Abstract
Aims
Recently, transcatheter aortic valve replacement (TAVR) has evolved as the standard treatment in patients with inoperable aortic valve stenosis. According to TAVR guidelines, body computed tomography (CT) is recommended for pre-procedural planning. Due to the advanced age of these patients, multiple radiological potentially malignant incidental findings (pmIFs) appear in this cohort. It is unknown how pmIFs influence the decision by the heart team to intervene and the mortality.
Methods and results
We evaluated in a retrospective single-centre observational study 414 participants screened for TAVR with dual-source CT between October 2010 and December 2012. pmIFs are common and appeared in 18.7% of all patients screened for TAVR. The decision to intervene by TAVR or surgical aortic valve replacement (SAVR) was made by an interdisciplinary heart team and the role of pmIF in decision-making and time to treatment with TAVR or SAVR was analysed, retrospectively. The appearance of a pmIF vs. no pmIF did not significantly influence therapeutic decisions [odds ratio (OR) 1.14; P = 0.835] or time to treatment (91 ± 152 vs. 61 ± 109 days, respectively). Several findings, which are highly suspicious for malignancy, were less likely associated with invasive treatment (OR 0.207; P = 0.046). Patient survival was evaluated for at least 2 years until January 2014. Two-year survival of patients after TAVR or SAVR, treated according to the heart team decision, was ∼75% and independent from the presence of a non-severe (P = 0.923) or severe (P = 0.823) pmIF.
Conclusion
The study indicates that frequently occurring radiologic pmIF did not influence 2-year survival after a decision to intervene was made by an interdisciplinary heart team.
Keywords: aortic valve stenosis, aortic valve replacement, TAVR, TAVI, incidental finding, malignancy, cancer, mortality, computed tomography
Introduction
Elderly patients with severe aortic valve stenosis and high operative risk can be treated by transcatheter aortic valve replacement (TAVR) with equivalent outcome to surgical aortic valve replacement (SAVR).1–4 Aortic valve stenosis is the most common valve disease in elderly patients, and TAVR is increasingly being performed.5 According to TAVR guidelines, pre-procedural planning requires a contrast-computed tomography (CT) from thorax to iliofemoral vessels.6–8 Due to the higher prevalence of tumours in elderly patients,9 more potential malignant incidental findings (pmIFs) are expected in CTs of these patients.
If a radiologic pmIF appears, the clinician must interpret its significance and the consequence for further treatment. On the one hand, potential malignancies could be diagnosed and treated, but conversely unnecessary diagnostic workup of possibly benign findings could increase costs,10,11 threaten patients by invasive diagnostics, and lead to increased anxiety.12,13 Furthermore, a pmIF with unclear significance could influence the decision of the heart team since TAVR guidelines do not recommend intervention in patients with an estimated survival below 1 year.7 The diagnostic workup triggered by the pmIF could delay intervention on the aortic valve and thus could raise mortality in patients with severe aortic valve stenosis,1,5,14 and the burden of symptoms of aortic valve stenosis limits quality of life of patients when an intervention with a fast recovery could be performed.
It is unknown whether the presence of suspicious pmIF with an estimated patient survival of >1 year correlates with mortality in elderly patients planned for aortic valve replacement. Therefore, the purpose of this systematic retrospective observational study is to evaluate the prevalence and relevance of potentially malignant unsuspected pmIF with respect to treatment decision, time to treatment, and 2-year survival.
Methods
Study design
The study is a retrospective, single-centre observational study in 414 participants who underwent CT for evaluation of TAVR between October 2010 and December 2012 at the University Heart Center Freiburg. Only patients with severe aortic valve stenosis were included for further analyses. Participants were retrospectively screened for pmIF, which were suspicious for malignancy as assessed by a senior radiologist. An interdisciplinary heart team consisting of cardiologist, heart surgeon, and radiologist made the decision for conservative, best medical therapy only (drug therapy), or invasive treatment, i.e. SAVR or TAVR. The follow-up period ended at January 2014. All participants gave informed consent. The ethical committee of the University of Freiburg approved the study design, and the study complies with the Declaration of Helsinki.
