Abstract
Stereotactic body radiotherapy (SBRT) is a high precision radiotherapy technique used for the treatment of small to moderate extra-cranial tumours. Early studies utilising SBRT have shown favourable outcomes. However, major disadvantages of static field SBRT include long treatment times and toxicity complications. Volumetric modulated arc therapy (VMAT) and intensity modulated radiotherapy (IMRT) may potentially mitigate these disadvantages. This review aims to assess the feasibility of emerging VMAT and IMRT-based SBRT treatment techniques and qualify which offers the best outcome for patients, whilst identifying any emerging and advantageous SBRT planning trends. A review and synthesis of data from current literature up to September 2013 was conducted on EMBASE, Medline, PubMed, Science Direct, Proquest central, Google Scholar and the Cochrane Database of Systematic reviews. Only full text papers comparing VMAT and or IMRT and or Static SBRT were included. Ten papers were identified that evaluated the results of VMAT/IMRT SBRT. Five related to medically inoperable stage 1 and 2 non-small-cell lung cancer (NSCLC), three to spinal metastasis, one related to abdominal lymph node malignancies, with the final one looking at pancreatic adenocarcinoma. Overall treatment times with VMAT were reduced by 66–70% for lung, 46–58% for spine, 42% and 21% for lymph node and pancreatic metastasis respectively, planning constraints were met with several studies showing improved organs at risk sparing with IMRT/VMAT to static SBRT. Both IMRT and VMAT were able to meet all planning constraints in the studies reviewed, with VMAT offering the greatest treatment efficiency. Early clinical outcomes with VMAT and IMRT SBRT have demonstrated excellent local control and favourable survival outcomes.
Keywords: Extracranial, inoperable early stage non-small-cell lung cancer (NSCLC), intensity modulated radiotherapy (IMRT), spinal metastases, stereotactic body radiotherapy (SBRT), volumetric modulated arc therapy (VMAT)
Introduction
Stereotactic body radiotherapy (SBRT) or stereotactic ablative radiotherapy (SABR) is a high-precision radiotherapy technique that utilises high doses of radiation in a few or single fractions for the treatment of small to moderate extra-cranial tumours.
Recent technological advancements in immobilisation, imaging and the ability to compensate for respiratory motion have led to an increase in the use of SBRT in a number of clinical settings.
SBRT is an emerging and evolving treatment modality where optimal dose, fractionation schedule and technique are still to be determined. However, early results of studies utilising SBRT for the treatment of inoperable early stage non-small cell-lung cancer (NSCLC) and spinal metastases have shown favourable results.1 SBRT has also been used for the treatment of other extra-cranial tumours like prostate cancer, liver metastasis, unresectable pancreatic cancer and other abdominal lesions.2,3 Limited experience in these studies suggests favourable local tumour control results.
However, many conformal SBRT/SABR studies have reported frequently occurring and clinically significant treatment related toxicity complications.4 Another drawback associated with SBRT is the long treatment times relating to patient setup and radiation delivery. Depending on equipment and dose utilised, patient setup time can take up to 22 min5 and 100 min for treatment delivery.6 Longer treatment times significantly increase the chances of intrafraction motion and error.7
Recently there has been much interest at mitigating the risks associated with SABR by delivering stereotactic doses through different techniques other than static non-coplanar/planer beams, most notably these include the use of SBRT with intensity modulated therapy (IMRT),8,9 volumetric modulated arc therapy (VMAT)10–12 also referred to as rapid arc (RA) and or other arc-related treatment techniques such as cyberknife.13
The purpose of this review was to assess the feasibility of emerging VMAT and IMRT-based SBRT treatment techniques. Furthermore, we aimed to identify any emerging and advantageous SBRT planning trends and in particular see which SBRT planning modality offered the best outcome for prospective patients.
Method
A review and synthesis of data from the current literature up to September 2013 was conducted on EMBASE, PubMed, Science Direct, Google Scholar, Proquest central, Medline and the Cochrane Database of Systematic reviews. A combination of relevant keywords and subject headings were used as shown in Figure1. No time or language restriction was applied. Articles that included SBRT with the words modulated, IMRT and or VMAT were all considered. For an article to be included, it firstly had to be a full text article and relate to SBRT/SABR. It then had to compare IMRT and VMAT dose distribution plans and or delivery times to each other, or evaluate SBRT VMAT/IMRT plans with other current SBRT treatment modalities which included: 3D conformal radiotherapy (3DCRT), Cyberknife or VMAT flattening filter free (FFF). Any SBRT studies that utilised VMAT and or IMRT that reported clinical outcomes of local control and or survival outcomes were also included. Notably a large amount of (36) supplementary articles and conference posters relevant to the research topic were excluded based on the limited data presented. Due to limited amount of full text data relating to this specific data, all papers that were considered eligible (21) were screened by all three authors to limit bias.
Figure 1.
