Abstract
BACKGROUND
Despite an estimated prevalence of 10% in women, the etiology of endometriosis remains poorly understood. Over recent decades, endometriosis has been associated with risk of several chronic diseases, such as cancer, autoimmune diseases, asthma/atopic diseases and cardiovascular diseases. A deeper understanding of these associations is needed as they may provide new leads into the causes or consequences of endometriosis. This review summarizes the available epidemiological findings on the associations between endometriosis and other chronic diseases and discusses hypotheses for underlying mechanisms, potential sources of bias and methodological complexities.
METHODS
We performed a comprehensive search of the PubMed/Medline and ISI Web of Knowledge databases for all studies reporting on the associations between endometriosis and other diseases published in English through to May 2014, using numerous search terms. We additionally examined the reference lists of all identified papers to capture any additional articles that were not identified through computer searches.
RESULTS
We identified 21 studies on the associations between endometriosis and ovarian cancer, 14 for breast cancer, 8 for endometrial cancer, 4 for cervical cancer, 12 for cutaneous melanoma and 3 for non-Hodgkin's lymphoma, as well as 9 on the links between endometriosis and autoimmune diseases, 6 on the links with asthma and atopic diseases, and 4 on the links with cardiovascular diseases. Endometriosis patients were reported to be at higher risk of ovarian and breast cancers, cutaneous melanoma, asthma, and some autoimmune, cardiovascular and atopic diseases, and at decreased risk of cervical cancer.
CONCLUSIONS
Increasing evidence suggests that endometriosis patients are at higher risk of several chronic diseases. Although the underlying mechanisms are not yet understood, the available data to date suggest that endometriosis is not harmless with respects to women's long-term health. If these relationships are confirmed, these findings may have important implications in screening practices and in the management and care of endometriosis patients.
Keywords: asthma, autoimmune diseases, cancer, cardiovascular diseases, endometriosis
Introduction
Endometriosis, defined as the presence of endometrial tissue outside of the uterus, is the third leading cause of gynecologic hospitalization in the USA (Eskenazi and Warner, 1997) and one of the main causes of infertility in women. Cyclical bleeding from ectopic endometrial implants leads to the development of inflammation, scarring and adhesions, which produce debilitating pain and symptoms (such as chronic pelvic pain, fatigue, dysmenorrhea, dyspareunia, dysuria or dyschezia (Nnoaham et al., 2011)). Unfortunately, there is delayed diagnosis, and treatment options are poor (Ballweg, 1987). Despite an estimated prevalence of 10% in women (Nnoaham et al., 2011), substantial associated costs (Simoens et al., 2011a, b) and considerable adverse impacts on quality of life (Nnoaham et al., 2011), the etiology of endometriosis remains largely unknown.
There is evidence for the involvement of female hormones, local inflammation and disruption of immunologic processes in endometriosis (Missmer and Cramer, 2003), although it is unclear whether immune dysfunction is a cause or a consequence of the disease (Herington et al., 2011). Familial aggregation of endometriosis additionally suggests a genetic contribution to the disease (Montgomery et al., 2008), and some genetic factors have been identified in genome-wide association studies (Rahmioglu et al., 2014). Recent evidence also suggests that environmental toxicants (such as phthalates, bisphenol A or organochlorinated pollutants) may play a role in the development of endometriosis (Buck Louis et al., 2013; Porpora et al., 2013). However, the only factors that have been robustly associated with endometriosis to date reflect increased exposure to menstruation (i.e. earlier menarche, shorter menstrual cycles and nulliparity) (Missmer and Cramer, 2003) and a low body mass index (Vigano et al., 2012). Modifiable risk factors remain to be identified to help primary prevention of endometriosis.
While little is understood about the causes of the disease, endometriosis has been associated with several types of cancer, autoimmune diseases, asthma/atopic diseases or cardiovascular diseases over recent decades, suggesting that endometriosis patients may represent a high-risk group for these chronic diseases. A deep understanding of the associations between endometriosis and other chronic disease outcomes may thus extend our knowledge and provide new leads into the causes or consequences of endometriosis. Also, given the prevalence of endometriosis, the development of targeted prevention and early detection guidelines for these chronic diseases may have a significant public health impact (Missmer, 2009).
This review summarizes the available epidemiological findings on the associations between endometriosis and major chronic diseases, and discusses hypotheses for underlying mechanisms, potential sources of bias and methodological complexities.
Methods
We performed a comprehensive search of the PubMed/Medline and ISI Web of Knowledge databases for all epidemiological studies reporting on the associations between endometriosis and other diseases published up to May 2014. The search terms included ‘endometriosis’ in combination with the following terms: ‘cancer’, ‘breast cancer’, ‘ovarian cancer’, ‘endometrial cancer’, ‘melanoma’, ‘skin cancer’, ‘colorectal cancer’, ‘lymphoma’, ‘thyroid cancer’, ‘autoimmune disease’, ‘systemic lupus erythematosus’, ‘Sjögren's syndrome’, ‘scleroderma’, ‘multiple sclerosis’, ‘rheumatoid arthritis’, ‘inflammatory bowel disease’, ‘celiac disease’, ‘asthma’, ‘allergy’, ‘atopic disease’, ‘cardiovascular disease’, ‘myocardial infarction’, ‘atherosclerosis’, ‘angina’. We additionally examined the reference lists of all identified papers to capture any additional articles that were not identified through computer searches.
We summarized the current literature on the topic, and based on the retrieved publications and the experience of our group on this topic, we identified and discussed hypotheses for underlying mechanisms, potential sources of bias, and methodological complexities related to the exploration of such associations.
Results
Cancer
While endometriosis is generally regarded as a benign condition, evidence suggests a potential link with some cancers. The disease has even been suggested to share some characteristics with malignant tumors, such as local and distant invasion, abnormal tissue growth, dysfunction of target organs and genetic damage (Garry, 2001). The epidemiological studies that have investigated the link between endometriosis and cancer are reviewed below.
Gynecological cancers
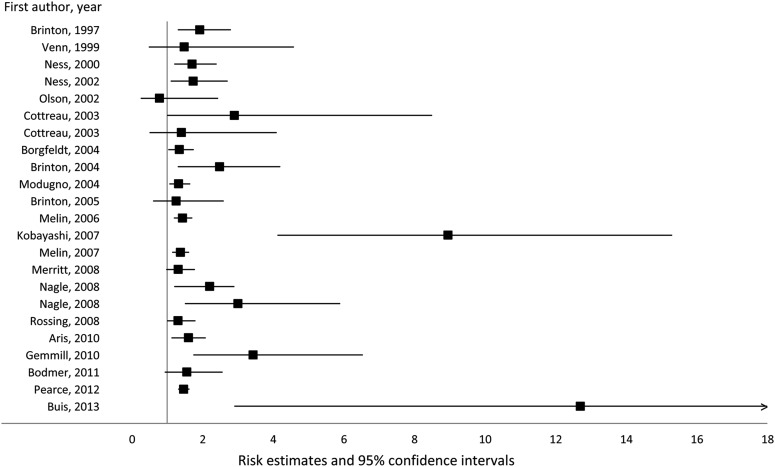
Of all neoplasms, ovarian cancer has been the most consistently associated with endometriosis. This association was first identified by Sampson in 1925 (Sampson 1925) and has been observed in a number of clinical case series reporting a high rate of endometriosis in ovarian cancer patients (Somigliana et al., 2006; Heidemann et al., 2014). Among the 21 epidemiological studies that have investigated endometriosis in relation to ovarian cancer risk to date (Fig. 1), 20 reported a positive association (including 16 which reported statistically significant findings) (Brinton et al., 1997, 2004, 2005; Venn et al., 1999; Ness et al., 2000, 2002; Cottreau et al., 2003; Borgfeldt and Andolf, 2004; Modugno et al., 2004; Melin et al., 2006, 2007; Kobayashi et al., 2007; Merritt et al., 2008; Nagle et al., 2008; Rossing et al., 2008; Aris, 2010; Gemmill et al., 2010; Bodmer et al., 2011; Pearce et al., 2012; Buis et al., 2013) (Table I). Most of these reports were based on a case–control or a retrospective cohort design, or were focused on specific populations (i.e. cohort of endometriosis patients, or cohort of women evaluated for infertility). The sole study reporting no association was based on a cohort of post-menopausal women (Olson et al., 2002).
Figure 1.
Association between endometriosis and ovarian cancer risk.
Table I.