Pre-procedural dual-source CT scanner
All examinations were performed on a first-generation dual-source CT scanner (DSCT, Somatom Definition, Siemens Healthcare) using the following scan parameters: 330 ms gantry rotation time, detector collimation 0.6 mm, and tube voltage of 120 kV. A scout view of thorax and abdomen was obtained to plan data acquisitions. After a single contrast medium injection, combined ECG-assisted scanning of the thorax and the upper chest (including 2–3 cm of the arteria carotis), and non-ECG-assisted scanning of the abdomen were performed. The total amount of contrast agent (Imeron 350®, Bracco, Konstanz, Germany) and flow rate were adapted to body weight: patients weighing <70 kg received 110 mL of contrast agent at 4 mL/s and those weighing 70 kg or more received 130 mL at 4.5 mL/s, followed by a saline bolus chaser of 50 mL administered at an equal flow rate. The reference tube current time product was twice as high for the lower part of the chest as for the upper part, with the boundary ∼2 cm below the carina. Attenuation-based tube current modulation (CareDOSE, Siemens Healthcare) and prospective ECG-triggered tube current modulation12 were used for radiation dose reduction, the latter with a pulsing window between 30 and 80% of the R–R cycle and tube current lowered to 20% of the maximum outside the pulsing window. The scanner was operated in the single-source mode for the abdomen. A preceding delay of 4 s was necessary to change the scanning mode. Patients were instructed to sustain their breath-hold if possible or to continue shallow breathing.
Follow-up
Follow-up period was from October 2010 until January 2014. Participants with a pmIF in the initial DSCT were followed up for 2 years and either contacted by telephone or presented in the outpatient clinic.
Classification of incidental findings
To distinguish highly suspicious from rather benign findings, the pmIFs were divided into two groups as the clinician and heart team must do to interpret the radiologic finding: ‘severe’ or ‘non-severe’. The findings of the ‘severe’ group reflected a potential limitation of the patients’ life expectancy, which has to be taken into account during the decision-making process by the heart team. These findings are clinically suspicious for malignant growth. The following characteristics defined a ‘severe’ pmIF: metastasis with primary tumour, infiltrating or progressive tumour with or without inhomogeneous contrast uptake, bulky lymph nodes, multiple metastases, or osteolysis. Non-severe findings are clinically less likely for malignant growth. The following characteristics defined a ‘non-severe’ pmIF, described as rather cystic or other disease, organ hyperplasia (e.g. prostate), small nodules with or without recommendation for a follow-up, inhomogeneous contrast uptaking organ, and single lymph nodes.
Statistical analysis
Quantitative continuous variables are described with means ± standard deviation and quantitative discrete variables with relative frequencies. Baseline characteristics were compared using the Students t-test, the Mann–Whitney U test, and the χ2 test when appropriate. To identify the incidental findings relevance for treatment decision, a multivariable logistic regression model was fitted including the logistic EuroScore I, age, as a classification of the clinical significance of the incidental finding. Variable selection took place according to clinical relevance as well as according to previous works on the relevance of patient-specific variables for Heart-team decision-making.15 For missing variables, multiple imputation was applied. Two-year cumulative survival rates were estimated by means of the Kaplan–Meier method. Differences between groups were determined using the Log-rank test. All analyses were performed using Stata 12 (StataCorp, College Station, TX, USA).