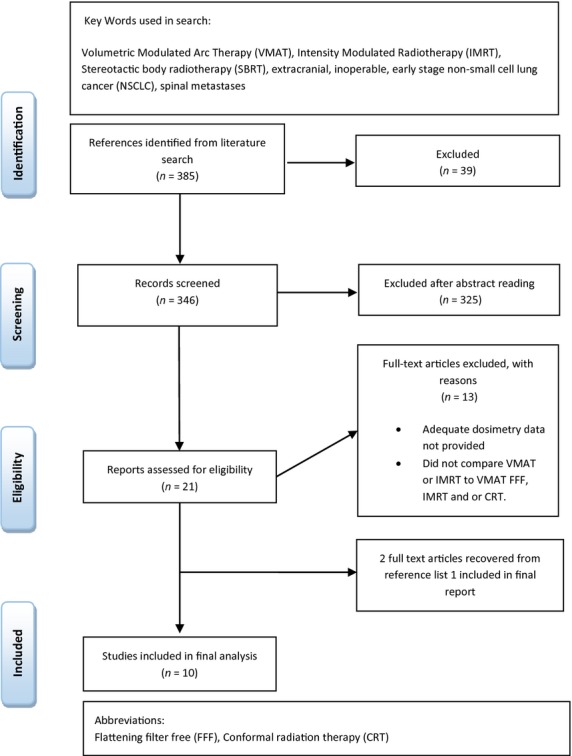
Search flow diagram.
Studies were analysed based on treatment times, organ dose, monitor units, conformity index, planning techniques and where applicable local tumour control and survival outcomes. Studies that were included reported at least three of these variables. A summary of the search methodology can be seen in Figure1.
Results
A search of the literature yielded 385 articles, of these 21 full text articles were retrieved and analysed against the inclusion criteria, with 10 studies included in the final analysis (Fig1). From these, five studies14–17,8 related to medically inoperable stage 1 and 2 NSCLC (T1-T2NOMO), three cases8–10 related to spinal metastases, with two directly comparing VMAT SBRT to IMRT SBRT the other comparing VMAT FFF SBRT to VMAT with flattening filter (FF) SBRT.8 One study11 looked at the feasibility of VMAT/IMRT SBRT to that of 3DCRT for the treatment of abdominal lymph node malignancies, and another reviewed the feasibility of IMRT/VMAT SBRT for the treatment of pancreatic adenocarcinoma.12 All the articles included in the review reported on a minimum of six dosimetric planning or treatment outcomes, articles with <6 dosimetric results were deemed not to provide an acceptable level of data for a qualitative comparison and not included in this review.
Use of VMAT and IMRT for SBRT in medically inoperable lung lesions
Five lung cancer studies14–17,8 were analysed and categorised in Table1. Unfortunately not all the studies presented the same variables; however, at least three common variables were reported throughout the studies which were used for comparison. Of the studies reviewed, only two SBRT studies16,18 that utilised VMAT and or IMRT reported clinical outcomes of local control or survival outcomes. Both these studies related to medically inoperable NSCLC (T1–T2NOMO). The earlier of these two studies18 utilised 7 beam IMRT SBRT for 25 peripheral lesions and 3 central lesions in 26 patients with a mean age of 24. The results of this study showed excellent local control and overall survival, where 3 year rates were 94.4% and 52% respectively. Median survival was 38.4 months. The latter of these studies16 utilised VMAT RA FFF and retrospectively compared results to previous patients treated with 3DCRT with the same dose and fractionation schedule. This is the only study to date that has presented clinical results of VMAT RA FFF for lung SBRT. The 1 year local control rate for VMAT compared to 3DCRT was 100% and 92.5% respectively. Analysis of pulmonary toxicity favoured the VMAT RA FFF cohort where: there were eight accounts (17.4%) of grade 1-grade 2 (G1-G2) and 2 accounts (4%) of grade 3 (G3) pulmonary toxicity. In comparison, the rate of G1, G2 and G3 pulmonary toxicity for the 3DCRT cohort was 24%, 42% and 9% respectively. The low pulmonary toxicity rate seen in the VMAT RA FFF cohort correlated with a decreased percentage in V20 and V5 lung dose for VMAT RA FFF patients. Of the five studies, two planning studies14,17 directly compared VMAT to IMRT; in both studies, treatment time was reduced by an average of 70% with VMAT to that of IMRT. The average dose to healthy lung V20 between VMAT and IMRT in both studies was comparable with only a 0.3%14 and 0.2%17 difference respectively. Lung V5 dose was slightly higher in the VMAT cohort in both studies – 1.4%14 and 3.7%17 respectively.
Table 1.