Epidemiological studies exploring the associations between endometriosis and cancer.
| Studies | Geographic location | Study period | Design | Endometriosis number and ascertainment | Cancer cases | Risk estimates (RR, OR or SIR and 95% CI) | Comments |
|---|---|---|---|---|---|---|---|
| Ovarian cancer | |||||||
| Brinton et al. (1997) | Sweden | 1969–1989 | Retrospective cohort | 20 686 Clinical records |
29 | 1.92 (1.3–2.8) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Venn et al. (1999) | Australia | 1986–1996 | Retrospective cohort | – Clinical records |
13 | 1.48 (0.48–4.59) | Cohort of women referred to an IVF clinic |
| Ness et al. (2000) | US | 1994–1998 | Case–control | 151 Self-report |
767 | 1.7 (1.2–2.4) | |
| Ness et al. (2002) | Multiple | 1989–1999 | Pooled analysis of case–control studies | 90 Self-report |
3678 | 1.73 (1.10–2.71) | |
| Olson et al. (2002) | US | 1986–1998 | Prospective cohort | 1392 Self-report |
188 | 0.78 (0.25–2.44) | Cohort of post-menopausal women Age-adjusted estimate |
| Cottreau et al. (2003) | Multiple | 1989–1999 | Case–control | 249 Self-report |
1373 | 2.9 (1.0–8.5) 1.4 (0.5–4.1) |
In danazol users In lupron or nafarelin users |
| Borgfeldt and Andolf (2004) | Sweden | 1969–1997 | Case–control | 28 163 Clinical records |
81 | 1.34 (1.03–1.75) | |
| Brinton et al. (2004) | US | 1965–1999 | Retrospective cohort | 1919 Clinical records |
45 | 2.48 (1.3–4.2) | Cohort of women evaluated for infertility |
| Modugno et al. (2004) | US | 1993–2001 | Case–control | 361 Self-report |
2098 | 1.32 (1.06–1.65) | |
| Brinton et al. (2005) | US | 1965–1999 | Retrospective cohort | – Clinical records |
45 | 1.25 (0.6–2.6) | Cohort of women evaluated for infertility |
| Melin et al. (2006) | Sweden | 1969–2000 | Retrospective cohort | 64 492 Clinical records |
122 | 1.43 (1.19–1.71) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Kobayashi et al. (2007) | Japan | 1985–2002 | Prospective cohort | 6398 Clinical records |
46 | 8.95 (4.12–15.3) | Cohort of women with clinically documented endometriomas |
| Melin et al. (2007) | Sweden | 1958–2002 | Retrospective cohort (update) | 63 630 Clinical records |
134 | 1.37 (1.14–1.62) | Cohort of women hospitalized for endometriosis |
| Merritt et al. (2008) | Australia | 2002–2005 | Case–control | 211 Self-report |
1576 | 1.31 (0.97–1.78) | Increased risks observed for endometrioid (1.85, 1.02–3.38) and clear cell (2.66, 1.31–5.44) subtypes, but not with mucinous or serous types |
| Nagle et al. (2008) | Australia | 2002–2005 | Case–control | 118 Self-report |
142 endometrioid 90 clear cell |
2.2 (1.2–3.9) 3.0 (1.5–5.9) |
|
| Rossing et al. (2008) | US | 2002–2005 | Case–control | 175 Self-report |
812 | 1.3 (1.0–1.8) | Association with endometrioid and clear cell invasive subtypes combined (2.8, 1.7–4.7) but not with other subtypes, and with invasive (1.5, 1.1–2.1) but not borderline tumors |
| Aris (2010) | Canada | 1997–2006 | Cross-sectional | 2521 Computerized patients' records |
292 Identified from cancer registry |
1.6 (1.12–2.09) | Endometriosis-associated ovarian cancer was mostly of the endometrioid (24.4%, P = 0.007) and clear-cell types (21.9%, P = 0.003) |
| Gemmill et al. (2010) | US | 1998 | Cross-sectional | – Self-report of a surgical diagnosis |
– | 3.43 (1.74–6.54) | Cohort of patient members of the Endometriosis Association |
| Bodmer et al. (2011) | UK | 1995–2009 | Case–control | 88 Recorded diagnosis from National General Practitioner Database |
1161 Recorded diagnosis from National General Practitioner Database |
1.55 (0.93–2.57) | |
| Pearce et al. (2012) | Multiple locations | 1992–2008 | Pooled analysis of case–control studies | 1556 Self-report |
7911 invasive 1907 borderline |
1.46 (1.31–1.63) | Associations with clear-cell (3.05, 2.43–3.84), endometrioid (2.04, 1.67–2.48) and low-grade serous (2.11, 1.39–3.20) tumors, but not with mucinous, high-grade serous or borderline tumors |
| Buis et al. (2013) | The Netherlands | 1980–2007 | Retrospective cohort | 3657 Clinical records |
19 | 12.7 (2.9–55.5) | Cohort of women with subfertility problems |
| Breast cancer | |||||||
| Moseson et al. (1993) | US | 1977–1981 | Case–control | 747 Self-report |
354 | 1.7 (0.6–5.1) | |
| Brinton et al. (1997) | Sweden | 1969–1989 | Retrospective cohort | 20 686 Clinical records |
297 | 1.27 (1.1–1.4) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Schairer et al. (1997) | Sweden | 1965–1987 | Retrospective cohort | 5 Clinical records |
295 | 3.2 (1.2–8.0) in those with hysterectomy indicated for endometriosis treatment 1.7 (0.7–4.1) in those with oophorectomy indicated for endometriosis treatment |
Cohort of women who underwent gynecological surgery |
| Venn et al. (1999) | Australia | 1986–1996 | Retrospective cohort | – Clinical records |
143 | 1.04 (0.71–1.54) | Cohort of women referred to an IVF clinic |
| Weiss et al. (1999) | US | 1990–1992 | Case–control | 96 Self-report of surgical diagnosis |
2173 | 1.14 (0.7–1.8) | |
| Baron et al. (2001) | US | 1990–1994 | Case–control | 541 Self-report |
5659 | 0.8 (0.7–1.0) | |
| Olson et al. (2002) | US | 1986–1998 | Prospective cohort | 1392 Self-report |
1795 | 0.96 (0.75–1.23) | Cohort of post-menopausal women |
| Borgfeldt and Andolf (2004) | Sweden | 1969–1997 | Case–control | 28 163 Clinical records |
427 | 1.10 (0.98–1.23) | |
| Brinton et al. (2005) | US | 1965–1999 | Retrospective cohort | – Clinical records |
292 | 0.78 (0.6–1.1) | Cohort of women evaluated for infertility |
| Melin et al. (2006) | Sweden | 1969–2000 | Retrospective cohort | 64 492 Clinical records |
1288 | 1.04 (0.98–1.09) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Bertelsen et al. (2007) | Denmark | 1978–1998 | Retrospective cohort | 1978 Clinical records |
16 983 | 0.97 (0.85–1.11) | Inverse associations in women with <40 years of age at endometriosis diagnosis; but positive association in those ≥50 years at endometriosis diagnosis |
| Melin et al. (2007) | Sweden | 1958–2002 | Retrospective cohort (update) | 63 630 Clinical records |
1465 | 1.08 (1.02–1.13) | Cohort of women hospitalized for endometriosis |
| Gemmill et al. (2010) | US | 1998 | Cross-sectional | – Self-report of a surgical diagnosis |
– | 0.54 (0.32–0.90) | Cohort of patient members of the Endometriosis Association |
| Matta et al. (2013) | Puerto Rico | 2006–2012 | Case–control | 80 Self-report of a surgical diagnosis |
385 | 0.5 (0.3–0.9) | |
| Endometrial cancer | |||||||
| Brinton et al. (1997) | Sweden | 1969–1989 | Retrospective cohort | 20 686 Clinical records |
12 | 1.09 (0.6–1.9) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Venn et al. (1999) | Australia | 1986–1996 | Retrospective cohort | – Clinical records |
12 | 0.91 (0.13–6.49) | Cohort of women referred to an IVF clinic |
| Olson et al. (2002) | US | 1986–1998 | Prospective cohort | 1392 Self-report |
– | 1.20 (0.57–2.53) | Cohort of post-menopausal women |
| Borgfeldt and Andolf (2004) | Sweden | 1969–1997 | Case–control | 28 163 Clinical records |
39 | 0.58 (0.42–0.81) | |
| Brinton et al. (2005) | US | 1965–1999 | Retrospective cohort | – Clinical records |
39 | 0.82 (0.3–1.9) | Cohort of women evaluated for infertility |