Results
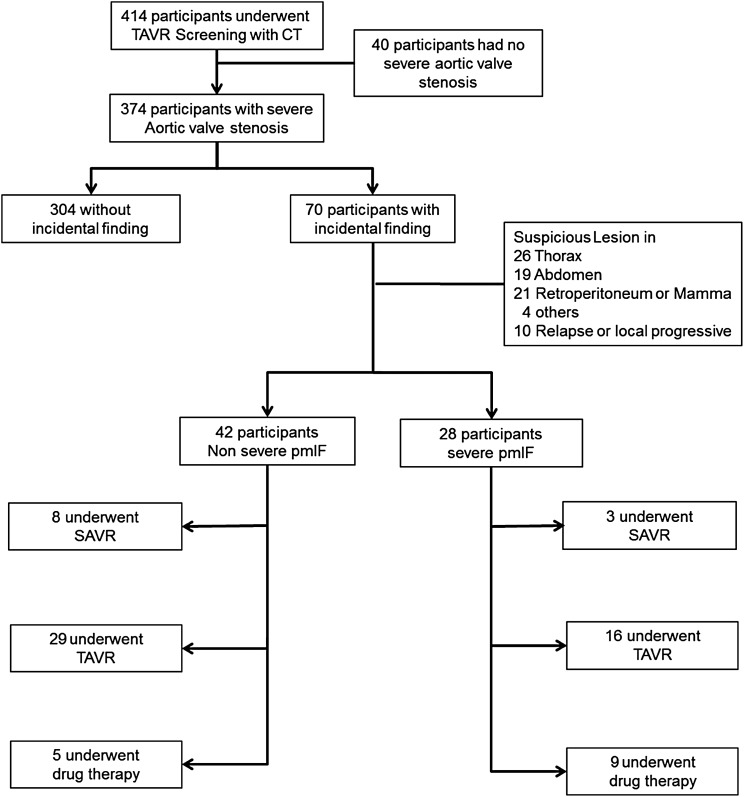
A total of 414 subjects, who underwent screening for TAVR with DSCT at the University Heart Center Freiburg between October 2010 and December 2012, were eligible. Severe aortic valve stenosis (mean valve area 0.7 ± 0.2 cm2) was diagnosed in 374 patients who participated in the study (Figure 1). The mean age of the study population was 79.8 ± 8.9 years and 55.3% were women. Unexpected pmIFs were detected in 70 (18.7%) patients (Table 1). Patients with pmIF were significantly older (mean age 82 vs. 79 years), other baseline parameters did not differ including kidney function, incidence of smoking, or diabetes.
Figure 1.
The flow diagram shows eligible participants. pmIFs were classified into ‘severe’ and ‘non-severe’. The interdisciplinary heart team made the decision for TAVR, SAVR, or drug therapy only.
Table 1.
Baseline characteristics were collected during the TAVR screening
| All |
No incidental finding |
Incidental finding |
P-value | ||||
|---|---|---|---|---|---|---|---|
|
n = 374 |
n = 304 |
n = 70 |
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Female | 55.3% | 55.3% | 55.7% | 0.945 | |||
| Age (years) | 79.83 | ±8.92 | 79.26 | ±9.31 | 82.33 | ±6.45 | 0.009 |
| Body weight (kg) | 70.90 | ±15.75 | 70.71 | ±15.92 | 71.74 | ±15.02 | 0.622 |
| NYHA Class | 2.63 | ±0.71 | 2.62 | ±0.71 | 2.66 | ±0.72 | 0.529 |
| Pulmonary hypertension | 21.4% | 21.4% | 21.4% | 0.993 | |||
| Ejection fraction % | 48.92 | ±10.69 | 48.75 | ±10.99 | 49.67 | ±9.35 | 0.515 |
| Aortic valve area (cm2) | 0.71 | ±0.21 | 0.70 | ±0.19 | 0.74 | ±0.28 | 0.218 |
| Log EuroScore I | 18.7% | ±13.3% | 18.9% | ±13.7% | 18.1% | ±11.5% | 0.645 |
| Arterial hypertension | 82.9% | 81.9% | 87.1% | 0.294 | |||
| Hyperlidaemia | 53.5% | 52.0% | 60.0% | 0.225 | |||
| Smoker (current) | 21.4% | 21.4% | 21.4% | 0.665 | |||
| Diabetes | 27.8% | 28.0% | 27.1% | 0.891 | |||
| Chronic lung disease | 15.8% | 16.1% | 14.3% | 0.704 | |||
| Stadium chronic renal failure (KDOQI CKF16) | 2.80 | ±0.86 | 2.80 | ±0.87 | 2.81 | ±0.80 | 0.945 |
| Dialysis | 2.9% | 3.0% | 2.9% | 0.963 | |||
| Liver disease | 4.0% | 4.6% | 1.4% | 0.221 | |||
Data are presented as mean ± SD or percentage/group; P-values were calculated by the Students t-test.