Summary of dosimetric results for studies utilising IMRT and VMAT SBRT in medically inoperable stage 1 NSCLC
| Reference | Holt et al.14 | Ong et al.17 | Navarria et al.16 | McGrath et al.15 | Ong et al.8 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target area | Medically inoperable NSCLC (T1–T2NOMO) | Medically inoperable stage 1 NSCLC | Medically inoperable stage 1 NSCLC | Medically inoperable stage 1A NSCLC | Medically inoperable stage 1 NSCLC | |||||||
| Dose/Fx | 54 Gy/3Fx (18 Gy/Fx) | (60 Gy/8Fx), (55 Gy/5Fx), (54 Gy/3F) (Results for delivery time represent delivery of single 7.5 Gy fraction) | 48 Gy/4Fx (12 Gy per Fx) | 48 Gy/4Fx (12 Gy/Fx) | 3 Fractionation schemes (54 Gy/3Fx), (55 Gy/5Fx), (60/8Fx) | |||||||
| Variable | VMAT | IMRT (coplanar) | VMAT RA | IMRT sliding window (coplanar) | VMAT RA | 3DCRT | VMAT RA with FFF | 3DCRT FF | VMAT | 3D-CRT | VMAT RA FF 6MV | VMAT RA FFF 10MV |
| Patients | 27 | 9 | 18 | 46 | 86 | 21 | 10 | |||||
| Arc length/beam number | 1 dual arc of 209° | 12–16 | 2 × 358° | 9–10 | 2 × 358° | 10 | 1–2 × 212 ± 37° | 4–6 × 360° conformal arcs | 1 × 180° | 7–10 | 2 × 360° | 2 × 360° |
| Mean PTV (cm3) | 44.5 (range 14.3–101.8 cm3) | 27.1 ± 13.4 (range 11.9–55.8 cm3) | 38.8 ± 21.3 (range 6.3–67.1 cm3) | 46.7 ± 27.7 (range 2.3–125.2 cm3) | 59.9 ± 7.7 (range 6.1–162.2 cm3) | 57.4 cm (range 22.2–125.2 cm3) | 58.2 (rage 8.9–153.4 cm3) | |||||
| Delivery time (min) | 6.6 | 23.7 | 3.9 | 12 | 3.9 | 11.6 | 1.5 ± 0.3 | 8.3 ± 1.3 | 6.1 | 11.9 | 3.6 ± 1.0 | 2.5 ± 0.1 |
| 0.66 | 0.82 | |||||||||||
| Lung V5 (%) | 18 | 19.4 | 17 ± 7.7 | 14.7 ± 6.9 | 18.3 ± 7.2 | 18.1 ± 6.8 | 25.3 ± 11.8 | 31.4 ± 11.9 | VMAT plan < V5 by 4.2 | NA | NA | |
| Lung V20 (%) | 5.4 | 5.7 | 4.4 ± 2.7 | 4.2 ± 2.5 | 5.4 ± 3.2 | 4.9 ± 2.9 | 7.3 ± 4.9 | 11.8 ± 7.0 | VMAT plan < V20 by 4.5 | NA | NA | |
| Spinal cord Dmax (Gy) | Dmax 8.3 | Dmax 8.1 | NA | NA | 10.8 ± 5.0 | 7.9 ± 3.8 | NA | NA | Dmax 11.46 | Dmax 11.78 | Dmax 13.5 ± 5.3 | Dmax 13.4 ± 5.4 |
| Chest wall | 36.2 V30Gy(cm3) | 36.1 V30Gy(cm3) | 8.6 ± 12.2 V30Gy(cm3) | 24.8 ± 11.5 V30Gy(cm3) | 1.2 ± 1.8 V45Gy(cm3) | 2.0 ± 2.7 V45Gy(cm3) | NA | NA | NA | NA | NA | NA |
| MUs | 3428Mus/1 Fx of 18Gy | 3335Mus/1 Fx of 18Gy | 234 ± 27MU/Gy | 445 ± 84MU/Gy | 240 ± 31MU/Gy | 179 ± 18MU/Gy | 1907 ± 632MU/min | 300MU/min | 2360mean | 2235mean | 228 ± 18MU/Gy | 247 ± 26MU/Gy |
| CI80 | NA | NA | 1.07 ± 0.02 | 1.14 ± 0.06 | 1.10 ± 0.07 | 1.18 ± 0.12 | NA | NA | 1.87 | 1.93 | 1.08 ± 0.3 | 1.08 ± 0.4 |
| CI50 | 5.17 | 5.31 | NA | NA | NA | NA | NA | NA | 5.19 | 5.65 | 1.97 ± 0.18 | 1.99 ± 0.20 |
MU, monitor units; PTV, planning target volume; NSCLC, non-small-cell lung cancer, IMRT, intensity modulated radiotherapy; VMAT, volumetric modulated arc therapy; 3DCRT, 3D conformal radiotherapy therapy; RA, rapid arc; FF flattening filter; FFF, flattening filter free; Dmax, maximum dose; CI, conformity index; Fx, fraction; Vn, volume of lung receiving n dose in Gy; NA, not applicable.
Three studies compared VMAT-SBRT with 3DCRT-SBRT,15–17 one of which utilised VMAT FFF.16 Between the three studies VMAT reduced the average treatment time by 66%, mean lung V5 by 8.1% and mean lung V20 by 11.2%. However, one of these studies17 reported a slight increase in lung V20 with VMAT and no significant difference (NSD) in lung V5 dose. All three studies reported an improvement with VMAT in plan conformity15,17 and or homogeneity16 compared to 3DCRT.
Finally one plan directly compared VMAT FF SBRT with VMAT FFF SBRT. Patients planed with FFF had a reduction in treatment delivery time by 31% whilst maintaining comparable plan quality. There was NSD in rib dose between the two modalities or any other reported organs at risk (OAR). However, the average monitor units (MU)/Gy needed was 8% higher with the FFF plans.
