previous studies have demonstrated that VEGF may act as a downstream effector of Wnt/β-catenin signaling [26,27]. By transducing human umbilical vein endothelial cells (HUVECs) with Ad-β-catenin, Skurk detected dose-dependent upregulation of VEGF-A and VEGF-C RNA. Consistent with increased VEGF signaling, upregulation of VEGF-R2 transcript and protein levels in endothelial cells was also demonstrated [28]. Another in vitro experiment also indicated that β-catenin signaling could upregulate expression of VEGF-A in colon cancer [29]. In the STZ-induced diabetic model, disruption of β-catenin in Müller cells was shown to attenuate overexpression of VEGF and decrease vascular leakage [8]. Additionally, for the first time, we found a significantly positive correlation between intravitreal LRP6 and VEGF from patients with PDR in the current study. All these findings suggested that LRP6 might play a critical role in promoting angiogenesis in diabetic retinopathy, possibly acting synergistically with VEGF.
LRP 5 and 6 are considered cell-surface endocytosis receptors essential for the canonical Wnt signaling pathway. Although LRP6 is not supposed to relocate out of the cells, our study detected LRP6 levels in the plasma and vitreous samples. However, the molecular mechanism is still unknown at present. Furthermore, in this study we found significantly higher intravitreal levels of LRP6 in eyes with PDR compared to the controls, which correlated well with VEGF levels in the vitreous. It is possible that LRP6 is derived from the breakdown of the blood–retinal barrier or blood contamination due to vitreous hemorrhage in PDR. However, LRP6 was also detected in the vitreous fluid of non-diabetic controls. In addition, patients with recent vitreous hemorrhage 3 months preceding the study were excluded, which helped to avoid the massive influx of serum proteins into the vitreous. Although the origin of LRP6 is still uncertain, we suspect that the elevated vitreous LRP6 in PDR might be produced locally from intraocular tissues. This can be supported in a previous study, which detected more intensive LRP5/6 signals in the inner retina of STZ-diabetic rats compared with nondiabetic controls with immunohistochemical analysis [7]. Further work, including histopathological analysis of postmortem eyes from patients with PDR, is needed to determine the exact origin of LRP6 and its role in inflammation and angiogenesis in PDR.
The present study was limited by the small sample size with heterogeneous eye conditions, including different stages of PDR and various durations of previous retinal photocoagulation. All these factors may result in the large range of VEGF levels in current study. However, our findings are consistent with previous studies [30,31]. In addition, we did not include diabetes patients without retinopathy or subjects with non-proliferative diabetic retinopathy. Nevertheless, our observations might be favorable for better understanding the pathogenesis of diabetic retinopathy.
In conclusion, our findings demonstrated that LRP6 levels were increased in the vitreous body from patients with PDR and there was a positive correlation between LRP6 and VEGF. Although further experiments are needed to elucidate the exact role of Wnt signaling in PDR, LRP6 may be a potentially new therapeutic target in such pathological angiogenesis.