Most pmIFs were found within the thorax (in 26 participants), 19 were within the abdomen, and 21 retroperitoneum or mamma. Ten patients with a history of malignant disease were incidentally diagnosed as having progressive or relapsing disease. Finally, four participants had pmIF in other regions such as thyroid or multiple osteolysis (Table 2).
Table 2.
Potential malignant incidental findings were classified in ‘severe’ or ‘non-severe’ and are assigned to different regions
| Region | n severe pmIF | n not-severe pmIF |
|---|---|---|
| Thorax n = 26 |
4 | 22 |
|
|
|
| Abdomen n = 19 |
6 | 13 |
|
|
|
| Retroperitoneum or Mamma n = 21 |
15 | 6 |
|
|
|
| Other n = 4 |
3 | 1 |
| 3 multiple osteolysis | 1 thyroid cyst | |
| Relapse/progress all regions n = 10 |
8 | 2 |
|
|
Incidental diagnosis of relapse or progressive of a known malignant disease are included to the regions and are stated additionally in the last line.
The heart team has to assess these findings and decide which patient has a life expectancy of over 2 years and should be assigned to aortic valve repair. Notably, 40% of the pmIFs were severe and therefore highly suspicious for malignancies, but only 21% (n = 15) were finally confirmed.
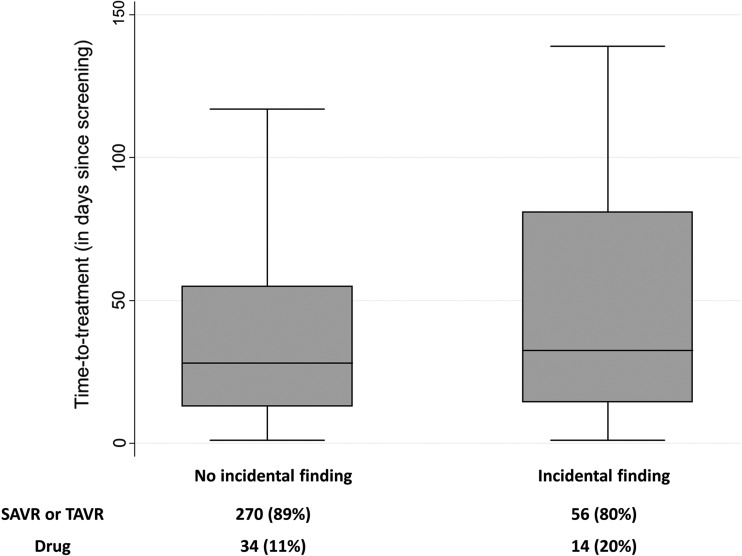
The average NYHA score of 2.6 indicated the symptomatic nature, apparent suffering, and need for intervention in this cohort. From 70 patients with pmIF, 56 underwent transcatheter or surgical treatment (45 TAVR, 11 SAVR) and 14 drug therapy (Figure 2).
Figure 2.
Time to treatment was retrospectively analysed in patients with or without pmIF. Results are presented in days after decision-making to the intervention. Decision of the interdisciplinary heart team is stated below.
As expected, age and EuroScore I had an impact on the decision to intervene in comparison to conservative drug therapy only, indicating that patients of high age and/or with high EuroScore I values were more likely to be assigned drug therapy only. The presence of any non-severe pmIF did not influence the decision to propose invasive treatment, but patients with an IF judged as ‘severe’ were much less likely to receive an intervention (OR 0.23; P = 0.019) (Table 3).
Table 3.