| Melin et al. (2006) | Sweden | 1969–2000 | Retrospective cohort | 64 492 Clinical records |
92 | 1.19 (0.96–1.46) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Melin et al. (2007) | Sweden | 1958–2002 | Retrospective cohort (update) | 63 630 Clinical records |
97 | 1.14 (0.93–1.39) | Cohort of women hospitalized for endometriosis |
| Zucchetto et al. (2009) | Italy | 1992–2006 | Case–control | 11 Self-report |
454 | 4.03 (1.04–15.52) | |
| Cervical cancer | |||||||
| Brinton et al. (1997) | Sweden | 1969–1989 | Retrospective cohort | 20 686 Clinical records |
11 | 0.72 (0.4–1.3) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Borgfeldt and Andolf (2004) | Sweden | 1969–1997 | Case–control | 28 163 Clinical records |
23 | 0.57 (0.37–0.90) | |
| Melin et al. (2006) | Sweden | 1969–2000 | Retrospective cohort | 64 492 Clinical records |
51 | 0.64 (0.47–0.84) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Melin et al. (2007) | Sweden | 1958–2002 | Retrospective cohort (update) | 63 630 Clinical records |
49 | 0.71 (0.53–0.94) | Cohort of women hospitalized for endometriosis |
| Cutaneous melanoma | |||||||
| Wyshak et al. (1989) | US | 1981 | Cross-sectional | 9 Self-report |
18 | 3.9 (1.2–12.4) | |
| Frisch et al. (1992) | US | – | Case–control | – Self-report |
71 | 1.1 (0.5–2.3) | |
| Holly et al. (1995) | US | 1981–1986 | Case–control | – Self-report |
452 | 0.9 (0.5–1.4) | |
| Brinton et al. (1997) | Sweden | 1969–1989 | Retrospective cohort | 20 686 Clinical records |
35 | 1.0 (0.7–1.5) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Wyshak and Frisch (2000) | US | – | Cross-sectional (update) | – Self-report |
62 | OR = 2.6, P = 0.3 in red-haired | |
| Young et al. (2001) | Australia | 1980–1990 | Retrospective case-cohort | 3 Clinical records |
14 | 0.62 (0.16–2.41) | |
| Olson et al. (2002) | US | 1986–1998 | Prospective cohort | 1392 Self-report |
– | 0.67 (0.25–1.80) | Cohort of post-menopausal women |
| Brinton et al. (2005) | US | 1965–1999 | Retrospective cohort | – Clinical records |
42 | 2.06 (1.0–4.4) | Cohort of women evaluated for infertility |
| Melin et al. (2006) | Sweden | 1969–2000 | Retrospective cohort | 64 492 Clinical records |
186 | 1.16 (1.00–1.33) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Kvaskoff et al. (2007) | France | 1990–2002 | Prospective cohort | 5949 Self-report of diagnosis or treatment through specific procedures |
363 | 1.62 (1.15–2.29) | |
| Melin et al. (2007) | Sweden | 1958–2002 | Retrospective cohort (update) | 63 630 Clinical records |
217 | 1.23 (1.07–1.40) | Cohort of women hospitalized for endometriosis |
| Gemmill et al. (2010) | US | 1998 | Cross-sectional | – Self-report of a surgical diagnosis |
– | 3.81 (2.60–5.56) | Cohort of patient members of the Endometriosis Association |
| Non-Hodgkin's lymphoma | |||||||
| Brinton et al. (1997) | Sweden | 1969–1989 | Retrospective cohort | 20 686 Clinical records |
28 | 1.79 (1.2–2.6) | Cohort of women hospitalized for endometriosis Data included in Melin et al. (2007) |
| Olson et al. (2002) | US | 1986–1998 | Prospective cohort | 1392 Self-report |
243 | 1.67 (0.97–2.87) | Cohort of post-menopausal women |
| Gemmill et al. (2010) | US | 1998 | Cross-sectional | – Self-report of a surgical diagnosis |
– | 0.84 (0.14–3.37) | Cohort of patient members of the Endometriosis Association |
CI, confidence interval; OR, odds ratio; RR, relative risk; SIR, standardized incidence ratio.
The reports suggesting a positive association include two large pooled analyses of case–control studies on ovarian cancer, which include the published analyses in individual studies and have overlapping analysis populations. Ness et al. pooled the data from eight case–control studies that ascertained 3678 ovarian cancer cases over 1989–1999 and observed a pooled odds-ratio (OR) of 1.73 (95% confidence interval (CI) = 1.10–2.71) for ovarian cancer risk in relation to self-reported endometriosis (Ness et al., 2002). Pearce et al. used data from the Ovarian Cancer Association Consortium in a pooled analysis of 13 case–control studies that included 7911 invasive ovarian cancer cases and 1907 borderline ovarian tumors ascertained over 1992–2008, constituting the largest evaluation of the association to date (Pearce et al., 2012). The analysis produced a pooled OR of 1.46 (95% CI = 1.31–1.63) for self-reported endometriosis in relation to invasive ovarian cancer risk. In that report, subtype analyses suggested 2-fold increased risks of endometrioid or low-grade serous subtypes, and a 3-fold increased risk of clear-cell ovarian cancer, but no significantly increased risk of mucinous or high-grade serous tumors, and there was no significant association between endometriosis and borderline ovarian cancer risk.
Recently, a meta-analysis evaluated the associations between endometriosis and risk and prognosis of ovarian cancer in studies published between 1990 and 2012 (Kim et al., 2014). In case–control or two-arm studies, the RR was 1.27 (95% CI = 1.21–1.32), and the standardized incidence ratio (SIR) was 1.80 (95% CI = 1.28–2.53) in single-arm cohort studies. A significant positive association was observed regardless of study design, assessment of endometriosis, quality score of study or number of adjustment factors. Interestingly, ovarian cancer associated with endometriosis was associated with better overall survival and early-stage, low-grade ovarian cancer, although progression-free ovarian cancer survival did not differ according to the presence of endometriosis.
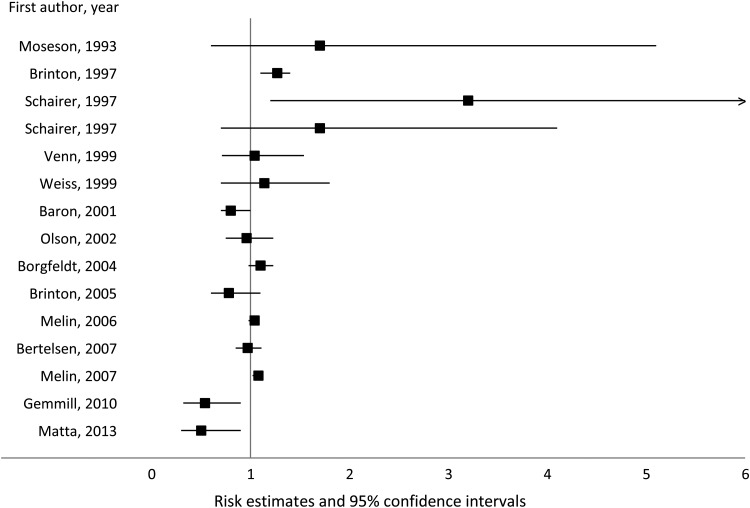
Findings for other gynecological cancers have been conflicting. While most studies suggest a modest positive association between endometriosis and the risk of breast cancer (albeit only three reported significant positive associations) (Moseson et al., 1993; Brinton et al., 1997; Schairer et al., 1997; Borgfeldt and Andolf, 2004; Melin et al., 2006, 2007), four observed no clear association (Venn et al., 1999; Weiss et al., 1999; Olson et al., 2002; Bertelsen et al., 2007) and four reported an inverse relation (Baron et al., 2001; Brinton et al., 2005; Gemmill et al., 2010; Matta et al., 2013) (Fig. 2). None of the available studies evaluated heterogeneity of the findings according to breast cancer type, hormone receptor status or molecular subtype.
Figure 2.
Association between endometriosis and breast cancer risk.
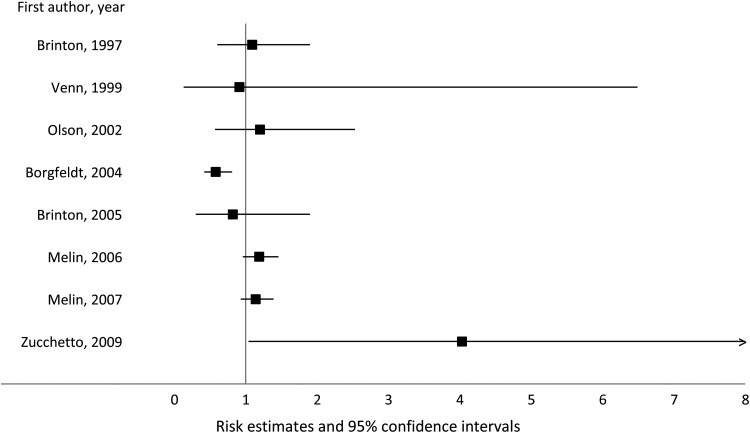
Regarding endometrial cancer, the main issue in previous investigations was the low number of cases, which offered little statistical power to adequately explore its relation with endometriosis. Most studies (with numbers of endometrial cancer cases ranging from 12 to 97) observed no association (Brinton et al., 1997, 2005; Venn et al., 1999; Melin et al., 2007), while two studies (including one with 454 cases (Zucchetto et al., 2009)) reported increased endometrial cancer risk in women with endometriosis (Melin et al., 2006; Zucchetto et al., 2009), in contrast to one report suggesting a significantly inverse association (Borgfeldt and Andolf, 2004) (Fig. 3).
Figure 3.
Association between endometriosis and endometrial cancer risk.