Use of VMAT and IMRT for stereotactic spine radiotherapy
The use of VMAT and IMRT for the delivery of SBRT in spinal metastasis was analysed in three retrospective studies.8–10 The dosimetric results of these studies were analysed and categorised accordingly in Table2. The earliest of these studies10 compared IMRT SBRT to VMAT SBRT with 1 and 2 arcs. The number of multi-leaf collimator (MLC) segments needed for the IMRT plans was 1131 ± 183. Compared to VMAT1arc (177) and VMAT2arc (354), the number of segments was considerably higher for the IMRT plans. Projected delivery time was reduced by 50% and 46% with the use of VMAT SBRT for 1 and 2 arcs respectively. A similar correlation was seen with a reduction in mean MUs by 11% and 27% with 1 and 2 arcs respectively. However, these results could not be replicated to the same extent with the study conducted by Kuijper et al.,9 where the delivery time for both IMRT and VMAT was comparable for 2 arcs and only slightly faster (18%) for 3 arcs compared to IMRT. There was NSD in planning target volume (PTV) coverage in all three studies. Spinal cord sparing was comparable between the studies but worst with VMAT using 1 arc.10 Plan conformity was the greatest in both studies utilising 2 arcs.9,10 The application of VMAT FFF SBRT reduced projected radiation delivery time by 58% compared to standard VMAT SBRT without compromising plan accuracy or quality.8
Table 2.
Summary of dosimetric results for studies utilising IMRT and VMAT SBRT for spinal metastasis
| Reference | Wu et al.10 | Kuijper et al.9 | Ong et al.8 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Target area | Spinal metastasis | Spinal metastasis | Spinal metastasis | ||||||
| Dose/Fx | 16 Gy/1Fx | 16 Gy/1Fx | 16 Gy/1Fx or 10 Gy/2Fx or 9 Gy/3Fx | ||||||
| Variable | IMRT | VMAT 1 arc | VMAT 2 arcs | Vertebral body IMRT VMAT 2 arcs | Entire vertebra IMRT VMAT 3 arcs | VMAT RA FF 6MV | VMAT RA FFF 10MV | ||
| Patients | 10 | 3 | 4 | 10 | |||||
| Arc length/beam number | 8–12 | 1 × 358° | 2 × 358° | 7–9 | 2 × 358° | 7–9 | 3 × 358° | 2 × 360° | |
| Mean PTV (cm3) | 104.56 (range 11.5–411.1 cm3) | 109 (range 82–147 cm3) | 181 (range 109–331 cm3) | 119 (range 34.13–225.9 cm3) | |||||
| Delivery time (min) | 15.9 | 7.9 | 8.6 | 12.5 | 13.5 | 19.5 | 16 | 6.7 ± 2.7 | 2.8 ± 0.4 |
| PTV Dmean | 16.7 ± 0.13 | 16.7 ± 0.20 | 16.7 ± 0.18 | 18.7 | 18.3 | 17.5 | 17.3 | 112 ± 4 (%) | 114 ± 4 (%) |
| PTV D95% | 15.5 ± 0.15 | 15.6 ± 0.16 | 15.5 ± 0.15 | 16 | 16 | 14.2 | 14.5 | NA | NA |
| Lung V5 | 130 ± 84 | 166 ± 104 | 164 ± 104 | NA | NA | NA | NA | NA | NA |
| Lung V10 | 35 ± 22 | 45 ± 27 | 43 ± 26 | NA | NA | NA | NA | NA | NA |
| Spinal cord Dmax/mean (Gy) | Dmean 4.48 ± 0.87 | Dmean 5.21 ± 0.92 | Dmean 4.81 ± 0.81 | Dmax 8.5 | Dmax 8.3 | Dmax 9.7 | Dmax 9.2 | Dmax 13.5 ± 5.3 | Dmax 13.4 ± 5.4 |
| Spinal cord D10 | 6.65 ± 1.05% | 7.75 ± 1.05% | 6.97 ± 0.91% | 3.6% | 3.6% | 11.5% | 9.4% | NA | NA |
| Oesophagus Dmean | 5.85 ± 2.48 | 5.34 ± 2.64 | 5.37 ± 2.45 | 4.5 | 5.0 | 6.4 | 6.7 | NA | NA |
| Skin Dmax Gy | NA | NA | NA | NA | NA | NA | NA | 11.1 ± 5.0 | 10.9 ± 4.9 |
| MUs | Mean + (SD) 8711 ± 1308 | Mean + (SD) 7730 ± 1843 | Mean + (SD) 6317 ± 1156 | Mean + (SD) 5660 | Mean + (SD) 7816 | Mean + (SD) 9399 | Mean + (SD) 9019 | 528 ± 113MU/Gy | 498 ± 91 MU/Gy |
| CIavg | 1.15 ± 0.06 | 1.12 ± 0.04 | 1.09 ± 0.03 | 1.10 | 1.02 | 1.10 | 0.90 | NA | NA |
MU, monitor units; PTV, planning target volume; IMRT, intensity modulated radiotherapy; VMAT, volumetric modulated arc therapy; 3DCRT, 3D conformal radiotherapy therapy; RA, rapid arc; FF, flattening filter; FFF, flattening filter free; Dmax, maximum dose; SD, standard deviation; CI, conformity index; Fx, fraction: Vn, volume of lung receiving n dose (Gy); NA, not applicable; Dxx%, dose (Gy) to xx% of volume.