The decision by the interdisciplinary heart team to intervene was retrospectively analysed with a multivariable logistic regression model
| Decision to intervene |
||
|---|---|---|
| Odds ratio | P-value | |
| EuroScore I | 0.95 | >0.001 |
| Age | 0.95 | 0.067 |
| Non-severe incidental finding | 1.14 | 0.826 |
| Severe incidental finding | 0.23 | 0.019 |
| Confirmed malignant disease | 1.17 | 0.85 |
| n | 374 | |
Since the mortality risk of patients with severe aortic valve stenosis is significantly elevated,5 treatment should not be delayed. As shown in Figure 2, the presence of a pmIF did not change time to treatment significantly, but it was prolonged somewhat (with IF: 91 ± 152 days, range 1–729 days; without IF: 61 ± 109 days, range 1–859 days).
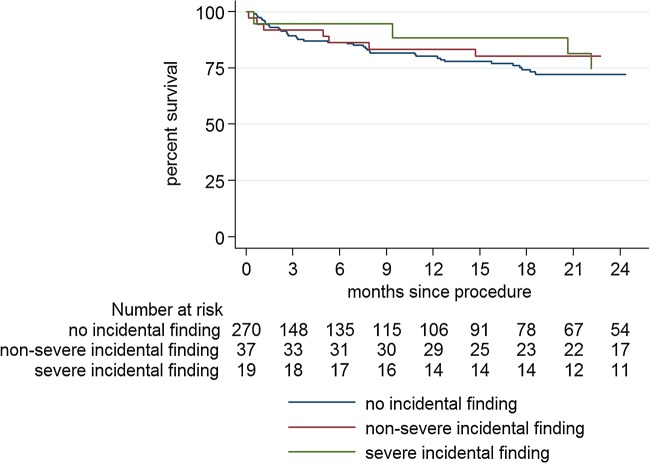
Follow-up period was over a period of 2 years. Two participants were lost to follow-up (one with severe, one with non-severe pmIF). If an individual's estimated survival was over 1 year and the heart team proposed invasive treatment, 2-year survival of these patients was around 75% and independent from the presence of an pmIF regardless of whether these findings were classified as non-severe (P = 0.923) or severe (P = 0.823) (Figure 3).
Figure 3.
Kaplan–Meier survival curves for patients without pmIF, with severe or non-severe pmIF receiving invasive treatment (TAVR or SAVR). P-values for comparison of the survival distributions between groups were calculated with the log-rank test.
Discussion
Elderly patients with severe aortic valve stenosis are increasingly being treated with TAVR.17 Since pre-procedural planning for this requires a whole-body CT,7 clinicians are more and more faced with equivocal pmIF. Essentially, this corresponds to an unscheduled screening for cancers in a cohort of patients with a high prevalence for pathologies and with all known medical-economic consequences.
In 374 patients with an average age of 80 years screened for TAVR, we found a prevalence of 18.7% for pmIF suspicious for malignant diseases. This prevalence corresponds broadly to previously published findings18 such as Gufler et al.19 who found 23.7% significant extravascular and 3.8% highly suspicious malignant findings in patients planned for TAVR and an average age of 82 years. Since the contrast was focused on arterial phase, incidence of pmIF could be even underestimated. Similarly, performing a cardiac CT leads to confirmation of previously unknown malignancies in 0.7%, assessed by a meta-analysis,20 and of overall IF (not only tumour suspicious) in 13%.21 IF appear in around 50% of patients with CT scans for trauma and in up to 90% of patients evaluated for an aortic aneurysm.22,23 But, clinical significance with respect to further needed diagnostic workup, change in therapy decision, or prognosis remain unclear. Particularly, the extent of radiologic findings is not obligatorily directly associated with clinical severity since the activity of the potential malignancy is unclear. Machaalany et al.10 showed direct costs for investigating IF of ∼US $80 000 per patient in a single-centre study in Canada. However, costs are subordinate, taking into account the potential impact of IF on treatment delay, changes in decision-making, patient anxiety and possibly positive outcomes, and expense of TAVR or SAVR.