While statistical power was even lower for cervical cancer in previous studies, all of the available investigations to date, all from Sweden, have reported decreased risks in women with endometriosis (Brinton et al., 1997; Borgfeldt and Andolf, 2004; Melin et al., 2006, 2007) (of note, data from two of these studies (Brinton et al., 1997; Melin et al., 2006) were included in the most recent historical cohort on this topic (Melin et al., 2007)).
Other cancers
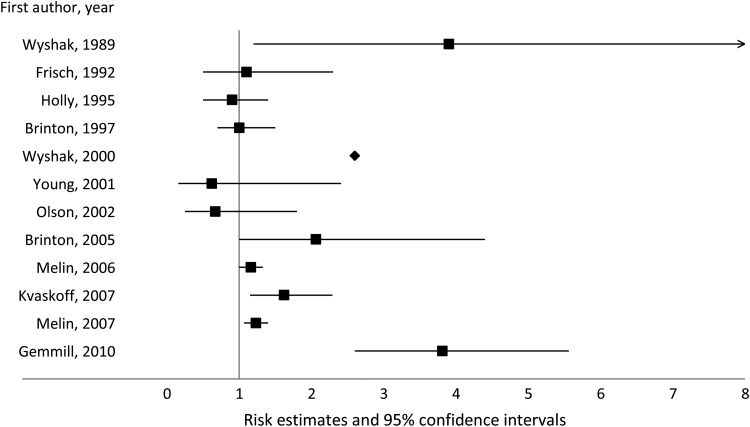
Among non-gynecological cancers, cutaneous melanoma has been the most studied in relation to a history of endometriosis. Out of the 12 studies that explored this topic, seven suggested a positive association (Wyshak et al., 1989; Wyshak and Frisch, 2000; Brinton et al., 2005; Melin et al., 2006, 2007; Kvaskoff et al., 2007; Gemmill et al., 2010), while 5 studies reported no clear relation between endometriosis and cutaneous melanoma risk (Frisch et al., 1992; Holly, Cress and Ahn, 1995; Brinton et al., 1997; Young et al., 2001; Olson et al., 2002) (Fig. 4). Only three studies to date have assessed endometriosis in relation to non-melanoma skin cancers (basal-cell carcinoma and squamous-cell carcinoma), none of which detected evidence of an association (Wyshak et al., 1989; Brinton et al., 1997; Melin et al., 2006).
Figure 4.
Association between endometriosis and cutaneous melanoma risk.
Explorations of endometriosis in relation to other types of cancer have been sparse. Two cohort studies have reported a higher risk of non-Hodgkin's lymphoma in women with endometriosis (Brinton et al., 1997; Olson et al., 2002), while a cross-sectional study among patient members of the Endometriosis Association observed no association (Gemmill et al., 2010). Women with endometriosis were reported to be at increased risk of brain cancer in three studies (Melin et al., 2006, 2007; Claus et al., 2011), of endocrine cancers in two studies (Melin et al., 2006, 2007), and of thyroid or kidney cancer in one study (Melin et al., 2007). However, other studies showed no association between endometriosis and any of these or other cancers (Brinton et al., 1997, 2005; Olson et al., 2002).
Autoimmune diseases
Women have been shown to have a higher prevalence than men of several autoimmune diseases, including systemic lupus erythematosus (SLE), Sjögren's syndrome (SS), scleroderma, multiple sclerosis (MS) and rheumatoid arthritis (RA) (Tiniakou et al., 2013). While part of this female preponderance has been proposed to reflect sex differences in genetic and environmental exposures (Quintero et al., 2012; Tiniakou et al., 2013), it has also led to the hypothesis that female hormones have a fundamental role in the pathogenesis of these diseases (Pennell et al., 2012). Consistently, estrogens and prolactin have been shown to act as immune stimulants, while androgens are immune suppressors, and experimental studies show that each of these hormones promotes specific immunologic events in different types of autoimmune diseases (Quintero et al., 2012). Women with endometriosis have been demonstrated to exhibit altered immune surveillance, with depressed cell-mediated immunity (high T-, B-, and natural killer cell counts but decreased activity) and heightened humoral immune response (high serum levels of IgG, IgA, IgM autoantibodies, and anti-endometrial antibodies) (Nothnick, 2001), abnormalities which are also observed in autoimmune diseases (Eisenberg et al., 2012).
Few studies have evaluated the co-morbidity of endometriosis with autoimmune diseases (Table II). A cross-sectional survey conducted among patient members of the Endometriosis Association first reported higher than expected rates of SLE, MS, RA and SS than in the general female US population (Sinaii et al., 2002). While the latter study relied on self-reported diagnosis of autoimmune diseases, a later Spanish case–control study based on clinical record information reported no significant association between endometriosis and risk of SLE or SS (Matorras et al., 2007). However, the most recent and largest report to date, based on a retrospective cohort relying on clinical records (n = 37 661), observed a significant increased risk of SLE (SIR = 1.6; n = 54 cases), SS (SIR = 1.6; n = 86 cases) and MS (SIR = 1.2; n = 130 cases) in Denmark (Nielsen et al., 2011). When the analyses were restricted to surgically verified endometriosis, associations were attenuated, except for MS, for which the association was slightly strengthened and statistically significant. The same group also reported significantly increased risks of inflammatory bowel diseases (ulcerative colitis (SIR = 1.5; n = 228 cases) or Crohn's disease (SIR = 1.6; n = 92 cases)), and these relations were stronger when restricted to surgically verified endometriosis (Jess et al., 2012). Three studies evaluated the association between endometriosis and celiac disease: two case–control studies, based on low numbers of celiac disease cases (n = 3 and n = 7, respectively), reported either a statistically significant positive association (OR = 3.8) (Aguiar et al., 2009) or a non-significant higher occurrence of celiac disease among endometriosis patients (2.2%) compared with controls (0.8%) (Santoro et al., 2014). A large Swedish cohort study, based on registry and population registers, confirmed a significantly positive association between celiac disease and endometriosis (HR = 1.39, 1.14–1.70) (Stephansson et al., 2011). Finally, no association was observed between endometriosis and risk of autoimmune thyroid disorders, hypo- or hyperthyroidism in a Brazilian cross-sectional study (Petta et al., 2007).
Table II.
Epidemiological studies exploring the associations between endometriosis and autoimmune diseases.
| Studies | Geographic location | Study period | Design | Endometriosis number and ascertainment | AID cases and ascertainment | Risk estimates (RR, OR or SIR and 95% CI) |
|---|---|---|---|---|---|---|
| Sinaii et al. (2002) | US | 1998 | Cross-sectional | 3680 Self-report of a surgical diagnosis |
31 SLE 19 MS 68 RA 23 SS Self-report |
SLE: 20.7 (14.3–29.9) MS: 7.1 (4.4–11.3) RA: 1.5 (1.2–1.9) SS: 23.9 (15.5–36.5) |
| Matorras et al. (2007) | Spain | 1990–2004 | Case–control | 342 Clinical records |
120 SLE 22 SS Clinical records |
SLE: 0.37 (0.09–1.59) SS: 2.17 (0.48–9.90) |
| Petta et al. (2007) | Brazil | 2005–2006 | Cross-sectional | 148 Surgically confirmed |
38 AITD 5 hyperthyroidism 30 hypothyroidism Clinical records |
0.52 (0.25–1.06) 0.09 (0.01–1.65) 1.49 (0.69–3.23) No difference in AITD/thyroid dysfunction risk according to endometriosis stage |
| Aguiar et al. (2009) | Brazil | 2000–2003 | Case–control | 120 Laparoscopically confirmed |
3 CeD Intestinal biopsy |
3.8 (1.03–14.08) |
| Nielsen et al. (2011) | Denmark | 1977–2007 | Retrospective cohort | 37 661 Clinical records |
130 MS 54 SLE 86 SS Clinical records |
MS: 1.2 (1.05–1.5) SLE: 1.6 (1.2–2.1) SS: 1.6 (1.3–2.0) In surgically verified endometriosis: MS: 1.4 (1.04–1.9) SLE: 1.1 (0.6–2.1) SS: 1.4 (0.9–2.3) |
| Stephansson et al. (2011) | Sweden | 1969–2008 | Prospective cohort | 517 Hospital registry records |
11 097 CeD Biopsy reports |
1.39 (1.14–1.70) 1.35 (1.07–1.69) in women aged 16–45 years at study entry |
| Jess et al. (2012) | Denmark | 1977–2007 | Retrospective cohort | 37 661 Clinical records |
228 UC 92 CD Clinical records |
IBD: 1.5 (1.4–1.7) UC: 1.5 (1.3–1.7) CD: 1.6 (1.3–2.0) In surgically verified endometriosis: UC: 1.8 (1.4–2.3) CD: 1.7 (1.2–2.5) |
| Santoro et al. (2014) | Italy | 2012 | Case–control | 223 Laparoscopically confirmed |
7 CeD Intestinal biopsy |
CeD occurrence in endometriosis patients (2.2%) higher than that among controls (0.8%) (P = 0.265) |
AI, autoimmune; AID, autoimmune disease; AITD, autoimmune thyroid disease; CD, Crohn's disease; CeD, celiac disease; CI, confidence interval; IBD, inflammatory bowel disease; MS, multiple sclerosis; OR, odds ratio; RA, rheumatoid arthritis; RR, relative risk; SIR, standardized incidence ratio; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome; UC, ulcerative colitis.