Use of VMAT and IMRT for SBRT in abdominal lymph node metastases and pancreatic adenocarcinoma
One retrospective planning study evaluated the feasibility of VMAT and IMRT for SBRT in abdominal lymph node metastases on a patient cohort previously treated with SBRT, using 3DCRT techniques.11 The dosimetric results of this study are presented in Table3. Review of these results demonstrated: Effective treatment time increased by 41% with IMRT and decreased by 42% with VMAT compared to 3DCRT. VMAT significantly improved PTV95(%) coverage by a rate of 9% and conformality was greatest with VMAT and slightly less with IMRT compared to that of 3DCRT. In relation to OARs and dose to healthy tissue IMRT and VMAT showed superior healthy tissue sparing to that of CRT. The rate of improvement for IMRT and VMAT respectively in the following parameters are as follows: healthy tissue V10 Gy (%): 37% and 51%, spinal cord Dmax(Gy): NSD and 30%, small bowel D1% (Gy):17% and 22%, spinal cord D1% (Gy): 22% and 40% and mean liver dose NSD and 16%.
Table 3.
Summary of dosimetric results for SBRT planning study comparing 3DCRT versus IMRT versus VMAT–RA for the treatment of abdominal metastasis
| Reference | Bignardi et al.11 | Kumar et al.12 | |||
|---|---|---|---|---|---|
| Target area | Lymph nodes (abdominal region) 45 Gy/6Fx | Locally advanced pancreatic cancer | |||
| Dose/Fx | Plans acceptable if PTV dose >36 Gy (spinal cord 53 ± 21 cm3) (small bowel 780 ± 633 cm3) | 25 Gy/1Fx | |||
| Variable | 3DCRT | IMRT (co-planar) | VMAT RA | DS VMAT | DS IMRT |
| Patients | 14 | 15 | |||
| Effective treatment time (min) | 6.3 ± 0.5 | 10.6 ± 1.2 | 3.7 ± 0.4 | 9 | 11.45 |
| PTV: mean volume (cm3) | 44.0 ± 0.4 | 44.26 ± 0.4 | 44.5 ± 0.3 | 135 | 135 |
| PTV V95 (%) | 82.5 ± 9.6 | 84.5 ± 8.2 | 90.2 ± 5.2 | 97.5 | 98.6 |
| Healthy tissue V10Gy (%) | 6.3 ± 4.4 | 4.0 ± 1.9 | 3.1 ± 1.81 | NA | NA |
| Healthy tissue integral dose (Gy cm3 10−5) | NA | NA | NA | 0.37 | 0.35 |
| Healthy tissue CI60% | 3.8 ± 1.49 | 3.2 ± 0.7 | 2.5 ± 0.3 | NA | NA |
| Left kidney V5 Gy (%) | NA | NA | NA | 7.6 | 17.6 |
| Small bowel D1% (Gy) or D1cc (Gy) | D1% (Gy) 23.02 ± 10.81 | D1% (Gy) 19.01 ± 11.50 | D1% (Gy) 18.01 ± 10.83 | D1cc (Gy) 24.7 | D1cc (Gy) 25.6 |
| Small bowel V36Gy or V20 Gy (%) | V36Gy 0.3 ± 0.7 | V36Gy 0.2 ± 0.4 | V36Gy 0.1 ± 0.2 | V20 Gy 18.8 | V20 Gy 21.9 |
| Spinal cord Dmax (Gy) | 13.7 ± 5.7 | 13.9 ± 3.2 | 9.6 ± 2.3 | 11.6 | 11.7 |
| Spinal cord D1% (Gy) or V5 (Gy) | D1% (Gy) 12.9 ± 5.9 | D1% (Gy) 10 ± 2.8 | D1% (Gy) 7.8 ± 2.3 | V5 (Gy) 22.2 | V5 (Gy) 23.8 |
| Liver mean (Gy) | 4.3 ± 4.3 | 3.8 ± 4.0 | 3.6 ± 3.9 | 2.8 | 2.5 |
| MUs | 1554 ± 153 | 2583 ± 699 | 2186 ± 211 | 5437 | 6894 |
MU, monitor units; PTV, planning target volume; IMRT, intensity modulated radiotherapy; VMAT, volumetric modulated arc therapy; 3DCRT, 3D conformal radiotherapy therapy; RA, rapid arc; Dmax, maximum dose; SD, standard deviation; CI, conformity index; Fx, fraction; DS, dose sparing; Vn, volume of tissue receiving n dose (Gy); NA, not applicable; Dxx%, dose (Gy) to xx% of volume.
The use of IMRT SBRT to that of VMAT SBRT for the treatment of locally advanced pancreatic adenocarcinoma was also compared in a retrospective planning study of 15 patients.12 The dosimetric parameters summarised in Table3 for both plan types were similar. There was, however, a significant improvement in dose sparing with VMAT compared to IMRT to the spinal cord V5 Gy dose (P < 0.001) and the left kidney dose measured by Dmean (P < 0.001), V5Gy and D25%. Treatment time and MU utilised were both reduced by 21% with VMAT. In retrospect IMRT showed superior Dmean dosimetric parameters for the liver (P < 0.003) and stomach (P < 0.05) respectively. There was also a notable trend observed between PTV size and volumetric sparing of the duodenum; that is as the PTV size increased or overlapped into healthy tissue, the ability for both IMRT and VMAT SBRT to spare the duodenum decreased. We further examined this relationship and found in the two lung14,17 and spinal9,10 studies that utilised the same treatment parameters within their cohort, the ability to spare healthy surrounding OAR such as healthy lung and oesophagus decreased as the PTV size increased, measured by lung V20 and oesophagus Dmean respectively.