Patients with severe aortic valve stenosis are typically highly symptomatic, demonstrated by an average NYHA score of 2.6 in our cohort. This results in a mandatory and timely need for treatment, otherwise associated with high mortality.5 Nevertheless, since IF could represent a potential malignancy, clinicians have to interpret the radiologic finding. In our study, ∼40% of the pmIF were judged ‘severe’, but only 21% were finally confirmed as cancer. This indicates a gap between the radiologic finding and clinical significance. Moreover, the ‘non-severe’ pmIFs such as pulmonary nodes, conspicuous lymph nodes, or organ augmentation (e.g. prostate) are likely to be benign, and their clinical significance seems to be rather low,24,25 but do generate uncertainty both to patients and physicians.26
As can be expected, some ‘severe’ findings limit life expectancy and thus argue against invasive treatment. Accordingly, patients with a ‘severe’ pmIF were less likely to be scheduled for SAVR or TAVR. However, half of the patients (53%) received some type of aortic valve intervention. The interdisciplinary decision for treatment was made according to the guidelines for TAVR, which recommend the procedure only in patients with an estimated survival of >1 year.7 We recently published an analysis of the decision-making process in our centre and could see appropriate decisions by the heart team, since survival was around 75% in patients treated either with TAVR or SAVR.15 We may draw two conclusions from this: first, decision-making requires an interdisciplinary heart team and in cases of pmIF a distinct consultation of a radiology and oncology specialist; secondly, the decision to intervene (SAVR or TAVR) can be made, as our data suggest, with no differences in outcome for patients regardless of ‘severe’ or ‘non-severe’ pmIF.
Time to treatment is especially critical in patients with high-grade aortic valve stenosis and in our study pmIF prolonged time to treatment, although overall there were no differences with or without IF.
Remarkably, the appearance of a pmIF did not influence overall mortality, even if it was judged as severe. At first glance, this appears counterintuitive but may be explained by the fact that the proportion of patients invasively treated decreased with the incidence of pmIF, if cancer was confirmed and/or the pmIF was defined as severe. Accordingly, the 75% survival of patients receiving TAVR or SAVR may be seen as the result of a successful treatment selection process and therefore confirms that the interdisciplinary heart team approach is crucial for optimal treatment of patients with aortic valve stenosis.
Limitations
There are some important limitations. First, the study is a single-centre study with relatively small number of participants with heterogeneous co-morbidities and the cause of death is not known. Furthermore, we have no information about new diagnosed cancer in patients without pmIF. Thus, it is not possible to calculate a relative risk of pmIF vs. no pmIF. The results are limited by not addressing a potential specific treatment of the pmIF and the sample size of patients assigned to drug therapy only is small and does not allow mortality analysis. Thus, patients with severe pmIF, who have a life expectation under 1 year and were not treated with TAVR or SAVR according to the decision by the heart team, are not included in the survival analysis. Thus, it could also be speculated that patients would even be better able to eventually undergo cancer treatment if AVR was performed. The decision-making process of the heart team to intervene was investigated only by the final decision, but criterions to the individual patient could not be respected.
Conclusion
We conclude that aortic valve stenosis can be treated with TAVR and SAVR as long as the patient's estimated survival exceeds 1 year and the interdisciplinary heart team approach is efficient and beneficial. A more holistic approach to health care for all should result in better outcomes and economic savings and so the next vital step will be to evaluate the impact of early detection of malignancies and confirm benefits to the patient and society.
Translational perspective
Elderly patients with severe aortic valve stenosis, either inoperable or at high operative risk, are increasingly treated with TAVR. Pre-procedural planning involves a body CT, and consequently, clinicians will be faced with more pmIF in the future. In our study, we found IF suspicious for malignancies in 18.7% of all patients. However, these did not significantly affect therapeutic decision or time to treatment, but severe findings were less likely assigned to invasive treatment. Two-year survival of patients, treated accordingly by TAVR or SAVR to a heart team decision was around 75% and independent of a pmIF. We conclude that aortic valve stenosis can be treated with TAVR and SAVR as long as the patient's estimated survival exceeds 1 year and the interdisciplinary heart team approach is efficient and beneficial.
Funding
Internal funding of the University Heart Center Freiburg supported the study. Funding to pay the Open Access publication charges for this article was provided by internal funding of the University Heart Center Freiburg.
Acknowledgements
We gratefully thank all the patients who gave their informed consent to participate in the study. Furthermore, we thank Gordon Goodall for proofreading.