Asthma/allergies
Pertinent to the aberrant immunologic response and heightened inflammatory reaction in women with endometriosis, endometriosis patients also tend to be more susceptible to allergic manifestations and to allergy-related conditions such as asthma or atopic diseases than women without endometriosis (Bungum et al., 2014). The first suggestion of such associations came from two early US studies from the same group, which reported higher rates of eczema, hay fever, food sensitivities and allergic reactions in endometriosis cases compared with controls (Lamb and Nichols, 1986; Nichols et al., 1987) (Table III). Then, the survey of patient members of the Endometriosis Association suggested a higher proportion of reported allergies and asthma as compared with the general US female population (Sinaii et al., 2002). While a later case–control study reported a similar prevalence of asthma among women with or without endometriosis (Ferrero et al., 2005), a recent study found significantly higher risks of endometriosis among women with allergies (OR = 4.3), asthma (OR = 2.2), allergic rhinitis (OR = 23.3), medication (OR = 4.7) or penicillin (OR = 7.0) allergy, or with allergic disease in first-degree relatives (OR = 8.8) (Matalliotakis et al., 2012). In addition, in a case–control study in Italy, Ammendola et al. observed a significantly higher proportion of allergies in women with endometriosis (Ammendola et al., 2008). In that study, carriers of the C allele of the acid phosphatase locus 1 (ACP1) polymorphism, suggested to have a role in allergic manifestations, were at higher risk of endometriosis than those carrying other genotypes, although this gene was not reported to be associated with endometriosis in genome-wide scans (Rahmioglu et al., 2014).
Table III.
Epidemiological studies exploring the associations between endometriosis and asthma and atopic diseases.
| Studies | Geographic location | Study period | Design | Asthma/atopic disease number and ascertainment | Endometriosis number and ascertainment | Risk estimates (RR, OR or SIR and 95% CI) or frequency | Comments |
|---|---|---|---|---|---|---|---|
| Lamb and Nichols (1986) | US | – | Case–control | – Self-report |
43 | Eczema, hay fever and food sensitivities were significantly more common in women with endometriosis than controls | Only the abstract could be viewed |
| Nichols et al. (1987) | US | – | Case–control | – Self-report |
88 | Women with endometriosis were significantly more likely to report allergic manifestations | Only the abstract could be viewed |
| Sinaii et al. (2002) | US | 1998 | Cross-sectional | Allergies: 2245 Asthma: 442 Self-report |
3680 Self-report of a surgical diagnosis |
|
|
| Ferrero et al. (2005) | US | 2001–2004 | Case–control | Asthma: 45 Self-report |
467 Histologically confirmed |
Similar prevalence of asthma in women with endometriosis (4.9%, CI = 3.1–7.3) and those without (5.3%, CI = 3.4–8.0), P = 0.78 | Asthma patients responded to LWAQ |
| Ammendola et al. (2008) | Italy | – | Case–control | Allergy tested by prick tests | 113 Laparoscopically confirmed |
|
|
| Matalliotakis et al. (2012) | US | 1996–2002 | Case–control | Allergies: 328 Self-report |
501 Surgically confirmed |
Allergies: 4.28 (2.9–6.3) Asthma: 2.22 (1.03–4.80) Allergic rhinitis: 23.32 (9.42–57.73) Medication allergy: 4.66 (3.09–7.02) Penicillin allergy: 7.01 (3.31–14.86) First-degree relative with allergic disease: 8.82 (5.27–14.79) No significant differences according to severity of endometriosis |
The 188 controls had surgical evaluation and were confirmed not to have endometriosis |
ACP1, acid phosphatase locus 1; CI, confidence interval; LWAQ, Living with Asthma Questionnaire; OR, odds ratio; RR, relative risk; SIR, standardized incidence ratio.
Cardiovascular diseases
Levels of various inflammatory factors, such as intracellular adhesion molecule 1 (ICAM-1), C-reactive protein (CRP), interleukin-1 and 6 (IL-1 and IL-6), tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF), have been shown to be elevated in the peritoneal fluid and peripheral blood of women with endometriosis (Koumantakis et al., 1994; Wu et al., 1998; Pizzo et al., 2002; Bedaiwy et al., 2006; Agic et al., 2007), suggesting that endometriosis is associated with local and systemic chronic inflammation. Recent evidence also suggests higher levels of markers of oxidative stress (Van Langendonckt et al., 2002; Szczepanska et al., 2003; Gupta et al., 2006) and higher serum levels of low-density lipoprotein in women with endometriosis (Melo et al., 2010). Because inflammation, oxidative stress and an atherogenic lipid profile play key roles in the pathogenesis of coronary heart disease (CHD) (Hansson, 2005; Bonomini et al., 2008), and because endometriosis and CHD may share a common genetic background (Mu et al., manuscript under consideration for publication), endometriosis patients may be at increased risk of cardiovascular diseases.
Pretta et al. examined whether women with endometriosis (n = 66) had more subclinical atherosclerosis than controls (n = 66) by comparing their intima-media thickness and distensibility coefficient on the common carotid artery (Pretta et al., 2007). While no significant difference was reported, it is important to note the small sample size and thus low power of the study. However, despite even smaller sample sizes (n = 41 cases/28 controls and n = 37 cases/31 controls, respectively), two subsequent studies showed significantly lower values of flow-mediated dilation in endometriosis patients compared with controls (Kinugasa et al., 2011; Santoro et al., 2012), although no significant differences were observed in common carotid intima-media thickness between groups in the second study (Santoro et al., 2012). Recently, a prospective analysis of endometriosis in relation to CHD risk in the Nurses' Health Study II showed increased risks of myocardial infarction (RR = 1.52, 95% CI = 1.17–1.98; n = 498), angiographically confirmed angina (RR = 1.91, 1.59–2.29; n = 891), and coronary artery bypass graft surgery/coronary angioplasty procedure/stent (RR = 1.35, 95% CI = 1.08–1.69; n = 690) associated with endometriosis (Mu et al., manuscript under consideration for publication). Endometriosis was also associated with a higher risk of any of the three CHD events combined (RR = 1.62, 95% CI = 1.39–1.89). Part of the associations was found to be statistically accounted for by endometriosis treatments that are risk factors for CHD, such as hysterectomy/oophorectomy and earlier age at surgery following endometriosis diagnosis.
Discussion
The available epidemiological evidence suggests that women with endometriosis are at higher risks of ovarian and breast cancers, cutaneous melanoma, asthma, and some autoimmune, cardiovascular and atopic diseases, and at a decreased risk of cervical cancer.
Hypotheses for underlying mechanisms
While the exact pathophysiology underlying these associations is unknown, the co-occurrence of endometriosis and other disease outcomes may reflect at least four potential explanations.
Endometriosis shares common risk factors or a common exposure profile with these outcomes
A first potential explanation is the existence of common risk factors between endometriosis and the explored disease outcomes. These risk factors could affect risk of both diseases and thus act as potential confounders of the association (i.e. factors associated with both the exposure and the outcome but that are not in the causal pathway between the exposure and the outcome; e.g. the relation between parity and Down syndrome is confounded by maternal age) or as mediators (i.e. factors associated with both the exposure and the outcome that are in the causal pathway between the exposure and the outcome and act as intermediate factors of the relation; e.g. the relation between having multiple sex partners and cervical cancer risk is mediated by the increased risk of HPV infection). They may also be unknown risk markers for endometriosis, which the association with the other disease would enable us to uncover. Multivariable modeling allowing for adjustment for multiple factors and mediation analyses may help to identify some of these factors.
Treatment for endometriosis is associated with these outcomes
Treatment for endometriosis may include hormonal treatment (oral contraceptives, gonadotrophin-releasing hormone (GnRH) agonists, medroxyprogesterone, danazol), surgical treatment (including hysterectomy), and use of analgesics. If any of these treatments were associated with the risk of the explored disease outcomes, then it would have the potential to act as a mediator of the association between endometriosis and this disease outcome. These issues can be explored through mediation analyses, and through testing for effect modification by conducting stratified analyses according to these treatments (see ‘Methodological complexities’ below).
In the report from our group cited in this review (Mu et al., under consideration for publication), mediation analyses showed that part of the association between endometriosis and myocardial infarction and angina could be partially attributed to medical treatments for endometriosis.