Discussion
Lung cancer
For patients with stage 1 NSCLC, surgical resection offers the best 5 year survival outcome of 60–70%.19 However, for patients with medically inoperable NSCLC who have undergone 3DCRT with standard fractionation and doses typically 45–60 Gy in 1.8–2.0 Gy/Fx over 6 weeks, the 5 year survival rate is only 10–30%.20 The use of SBRT for the treatment of medically inoperable stage 1 NSCLC has seen some more favourable results. The results of SBRT are notably illustrated by two prospective phase ll studies: The Radiation Therapy Oncology Group (RTOG 0236)21 and The Nordic Cooperative Group (NCG).22 Both studies investigated SBRT for peripheral stage 1 T1T2N0M0 tumours which were treated with 54 Gy/3Fx (RTOG 0236) or 45 Gy/3Fx (NCG), local control and overall survival for the RTOG 0236 trial at 3 years were reported to be 91% and 48% (T1N0M0) and 56% (T2N0M0) respectively, grade 3 and 4 treatment-related toxicity was seen in 28% of patients. The NCG group reported 3 year local control and overall survival to be 92% and 60% (T1N0M0) and 88% (T2N0M0) respectively.
As with the above studies, SBRT is most commonly employed using multiple static beams where treatment delivery can take up to 100 min.6 As treatment time increases so does the probability of tumour shifts from that of the initial setup. Recent evidence suggests that longer treatment times increase the rate of secondary cancers which are associated with cell repopulation during treatment.23 Faster application of SBRT possibly through VMAT, could therefore improve patient comfort, treatment accuracy and patient outcomes. Of the studies reviewed, VMAT FFF offered the greatest time improvement for both VMAT versus IMRT and VMAT versus CRT. Uncertainties in tumour position caused by intra-fractional motion have been of concern for SBRT treatments. However, adaptation of breath hold techniques with Active Breathing Coordinator™ (ABC; Elekta, Stockholm, Sweden) has been shown to reduce intra-fractional motion uncertainty, this was demonstrated in a prospective phase I/II study by the group at Royal Marsden Hospital in London,24 which have successfully used the combination of cone beam computer tomography, VMAT and ABC to reduce overall treatment time of lung SBRT and significantly reduce dose to healthy lung.
Proximal lesions (tumours that are within 2 cm of the proximal bronchial tree) have also been treated with SBRT with reasonable results; however, they have been associated with a larger degree of treatment-related toxicity.1 Patients with central tumours compared to those with peripheral tumours have a 11-fold higher risk of developing severe toxicity.1 Factors such as increased mean dose to the ipsilateral lung and V5 (%) of the contralateral lung have been associated with the development of radiation pneumonitis.2 In the studies that compared VMAT to 3DCRT two15,16 of these saw significant reductions in lung V5 and lung V20; however, the third study17 did not show a significant difference in lung V5 and showed better volume sparing for lung V20 in the 3DCRT cohort. However, in this study, the plans were optimised for chest wall sparing which resulted in increased lung dose. The results of these studies indicate that VMAT is a suitable alternative to 3DCRT SBRT for the treatment of medically inoperable NSCLC.
Spine
Spinal metastasis and spinal cord compression is commonly treated with conventional anterior posterior (AP) posterior anterior (PA) RT techniques; however treatment outcomes of conventional techniques have been sub-optimal.25 SBRT studies26 have shown significant improvements in pain management and long-term control. A prospective RTOG trial27 is currently further examining the feasibility of spinal SBRT. The planning of spinal SBRT requires steep dose constraints and a highly conformal dose distribution around the PTV which surrounds the spinal cord. One of the main complications of 3DCRT spinal SBRT treatment is radiation myelopathy,4 a recently developed logistic model has recommended a number of dose constraints to the thecal sac to maintain radiation myelopathy under 5%: The Dmax to the thecal sac for a single fraction should be no more than 12.4 Gy, for 2 fractions 17 Gy, 20.3 Gy for 3 fractions and 23.0 Gy for 4 fractions.28 In comparison, all three spinal studies reviewed8–10 were able to maintain this dose constraint whilst meeting 3DCRT SBRT planning constraints.
Closer analysis of the spinal studies suggests as first proposed by Wu et al.10 that there is a correlation between the number of MLC segments and the dose drop-off rate within the cord. Intensity map manipulation is inadvertently affected by the number of segments in a beam and therefore more segments allows for finer intensity manipulation and a sharper dose gradient at the PTV spinal cord junction. As reported earlier, VMAT utilises fewer segments than IMRT but this increases with the number of arcs, in the spinal studies analysed although cord dose was comparable, cord sparing improved when more arcs were implemented. Based on analysis of these results, it could be suggested that at least 2 arcs should be used for future planning and treatment studies, however higher doses may require 3 arcs to avoid delivery times >3 min per arc.9 All FFF vertebral plans required only 2 arcs.