Conflict of interest: None declared.
References
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. [DOI] [PubMed] [Google Scholar]
- 3.Reinohl J, von Zur Muhlen C, Moser M, Sorg S, Bode C, Zehender M. TAVI 2012: state of the art. J Thromb Thrombolysis 2013;35:419–35. [DOI] [PubMed] [Google Scholar]
- 4.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790–8. [DOI] [PubMed] [Google Scholar]
- 5.Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714–20. [DOI] [PubMed] [Google Scholar]
- 6.Holmes DR, Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200–54. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 8.Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012;6:366–80. [DOI] [PubMed] [Google Scholar]
- 9.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137–50. [DOI] [PubMed] [Google Scholar]
- 10.Machaalany J, Yam Y, Ruddy TD, Abraham A, Chen L, Beanlands RS, et al. Potential clinical and economic consequences of noncardiac incidental findings on cardiac computed tomography. J Am Coll Cardiol 2009;54:1533–41. [DOI] [PubMed] [Google Scholar]
- 11.Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone JH. Incidentalomas--clinical correlation and translational science required. N Engl J Med 2006;354:2748–9. [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Fischer H, Gopal A. Incidental findings with cardiac CT evaluation: should we read beyond the heart? Catheter Cardiovasc Interv 2006;68:965–73. [DOI] [PubMed] [Google Scholar]
- 14.Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956–66. [DOI] [PubMed] [Google Scholar]
- 15.Kaier K, Gutmann A, Vach W, Sorg S, Siepe M, von Zur Muhlen C, et al. “Heart Team” decision making in elderly patients with symptomatic aortic valve stenosis who underwent AVR or TAVI - a look behind the curtain. Results of the prospective TAVI Calculation of Costs Trial (TCCT). EuroIntervention 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–100. [DOI] [PubMed] [Google Scholar]
- 17.Khatri PJ, Webb JG, Rodes-Cabau J, Fremes SE, Ruel M, Lau K, et al. Adverse effects associated with transcatheter aortic valve implantation: a meta-analysis of contemporary studies. Ann Intern Med 2013;158:35–46. [DOI] [PubMed] [Google Scholar]
- 18.Burt JR, Iribarren C, Fair JM, Norton LC, Mahbouba M, Rubin GD, et al. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med 2008;168:756–61. [DOI] [PubMed] [Google Scholar]
- 19.Gufler H, Schulze CG, Wagner S. Incidental findings in computed tomographic angiography for planning percutaneous aortic valve replacement: advanced age, increased cancer prevalence? Acta Radiol 2014;55:420–6. [DOI] [PubMed] [Google Scholar]
- 20.Flor N, Di Leo G, Squarza SA, Tresoldi S, Rulli E, Cornalba G, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol 2013;201:555–64. [DOI] [PubMed] [Google Scholar]
- 21.Buckens CF, Verkooijen HM, Gondrie MJ, Jairam P, Mali WP, van der Graaf Y. Unrequested findings on cardiac computed tomography: looking beyond the heart. PLoS ONE 2012;7:e32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Indes JE, Lipsitz EC, Veith FJ, Gargiulo NJ, 3rd, Privrat AI, Eisdorfer J, et al. Incidence and significance of nonaneurysmal-related computed tomography scan findings in patients undergoing endovascular aortic aneurysm repair. J Vasc Surg 2008;48:286–90. [DOI] [PubMed] [Google Scholar]
- 23.Sierink JC, Saltzherr TP, Russchen MJ, de Castro SM, Beenen LF, Schep NW, et al. Incidental findings on total-body CT scans in trauma patients. Injury 2014;45:840–4. [DOI] [PubMed] [Google Scholar]
- 24.Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178:956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iribarren C, Hlatky MA, Chandra M, Fair JM, Rubin GD, Go AS, et al. Incidental pulmonary nodules on cardiac computed tomography: prognosis and use. Am J Med 2008;121:989–96. [DOI] [PubMed] [Google Scholar]
- 26.Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 2010;7:754–73. [DOI] [PubMed] [Google Scholar]