Endometriosis induces a systemic change that is associated with these outcomes
It is possible that some consequences of endometriosis, such as infertility, aberrant hormonal milieu, chronic inflammation, and deficient immunologic response, as well as behavioral changes following endometriosis diagnosis (e.g. diet, physical activity), influence the long-term risk of other diseases, thus mediating the studied relationships between endometriosis and the explored disease outcomes. It is thus important to investigate this potential effect while exploring the associations between endometriosis and other diseases, which, again, may be explored through mediation or stratification analyses.
Bias
Because endometriosis and some of the explored disease outcomes (e.g. autoimmune diseases) are poorly characterized, there is potential for misclassification, which may either induce spurious associations that are only due to systematic error, or attenuate any existing association (see ‘Methodological complexities’ below). This bias can be minimized by selecting laparoscopically confirmed endometriosis and medically confirmed disease outcomes whenever possible. Since associations between endometriosis and the considered outcomes are likely not known from the general public, misclassification would be mostly non-differential and would thus result in an underestimation of effects. However, diagnostic, recall or other biases may also alter results in an unpredictable direction. Another bias that may be considered is the possibility that women with endometriosis may be at a higher probability of being diagnosed with one of the studied outcomes, and vice versa, because of a higher exposure to the medical system.
Methodological complexities
Temporality
Perhaps the most critical methodological aspect of evaluating the co-occurrence of endometriosis and other chronic diseases is the issue of temporality. In epidemiology, temporality is one of the criteria that support causal inference of an association. While observational studies do not allow the establishment of causal associations, prospective studies are associated with a higher level of evidence for causality, mostly because exposures are recorded prior to outcome data. However, within the context of the potential co-occurrence of endometriosis and other disease outcomes, the notion of temporality becomes more complex. We lack understanding about endometriosis initiation and progression; a mean delay of 7 years has indeed been estimated between onset of endometriosis symptoms and laparoscopic diagnosis (Nnoaham et al., 2011). There is also uncertainty in the diagnosis and onset of some of the disease outcomes of interest, such as autoimmune diseases, asthma/atopic diseases, cardiovascular diseases and some slow-growing cancer diagnoses. This double imprecision, along with the potential overlapping age-specific incidence curves of endometriosis and the considered outcome, renders it difficult to evaluate the direction of the temporal association, as it is uncertain whether endometriosis precedes these disease outcomes, endometriosis and the outcome of interest occur at a similar point in time, or endometriosis represents a consequence of the altered milieu resulting from the presence of these diseases. Therefore, associations should be interpreted with caution with regards to causality.
Misclassification
Self-reported endometriosis has a high potential to be misclassified in the general population. In nurses participating in the Nurses' Health Study II cohort, ascertainment rates for endometriosis were 96% in women reporting laparoscopically confirmed endometriosis, and 54% in those without laparoscopic confirmation (Missmer et al., 2010). Since this population is health-oriented, the true ascertainment rate in those with no laparoscopic confirmation is probably lower in the general female population. Outcome misclassification is usually low with respect to cancer outcomes, which are ascertained through pathology reports or cancer registry records with often high confirmation rates. However, confirmation of autoimmune diseases has the potential to be very low. In the Nurses' Health Study II, confirmation rates were 69% for SLE and 29% for RA after examination of clinical records in those who had screened positive to the Connective Tissue Disease Questionnaire, while only 7% of the original self-reports for any SLE or RA could be confirmed (Karlson et al., 2004; Costenbader et al., 2007).
Another issue to be considered with regards to misclassification is the choice of the comparison group in epidemiologic studies of endometriosis (Missmer and Cramer, 2003). Because of the invasive nature of laparoscopic diagnosis, and because endometriosis may be asymptomatic, there may be a fraction of undiagnosed cases in the comparison group, whether endometriosis is evaluated as an exposure or as the outcome of interest and regardless of study design, with a dilution of the association between endometriosis and the outcome of interest as a consequence. However, it has been estimated that the community prevalence of severe/symptomatic endometriosis is likely <2% (Zondervan et al., 2002), suggesting that population-based control groups in case–control or cohort studies are unlikely to contain many undiagnosed cases, and thus that the risk for this bias is low in this context. In hospital-based case–control studies, control groups selected among patients diagnosed with various conditions may represent a biased sample that is unrepresentative of the exposure distribution in the source population of cases. In addition, given the evidence reviewed above suggesting a higher risk of common chronic diseases in endometriosis patients, the inclusion of patients with other conditions in the control group has the potential to lead to biased evaluations of the associations between endometriosis and chronic disease outcomes. In retrospective cohorts, study populations are usually unrepresentative of the general female population as they are focused on a selected sample, e.g. endometriosis patients, women who underwent hysterectomy or oophorectomy, or infertile women. In addition, these samples may have particular characteristics that could either mediate or modify the amplitude of the associations between endometriosis and the considered chronic disease outcome (e.g. infertile women may have received ovarian-stimulating treatments that may be in the causal pathway of the associations under study).
Confounding and mediation
Another possible source of complexity in evaluating such associations is the potential for confounding or mediation of the relation between endometriosis and other diseases. In the assessment of co-occurrence between endometriosis and other disease outcomes, it is important to evaluate if these associations are driven by common risk factors (should they be in the causal pathway between endometriosis and these outcomes or not) rather than causal biology.
Robustness
Most previous studies exploring the relations between endometriosis and other morbidities have relied on a self-reported diagnosis of endometriosis or of autoimmune/allergic diseases, retrospective designs, or limited statistical power for rarer disease outcomes (e.g. endometrial and cervical cancers). Moreover, the studies that have assessed the associations between endometriosis and cancer to date were mostly within population historical cohorts, in which potential confounding factors or mediators of the associations could not be taken into account. Ideally, future studies should explore these relations using a prospective cohort design, large sample sizes, with laparoscopically confirmed endometriosis diagnosis and medically confirmed disease outcomes, and allowing for the assessment of temporality and potential confounders and mediators of these associations.
Very few of the reviewed studies included data on endometriosis stage (Matorras et al., 2007; Ferrero et al., 2005; Petta et al., 2007; Pretta et al., 2007; Aguiar et al., 2009; Kinugasa et al., 2011; Matalliotakis et al., 2012; Santoro et al., 2012, 2014), and such data were available on too few cases to allow stage-specific comparisons with adequate statistical power (and data were restricted to severe stages (III–IV) in one study (Kinugasa et al., 2011)). Two key components on the severity scale of endometriosis are the presence of endometriomas and the presence of scarring or adhesions. It is possible that the associations between endometriosis and chronic diseases are specific to one or the other feature, which would make stage III–IV endometriosis particularly correlated with the considered comorbidities. Unfortunately, due to the paucity of data on severity, type, or location of endometriosis in the available epidemiologic studies, it is currently unknown whether the associations reviewed above involve all endometriosis cases, or if they are restricted to particular subgroups of patients.
In light of the evidence reviewed above, it is legitimate to question whether the relations between endometriosis and morbidity are unique to this condition, or if women with other gynecological disorders are at higher risk of major chronic diseases also. To our knowledge, while androgen excess disorders such as polycystic ovary syndrome (PCOS) have been associated with increased cardiovascular risk (Macut et al., 2014), data seem limited to date regarding reproductive disorders other than endometriosis. Some evidence suggests that PCOS is associated with the risk of certain cancer types (Barry et al., 2014; Gottschau et al., 2015) and thyroiditis (Du and Li, 2013); women with uterine fibroids were reported to have a possible increased risk of cardiovascular diseases (Aksoy et al., 2014; Haan et al., 2015) and of overt hypothyroidism (Ott et al., 2014) in emerging studies; and premature ovarian insufficiency may be associated with cardiovascular risk (Roeters van Lennep et al., 2014) as well as autoimmune diseases and cancer-specific mortality (Wu et al., 2014). As the research in this area develops, we suggest that the methodological issues and recommendations outlined in the present review should most generally apply to the exploration of these other gynecological conditions in relation to chronic disease risk.
Conclusion
In conclusion, increasing evidence suggests that women with endometriosis are at higher risk of a number of chronic diseases. Although the underlying mechanisms are not understood, the available data to date suggest that endometriosis is not harmless with respects to women's long-term health and may have important consequences. Whether these mechanisms involve hormones, aberrant immune or inflammatory responses, genetic or environmental factors, a combination of these factors, or the reflection of methodological bias, needs to be investigated further. Future studies should take into account the methodological considerations involved in this type of analysis. Of note, very few of the previous studies on this topic were able to use information on stage/severity or type of endometriosis, and whether the observed associations are true for a particular type or stage of disease only is currently unknown. The ability to conduct analyses by type and stage is critical, however, to increase our understanding of endometriosis and its association with other chronic diseases. It will take large, geographically diverse, multidisciplinary collaborative efforts to disentangle these complex associations. If relationships between endometriosis and these disease outcomes are confirmed, these findings will have important implications in the management and care of endometriosis patients.
Authors' roles
M.K. and S.A.M. conceived and designed the study. M.K. drafted the original manuscript. M.K., F.M., K.L.T., H.R.H., E.M.P., L.F., and S.A.M. contributed to the interpretation of data discussed in the manuscript, revised the manuscript and approved its final version.