There has been some concern that the use of FFF beams can increase the dose at the build-up regions due to an increase in lower mean energy from the lack of beam hardening.29 Ong et al.8 compared skin doses between 6-MV FF and 10-MV FFF and found no significant increase to skin dose.
An observed relationship throughout the studies utilising VMAT was the collimator angle used. Several studies8,9,11 reported the use of a collimator angle between 30° and 45°; Kuijper et al.9 suggested a collimator range of 30–45° achieved optimal sparing.
Lymph node lesions and pancreatic adenocarcinoma
The use of SBRT for the treatment of metastatic abdominal malignancies is very limited nevertheless recent results13 have shown improvement in local control and improved quality of life; however, studies such as these have been limited to the use of cyberknife.13 Results of the retrospective planning study reviewed, showed favourable outcomes for the use of IMRT and VMAT for the delivery of SBRT in abdominal malignancies.11 Most notably was the improvement in treatment time efficiency, conformality and reduction in dose to OAR. Organ motion relating to nodes in the hepatic hilus is still a concern; one solution is the implementation of an ABC technique in combination with VMAT FFF in patients who can hold their breath for 15 sec. Centres that do not have access to ABC could feasibly treat retroperitoneal nodes bordering large vessels effectively due to the limited organ motion in these areas.11 A current multicentre prospective study with VMAT-RA as the treatment modality should establish a more conclusive understanding of VMAT-FF treatment efficacy in this line of patients.
The use of SBRT for the treatment of unresectable pancreatic adenocarcinoma was studied by Kumar et al.,12 with the exception of spinal cord Dmax both IMRT/VMAT SBRT were able to obtain the normal tissue constraints used for treatment planning constraints utilised in previous Cyberknife studies.30
One of the main concerns in previous studies such as the one conducted by Chang et al.30 is treatment-related toxicity; however, the dosimetric parameters presented by Kumar et al.12 suggest advantageous dosimetric sparing to that of Cyberknife SBRT, specifically significant reductions in kidney, stomach and liver dose. VMAT SBRT offered significant improvements in treatment time and MU over the other modalities.
Similar to the findings of Kumar et al.,12 we suggest a relationship between PTV size and the ability to spare healthy tissue. As the PTV size increases for both IMRT and VMAT, efficiency of volumetric sparing of healthy tissues decreases. We suggest future studies look at this relationship in more detail.
Limitations
Limitations of this review included the small population numbers in some of the studies reviewed and some studies did not present the same comparative parameters. Where adequate data were given, we tabulated and recorded this in the appropriate tables. As data for large SBRT studies are only recently emerging, future studies should look at the trends presented in these studies in more detail when developing clinical guidelines. Future studies should also place a greater emphasis on survival and toxicity outcomes between SBRT treatment modalities, specifically assessing planning constraints like number of arcs used and length of delivery time to that of late sequel side effects. This should result in the development of more defined planning constraints and guidelines for SBRT treatment modalities.
Conclusion
In all the studies that were reviewed, the use of IMRT and VMAT for SBRT compared to 3DCRT SBRT showed improvements in dose conformality and homogeneity. Dose conformality was greatest with VMAT. Treatment time was markedly quicker with VMAT versus 3DCRT and IMRT in all cases except one. Overall both IMRT and VMAT were able to meet all planning constraints in the studies reviewed; however, treatment efficiency was greatest with VMAT. Notably cord sparing improved with a greater number of arcs, we suggest a minimum of 2 arcs be used for future planning and treatment studies. The relationship between PTV size and volumetric sparing efficiency should be further examined to form a conclusive understanding of this relationship. Overall VMAT and IMRT have been demonstrated as feasible alternatives to traditional static field RT SBRT.
Conflict of Interest
The authors declare no conflict of interest.