Acknowledgement
Supported by National Natural Science Foundation grants (30872820, 81271034)
References
- 1.Pollina EA, Legesse-Miller A, Haley EM, Goodpaster T, Randolph-Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7:2056–70. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 4.Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications. 2012;26:435–41. doi: 10.1016/j.jdiacomp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Abcouwer SF. Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol. 2013;11:1–12. doi: 10.4172/2155-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negi A, Vernon SA. An overview of the eye in diabetes. J R Soc Med. 2003;96:266–72. doi: 10.1258/jrsm.96.6.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, Boulton M, Lyons TJ, Gao G, Ma JX. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol. 2009;175:2676–85. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou KK, Benyajati S, Le Y, Cheng R, Zhang W, Ma JX. Interruption of Wnt Signaling in Müller Cells Ameliorates Ischemia-Induced Retinal Neovascularization. PLoS ONE. 2014;9:e108454. doi: 10.1371/journal.pone.0108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell. 2014;31:248–56. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Liddo R, Bertalot T, Schuster A, Schrenk S, Tasso A, Zanusso I, Conconi MT, Schäfer KH. Anti-inflammatory activity of Wnt signaling in enteric nervous system: in vitro preliminary evidences in rat primary cultures. J Neuroinflammation. 2015;12:23. doi: 10.1186/s12974-015-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329:209–23. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, Gerhardt H. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16:70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tachikawa K, Schroder O, Frey G, Briggs SP, Sera T. Regulation of the endogenous VEGF-A gene by exogenous designed regulatory proteins. Proc Natl Acad Sci USA. 2004;101:15225–30. doi: 10.1073/pnas.0406473101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LE. Wnt Signaling Mediates Pathological Vascular Growth in Proliferative Retinopathy. Circulation. 2011;124:1871–81. doi: 10.1161/CIRCULATIONAHA.111.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu F, He J, Zhou Y, Bai X, Wu G, Wang X, Liu Z, Chen Y, Ma JX, Liu Z. Plasma and vitreous fluid levels of Dickkopf-1 in patients with diabetic retinopathy. Eye (Lond) 2014;28:402–9. doi: 10.1038/eye.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, Ma JX. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes. 2012;61:2948–57. doi: 10.2337/db11-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 18.Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma JX. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4371–9. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi Y, Chen Q, Rajala RV, Ma JX. MicroRNA-184 modulates canonical Wnt signaling through the regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Lett. 2015;589:1143–9. doi: 10.1016/j.febslet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Abreu JG, Zhou K, Chen Y, Hu Y, Zhou T, He X, Ma JX. Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proc Natl Acad Sci USA. 2010;107:6900–5. doi: 10.1073/pnas.0906764107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Hu Y, Ma JX. Anti-inflammatory and antioxidant effects of SERPINA3K in the retina. Invest Ophthalmol Vis Sci. 2009;50:3943–52. doi: 10.1167/iovs.08-2954. [DOI] [PubMed] [Google Scholar]
- 22.Park K, Lee K, Zhang B, Zhou T, He X, Gao G, Murray AR, Ma JX. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol. 2011;31:3038–51. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Zhang B, McBride JD, Zhou K, Lee K, Zhou Y, Liu Z, Ma JX. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes. 2013;62:4228–38. doi: 10.2337/db12-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiello LP. Clinical implications of vascular growth factors in proliferative retinopathies. Curr Opin Ophthalmol. 1997;8:19–31. doi: 10.1097/00055735-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Funatsu H, Yamashita H, Mimura T, Noma H, Nakamura S, Hori S. Risk evaluation of outcome of vitreous surgery based on vitreous levels of cytokines. Eye (Lond) 2007;21:377–82. doi: 10.1038/sj.eye.6702213. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–4. [PubMed] [Google Scholar]
- 27.Sen M, Ghosh G. Transcriptional outcome of Wnt-Frizzled signal transduction in inflammation: evolving concepts. J Immunol. 2008;181:4441–5. doi: 10.4049/jimmunol.181.7.4441. [DOI] [PubMed] [Google Scholar]
- 28.Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. Glycogen-Synthase Kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96:308–18. doi: 10.1161/01.RES.0000156273.30274.f7. [DOI] [PubMed] [Google Scholar]
- 29.Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, Randazzo F, Gundel R, Warren RS, Escobedo J, Aukerman SL, Taylor RN, Fantl WJ. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–53. [PubMed] [Google Scholar]
- 30.Izuta H, Matsunaga N, Shimazawa M, Sugiyama T, Ikeda T, Hara H. Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol Vis. 2010;16:130–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Abu El-Asrar AM, Nawaz MI, De Hertogh G, Alam K, Siddiquei MM. Van den Eynde K2 Mousa A, Mohammad G, Geboes K, Opdenakker G. S100A4 is upregulated in proliferative diabetic retinopathy and correlates with markers of angiogenesis and fibrogenesis. Mol Vis. 2014;20:1209–24. [PMC free article] [PubMed] [Google Scholar]