Funding
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health Grant HD57210; and Dana-Farber/Harvard Cancer Center (DF/HCC) – A. David Mazzone Research Award. M.K. is financially supported by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme (#PIOF-GA-2011-302078). L.F. was supported by Training Grant T32HD060454 in Reproductive, Perinatal and Pediatric Epidemiology from the National Institute of Child Health and Human Development, National Institutes of Health.
Conflict of interest
None declared.
References
- Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, Friedrich M, Taylor RN, Hornung D. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci 2007;14:486–497. [DOI] [PubMed] [Google Scholar]
- Aguiar FM, Melo SB, Galvao LC, Rosa-e-Silva JC, dos Reis RM, Ferriani RA. Serological testing for celiac disease in women with endometriosis. A pilot study. Clin Exp Obstet Gynecol 2009;36:23–25. [PubMed] [Google Scholar]
- Aksoy Y, Sivri N, Karaoz B, Sayin C, Yetkin E. Carotid intima-media thickness: a new marker of patients with uterine leiomyoma. Eur J Obstet Gynecol Reprod Biol 2014;175:54–57. [DOI] [PubMed] [Google Scholar]
- Ammendola M, Pietropolli A, Saccucci P, Piccione E, Bottini E, Gloria-Bottini F. Acid phosphatase locus 1 genetic polymorphism, endometriosis, and allergy. Fertil Steril 2008;90:1203–1205. [DOI] [PubMed] [Google Scholar]
- Aris A. Endometriosis-associated ovarian cancer: a ten-year cohort study of women living in the Estrie Region of Quebec, Canada. J Ovarian Res 2010;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballweg ML. Overcoming Endometriosis: New Help From the Endometriosis Association. New-York: Congdon & Weed, 1987. [Google Scholar]
- Baron JA, Weiderpass E, Newcomb PA, Stampfer M, Titus-Ernstoff L, Egan KM, Greenberg ER. Metabolic disorders and breast cancer risk (United States). Cancer Causes Control 2001;12:875–880. [DOI] [PubMed] [Google Scholar]
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2014;20:748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T, Mascha EJ, Casper RF. Genetic polymorphism in the fibrinolytic system and endometriosis. Obstet Gynecol 2006;108:162–168. [DOI] [PubMed] [Google Scholar]
- Bertelsen L, Mellemkjaer L, Frederiksen K, Kjaer SK, Brinton LA, Sakoda LC, van Valkengoed I, Olsen JH. Risk for breast cancer among women with endometriosis. Int J Cancer 2007;120:1372–1375. [DOI] [PubMed] [Google Scholar]
- Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol 2011;123:200–204. [DOI] [PubMed] [Google Scholar]
- Bonomini F, Tengattini S, Fabiano A, Bianchi R, Rezzani R. Atherosclerosis and oxidative stress. Histol Histopathol 2008;23:381–390. [DOI] [PubMed] [Google Scholar]
- Borgfeldt C, Andolf E. Cancer risk after hospital discharge diagnosis of benign ovarian cysts and endometriosis. Acta Obstet Gynecol Scand 2004;83:395–400. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol 1997;176:572–579. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, Westhoff CL. Ovarian cancer risk associated with varying causes of infertility. Fertil Steril 2004;82:405–414. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Westhoff CL, Scoccia B, Lamb EJ, Althuis MD, Mabie JE, Moghissi KS. Causes of infertility as predictors of subsequent cancer risk. Epidemiology 2005;16:500–507. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, Varner MW, Kennedy A, Giudice L, Fujimoto VY, et al. Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil Steril 2013;100:162–169, e161–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buis CC, van Leeuwen FE, Mooij TM, Burger CW. Increased risk for ovarian cancer and borderline ovarian tumours in subfertile women with endometriosis. Hum Reprod 2013;28:3358–3369. [DOI] [PubMed] [Google Scholar]
- Bungum HF, Vestergaard C, Knudsen UB. Endometriosis and type 1 allergies/immediate type hypersensitivity: a systematic review. Eur J Obstet Gynecol Reprod Biol 2014;179:209–215. [DOI] [PubMed] [Google Scholar]
- Claus EB, Calvocoressi L, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M. Family and personal medical history and risk of meningioma. J Neurosurg 2011;115:1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum 2007;56:1251–1262. [DOI] [PubMed] [Google Scholar]
- Cottreau CM, Ness RB, Modugno F, Allen GO, Goodman MT. Endometriosis and its treatment with danazol or lupron in relation to ovarian cancer. Clin Cancer Res 2003;9:5142–5144. [PubMed] [Google Scholar]
- Du D, Li X. The relationship between thyroiditis and polycystic ovary syndrome: a meta-analysis. Int J Clin Exp Med 2013;6:880–889. [PMC free article] [PubMed] [Google Scholar]
- Eisenberg VH, Zolti M, Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun Rev 2012;11:806–814. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 1997;24:235–258. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Petrera P, Colombo BM, Navaratnarajah R, Parisi M, Anserini P, Remorgida V, Ragni N. Asthma in women with endometriosis. Hum Reprod 2005;20:3514–3517. [DOI] [PubMed] [Google Scholar]
- Frisch RE, Wyshak G, Albert LS, Sober AJ. Dysplastic nevi, cutaneous melanoma, and gynecologic disorders. Int J Dermatol 1992;31:331–335. [DOI] [PubMed] [Google Scholar]
- Garry R. Endometriosis: an invasive disease. Gynecol Endosc 2001;10:79–82. [Google Scholar]
- Gemmill JA, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Cancers, infections, and endocrine diseases in women with endometriosis. Fertil Steril 2010;94:1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol 2015;136:99–103. [DOI] [PubMed] [Google Scholar]
- Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online 2006;13:126–134. [DOI] [PubMed] [Google Scholar]
- Haan YC, Oudman I, de Lange ME, Timmermans A, Ankum WM, van Montfrans GA, Brewster LM. Hypertension risk in Dutch women with symptomatic uterine fibroids. Am J Hypertens 2015;28:487–492. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- Heidemann LN, Hartwell D, Heidemann CH, Jochumsen KM. The relation between endometriosis and ovarian cancer—a review. Acta Obstet Gynecol Scand 2014;93:20–31. [DOI] [PubMed] [Google Scholar]
- Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol 2011;7:611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EA, Cress RD, Ahn DK. Cutaneous melanoma in women. III. Reproductive factors and oral contraceptive use. Am J Epidemiol 1995;141:943–950. [DOI] [PubMed] [Google Scholar]
- Jess T, Frisch M, Jorgensen KT, Pedersen BV, Nielsen NM. Increased risk of inflammatory bowel disease in women with endometriosis: a nationwide Danish cohort study. Gut 2012;61:1279–1283. [DOI] [PubMed] [Google Scholar]
- Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum 2004;50:3458–3467. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br J Cancer 2014;110:1878–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugasa S, Shinohara K, Wakatsuki A. Increased asymmetric dimethylarginine and enhanced inflammation are associated with impaired vascular reactivity in women with endometriosis. Atherosclerosis 2011;219:784–788. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Sumimoto K, Moniwa N, Imai M, Takakura K, Kuromaki T, Morioka E, Arisawa K, Terao T. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol Cancer 2007;17:37–43. [DOI] [PubMed] [Google Scholar]
- Koumantakis E, Matalliotakis I, Neonaki M, Froudarakis G, Georgoulias V. Soluble serum interleukin-2 receptor, interleukin-6 and interleukin-1a in patients with endometriosis and in controls. Arch Gynecol Obstet 1994;255:107–112. [DOI] [PubMed] [Google Scholar]
- Kvaskoff M, Mesrine S, Fournier A, Boutron-Ruault MC, Clavel-Chapelon F. Personal history of endometriosis and risk of cutaneous melanoma in a large prospective cohort of French women. Arch Intern Med 2007;167:2061–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb K, Nichols TR. Endometriosis: a comparison of associated disease histories. Am J Prev Med 1986;2:324–329. [PubMed] [Google Scholar]
- Macut D, Antic IB, Bjekic-Macut J. Cardiovascular risk factors and events in women with androgen excess. J Endocrinol Invest 2014. [Epub ahead of print]. [DOI] [PubMed]
- Matalliotakis I, Cakmak H, Matalliotakis M, Kappou D, Arici A. High rate of allergies among women with endometriosis. J Obstet Gynaecol 2012;32:291–293. [DOI] [PubMed] [Google Scholar]
- Matorras R, Ocerin I, Unamuno M, Nieto A, Peiro E, Burgos J, Exposito A. Prevalence of endometriosis in women with systemic lupus erythematosus and Sjogren's syndrome. Lupus 2007;16:736–740. [DOI] [PubMed] [Google Scholar]