References
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- Ong CL, Palma D, Verbakel WFAR, Slotman BJ, Senan S. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): Planning considerations and early toxicity. Radiother Oncol. 2010;97:431–6. doi: 10.1016/j.radonc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Williams SG, Taylor JMG, Liu N, et al. Use of individual fraction size data from 3756 patients to directly determine the αβ ratio of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:24–33. doi: 10.1016/j.ijrobp.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Lo SS, Sahgal A, Chang EL, et al. Serious complications associated with stereotactic ablative radiotherapy and strategies to mitigate the risk. J Clin Oncol. 2013;25:378–87. doi: 10.1016/j.clon.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Hodge W, Tome WA, Jaradat HA, et al. Feasibility report of image guided stereotactic body radiotherapy (IG-SBRT) with tomotherapy for early stage medically inoperable lung cancer using extreme hypofractionation. Acta Oncol. 2006;45:890–6. doi: 10.1080/02841860600907329. [DOI] [PubMed] [Google Scholar]
- van der Voort van Zyp NC, Prévost J-B, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: Clinical outcome. Radiother Oncol. 2009;91:296–300. doi: 10.1016/j.radonc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Hoogeman MS, Nuyttens JJ, Levendag PC, Heijmen BJ. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 2008;70:609–18. doi: 10.1016/j.ijrobp.2007.08.066. [DOI] [PubMed] [Google Scholar]
- Ong CL, Verbakel WFAR, Dahele M, Cuijpers JP, Slotman BJ, Senan S. Fast arc delivery for stereotactic body radiotherapy of vertebral and lung tumors. Int J Radiat Oncol Biol Phys. 2012;83:e137–43. doi: 10.1016/j.ijrobp.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Kuijper IT, Dahele M, Senan S, Verbakel WFAR. Volumetric modulated arc therapy versus conventional intensity modulated radiation therapy for stereotactic spine radiotherapy: A planning study and early clinical data. Radiother Oncol. 2010;94:224–8. doi: 10.1016/j.radonc.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Wu QJ, Yoo S, Kirkpatrick JP, Thongphiew D, Yin F-F. Volumetric arc intensity-modulated therapy for spine body radiotherapy: Comparison with static intensity-modulated treatment. Int J Radiat Oncol Biol Phys. 2009;75:1596–604. doi: 10.1016/j.ijrobp.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Bignardi M, Cozzi L, Fogliata A, et al. Critical appraisal of volumetric modulated arc therapy in stereotactic body radiation therapy for metastases to abdominal lymph nodes. Int J Radiat Oncol Biol Phys. 2009;75:1570–7. doi: 10.1016/j.ijrobp.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Kumar R, Wild AT, Ziegler MA, et al. Stereotactic body radiation therapy planning with duodenal sparing using volumetric-modulated arc therapy vs intensity-modulated radiation therapy in locally advanced pancreatic cancer: A dosimetric analysis. Med Dosim. 2013;38:243–50. doi: 10.1016/j.meddos.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CW, Cho CK, Yoo SY, et al. Image-guided stereotactic body radiation therapy in patients with isolated para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys. 2009;74:147–53. doi: 10.1016/j.ijrobp.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Holt A, van Vliet-Vroegindeweij C, Mans A, Belderbos JS, Damen EMF. Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: A comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys. 2011;81:1560–7. doi: 10.1016/j.ijrobp.2010.09.014. [DOI] [PubMed] [Google Scholar]
- McGrath SD, Matuszak MM, Yan D, Kestin LL, Martinez AA, Grills IS. Volumetric modulated arc therapy for delivery of hypofractionated stereotactic lung radiotherapy: A dosimetric and treatment efficiency analysis. Radiother Oncol. 2010;95:153–7. doi: 10.1016/j.radonc.2009.12.039. [DOI] [PubMed] [Google Scholar]
- Navarria P, Ascolese AM, Mancosu P, et al. Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC) Radiother Oncol. 2013;107:414–8. doi: 10.1016/j.radonc.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Ong CL, Verbakel WFAR, Cuijpers JP, Slotman BJ, Lagerwaard FJ, Senan S. Stereotactic radiotherapy for peripheral lung tumors: A comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol. 2010;97:437–42. doi: 10.1016/j.radonc.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Videtic GMM, Stephans K, Reddy C, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: Excellent local control. Int J Radiat Oncol Biol Phys. 2010;77:344–9. doi: 10.1016/j.ijrobp.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Agarwal M, Brahmanday G, Chmielewski GW, Welsh RJ, Ravikrishnan KP. Age, tumor size, type of surgery, and gender predict survival in early stage (stage I and II) non-small cell lung cancer after surgical resection. Lung Cancer. 2010;68:398–402. doi: 10.1016/j.lungcan.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Cai X-W, Xu L-Y, Wang L, et al. Comparative survival in patients with postresection recurrent versus newly diagnosed non–small-cell lung cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1100–5. doi: 10.1016/j.ijrobp.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Timmerman RD, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for medically inoperable early-stage lung cancer patients. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- Sachs RK, Shuryak I, Brenner D, Fakir H, Hlatky L, Hahnfeldt P. Second cancers after fractionated radiotherapy: Stochastic population dynamics effects. J Theor Biol. 2007;249:518–31. doi: 10.1016/j.jtbi.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald F, McNair H, Brock J, et al. VISUAL: Clinical implementation of volumetric modulated arc therapy (VMAT) image-guided stereotactic radiotherapy using active breathing control (ABC) for small volume lung tumours. Lung Cancer. 2010;67(Supplement 1):S29. [Google Scholar]
- Maranzano E, Trippa F, Casale M, et al. 8 Gy single-dose radiotherapy is effective in metastatic spinal cord compression: Results of a phase III randomized multicentre Italian trial. Radiother Oncol. 2009;93:174–9. doi: 10.1016/j.radonc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Gibbs IC, Kamnerdsupaphon P, Ryu M-R, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–90. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase II/III study of image-guided stereotactic radiosurgery for localized (1-3) spine metastases: Phase II results. Int J Radiat Oncol Biol Phys. 2011;81:S131–2. doi: 10.1016/j.prro.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85:341–7. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Kragl G, af WetterstedtS, Knäusl B, et al. Dosimetric characteristics of 6 and 10MV unflattened photon beams. Radiother Oncol. 2009;93:141–6. doi: 10.1016/j.radonc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–72. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]