- Matta JL, Flores I, Morales LM, Monteiro J, Alvarez-Garriga C, Bayona M. Women with endometriosis have a higher DNA repair capacity and diminished breast cancer risk. Mol Cancer Biol 2013;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin A, Sparen P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod 2006;21:1237–1242. [DOI] [PubMed] [Google Scholar]
- Melin A, Sparen P, Bergqvist A. The risk of cancer and the role of parity among women with endometriosis. Hum Reprod 2007;22:3021–3026. [DOI] [PubMed] [Google Scholar]
- Melo AS, Rosa-e-Silva JC, Rosa-e-Silva AC, Poli-Neto OB, Ferriani RA, Vieira CS. Unfavorable lipid profile in women with endometriosis. Fertil Steril 2010;93:2433–2436. [DOI] [PubMed] [Google Scholar]
- Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer 2008;122:170–176. [DOI] [PubMed] [Google Scholar]
- Missmer SA. Commentary: Endometriosis—epidemiologic considerations for a potentially ‘high-risk’ population. Int J Epidemiol 2009;38:1154–1155. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am 2003;30:1–19, vii. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, Barbieri RL, Willett WC, Hankinson SE. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod 2010;25:1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modugno F, Ness RB, Allen GO, Schildkraut JM, Davis FG, Goodman MT. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am J Obstet Gynecol 2004;191:733–740. [DOI] [PubMed] [Google Scholar]
- Montgomery GW, Nyholt DR, Zhao ZZ, Treloar SA, Painter JN, Missmer SA, Kennedy SH, Zondervan KT. The search for genes contributing to endometriosis risk. Hum Reprod Update 2008;14:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseson M, Koenig KL, Shore RE, Pasternack BS. The influence of medical conditions associated with hormones on the risk of breast cancer. Int J Epidemiol 1993;22:1000–1009. [DOI] [PubMed] [Google Scholar]
- Nagle CM, Olsen CM, Webb PM, Jordan SJ, Whiteman DC, Green AC. Endometrioid and clear cell ovarian cancers: a comparative analysis of risk factors. Eur J Cancer 2008;44:2477–2484. [DOI] [PubMed] [Google Scholar]
- Ness RB, Grisso JA, Cottreau C, Klapper J, Vergona R, Wheeler JE, Morgan M, Schlesselman JJ. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology 2000;11:111–117. [DOI] [PubMed] [Google Scholar]
- Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, Purdie DM, Risch HA, Vergona R, Wu AH. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol 2002;155:217–224. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Lamb K, Arkins JA. The association of atopic diseases with endometriosis. Ann Allergy 1987;59:360–363. [PubMed] [Google Scholar]
- Nielsen NM, Jorgensen KT, Pedersen BV, Rostgaard K, Frisch M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum Reprod 2011;26:1555–1559. [DOI] [PubMed] [Google Scholar]
- Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnick WB. Treating endometriosis as an autoimmune disease. Fertil Steril 2001;76:223–231. [DOI] [PubMed] [Google Scholar]
- Olson JE, Cerhan JR, Janney CA, Anderson KE, Vachon CM, Sellers TA. Postmenopausal cancer risk after self-reported endometriosis diagnosis in the Iowa Women's Health Study. Cancer 2002;94:1612–1618. [DOI] [PubMed] [Google Scholar]
- Ott J, Kurz C, Braun R, Promberger R, Seemann R, Vytiska-Binstorfer E, Walch K. Overt hypothyroidism is associated with the presence of uterine leiomyoma: a retrospective analysis. Eur J Obstet Gynecol Reprod Biol 2014;177:19–22. [DOI] [PubMed] [Google Scholar]
- Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol 2012;13:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun 2012;38:J282–J291. [DOI] [PubMed] [Google Scholar]
- Petta CA, Arruda MS, Zantut-Wittmann DE, Benetti-Pinto CL. Thyroid autoimmunity and thyroid dysfunction in women with endometriosis. Hum Reprod 2007;22:2693–2697. [DOI] [PubMed] [Google Scholar]
- Pizzo A, Salmeri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest 2002;54:82–87. [DOI] [PubMed] [Google Scholar]
- Porpora MG, Resta S, Fuggetta E, Storelli P, Megiorni F, Manganaro L, De Felip E. Role of environmental organochlorinated pollutants in the development of endometriosis. Clin Exp Obstet Gynecol 2013;40:565–567. [PubMed] [Google Scholar]
- Pretta S, Remorgida V, Abbamonte LH, Anserini P, Ragni N, Del Sette M, Gandolfo C, Ferrero S. Atherosclerosis in women with endometriosis. Eur J Obstet Gynecol Reprod Biol 2007;132:226–231. [DOI] [PubMed] [Google Scholar]
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun 2012;38:J109–J119. [DOI] [PubMed] [Google Scholar]
- Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update 2014;20:702–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeters van Lennep JE, Heida KY, Bots ML, Hoek A; on behalf of the collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol 2014. [Epub ahead of print]. [DOI] [PubMed]
- Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Risk of epithelial ovarian cancer in relation to benign ovarian conditions and ovarian surgery. Cancer Causes Control 2008;19:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg 1925;10:1–72. [Google Scholar]
- Santoro L, D'Onofrio F, Campo S, Ferraro PM, Tondi P, Campo V, Flex A, Gasbarrini A, Santoliquido A. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Hum Reprod 2012;27:1320–1326. [DOI] [PubMed] [Google Scholar]
- Santoro L, Campo S, D'Onofrio F, Gallo A, Covino M, Campo V, Palombini G, Santoliquido A, Gasbarrini G, Montalto M. Looking for celiac disease in Italian women with endometriosis: a case control study. Biomed Res Int 2014;2014:236821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer C, Persson I, Falkeborn M, Naessen T, Troisi R, Brinton LA. Breast cancer risk associated with gynecologic surgery and indications for such surgery. Int J Cancer 1997;70:150–154. [DOI] [PubMed] [Google Scholar]
- Simoens S, Hummelshoj L, Dunselman G, Brandes I, Dirksen C, D'Hooghe T. Endometriosis cost assessment (the EndoCost study): a cost-of-illness study protocol. Gynecol Obstet Invest 2011a;71:170–176. [DOI] [PubMed] [Google Scholar]
- Simoens S, Meuleman C, D'Hooghe T. Non-health-care costs associated with endometriosis. Hum Reprod 2011b;26:2363–2367. [DOI] [PubMed] [Google Scholar]
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod 2002;17:2715–2724. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol 2006;101:331–341. [DOI] [PubMed] [Google Scholar]
- Stephansson O, Falconer H, Ludvigsson JF. Risk of endometriosis in 11,000 women with celiac disease. Hum Reprod 2011;26:2896–2901. [DOI] [PubMed] [Google Scholar]
- Szczepanska M, Kozlik J, Skrzypczak J, Mikolajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril 2003;79:1288–1293. [DOI] [PubMed] [Google Scholar]
- Tiniakou E, Costenbader KH, Kriegel MA. Sex-specific environmental influences on the development of autoimmune diseases. Clin Immunol 2013;149:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril 2002;77:861–870. [DOI] [PubMed] [Google Scholar]
- Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet 1999;354:1586–1590. [DOI] [PubMed] [Google Scholar]
- Vigano P, Somigliana E, Panina P, Rabellotti E, Vercellini P, Candiani M. Principles of phenomics in endometriosis. Hum Reprod Update 2012;18:248–259. [DOI] [PubMed] [Google Scholar]
- Weiss HA, Brinton LA, Potischman NA, Brogan D, Coates RJ, Gammon MD, Malone KE, Schoenberg JB. Breast cancer risk in young women and history of selected medical conditions. Int J Epidemiol 1999;28:816–823. [DOI] [PubMed] [Google Scholar]
- Wu MH, Yang BC, Hsu CC, Lee YC, Huang KE. The expression of soluble intercellular adhesion molecule-1 in endometriosis. Fertil Steril 1998;70:1139–1142. [DOI] [PubMed] [Google Scholar]
- Wu X, Cai H, Kallianpur A, Li H, Yang G, Gao J, Xiang YB, Ji BT, Yu T, Zheng W, et al. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PLoS One 2014;9:e89597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyshak G, Frisch RE. Red hair color, melanoma, and endometriosis: suggestive associations. Int J Dermatol 2000;39:798. [DOI] [PubMed] [Google Scholar]
- Wyshak G, Frisch RE, Albright NL, Albright TE, Schiff I. Reproductive factors and melanoma of the skin among women. Int J Dermatol 1989;28:527–530. [DOI] [PubMed] [Google Scholar]
- Young P, Purdie D, Jackman L, Molloy D, Green A. A study of infertility treatment and melanoma. Melanoma Res 2001;11:535–541. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum Reprod 2002;17:1415–1423. [DOI] [PubMed] [Google Scholar]
- Zucchetto A, Serraino D, Polesel J, Negri E, De Paoli A, Dal Maso L, Montella M, La Vecchia C, Franceschi S, Talamini R. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev 2009;18:316–321. [DOI] [PubMed] [Google Scholar]