Abstract
The beta-ketoacyl-ACP synthase II (KASII) is an enzyme in fatty acid biosynthesis, catalyzing the elongation of 16:0-acyl carrier protein (ACP) to 18:0-ACP in plastids. Mutations in KASII genes in higher plants can lead to lethality, which makes it difficult to utilize the gene for lipid metabolic engineering. We demonstrated previously that transient expression of plastid-directed fatty acyl reductases and wax ester synthases could result in different compositions of wax esters. We hypothesized that changing the ratio between C16 (palmitoyl-compounds) and C18 (stearoyl-compounds) in the plastidic acyl-ACP pool by inhibition of KASII expression would change the yield and composition of wax esters via substrate preference of the introduced enzymes. Here, we report that transient inhibition of KASII expression by three different RNAi constructs in leaves of N. benthamiana results in almost complete inhibition of KASII expression. The transient RNAi approach led to a shift of carbon flux from a pool of C18 fatty acids to C16, which significantly increased wax ester production in AtFAR6-containing combinations. The results demonstrate that transient inhibition of KASII in vegetative tissues of higher plants enables metabolic studies towards industrial production of lipids such as wax esters with specific quality and composition.
Oil crops are of great interest since they can provide a sustainable production of high-value oleochemicals such as wax esters and fatty alcohols for the chemical industry with similar hydrocarbon structures to those of conventional petrochemical products. Wax esters (WE) are highly hydrophobic neutral lipids that are composed of medium to very long chain alcohols esterified to a fatty acid moiety. They have excellent lubrication properties, oxidation stability, and high resistance to hydrolytic degradation. The unique physical properties of wax esters bring a high value to industrial applications within, for instance, the textile, cosmetic, packaging, ink, candle, drug, and food industries1,2,3. Wax esters are present in plants, animals, and microorganisms, where they serve a number of biological functions including prevention of water loss, protection against pathogens, insects, and UV radiation4,5, energy storage6, buoyancy density regulation7, and gland secretion in bird species8. Historically, the main source of wax esters for industrial applications was the spermaceti organ of sperm whales, until hunting of these animals was banned because the species came close to extinction. Nowadays, the industrial demand for wax esters from biological sources is mainly dependent on the carnauba palm (Copernicia prunifera) or the desert shrub jojoba (Simmondsia chinensis)9. The limited natural resources for plant wax esters, compared with the potential applications, provide high motivation to design and develop specialized wax esters for industrial purposes by metabolic engineering of additional plant species.
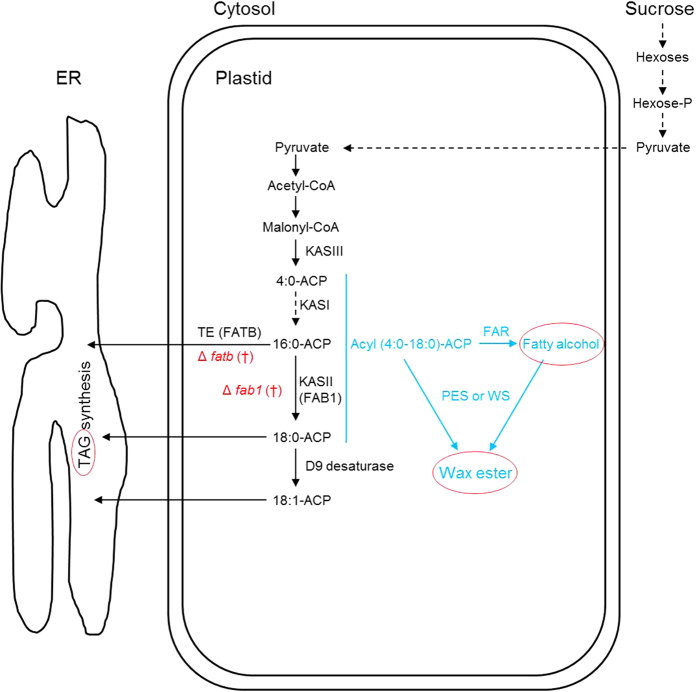
In plants, de novo fatty acid biosynthesis consists of a number of enzymatic reactions10,11 where C2-units (two carbon units) are added to a growing fatty acyl chain by a small β-ketoacyl-ACP synthase (KAS) family in plastids12,13. The reaction series commonly results in the formation of C16:0-ACP (palmitic) and C18:0-ACP (stearic) fatty acids. Three plastidial classes of KAS enzymes have been characterized. KASIII is responsible for condensation of C2:0-ACP to C4:0-ACP, KASI catalyzes reactions from C4:0-ACP up to C16:0-ACP, and finally KASII mediates the extension of C16:0-ACP to C18:0-ACP13. KAS families play a central role in determining the range of substrates for other enzymes14. Newly produced fatty acids are either released from plastids by the activity of acyl-ACP thioesterase (TE), known as FATA and FATB10, or further desaturated from C18:0-ACP to C18:1-ACP by stearoyl-ACP desaturase (Δ9 desaturase or SAD) activity15,16 (Fig. 1). The fatty acid 16:0-ACP is a substrate not only for KASII and FATB, but also for SAD17 and lysophosphatidyl acyltransferase (LPAAT)18. These enzymes together are important for determination of the fatty acid pool available to the lipid biosynthetic machinery in the cytosol. Research with the aim of characterizing KASII function and specificity has been performed in various species12,13,14,15, and has demonstrated that KASII has the highest affinity for C16:0 substrates12. In mesophyll cells of Arabidopsis, 69% of the C16:0-ACP pool was shown to be converted into C18:0-ACP19 by the function of KASII. Moreover, a partially deficient Arabidopsis mutant, fab1, which caused a reduction in KASII activity of 38.5%, led to an increase of 43.1% in leaf C16:0-ACP (palmitic aid) content20. However, complete inhibition of KASII activity in plants seems unattainable, as deficiency of KASII is lethal12,15.
Figure 1. Schematic overview of manipulation of enzymatic steps and estimated co-integration of fatty acids into different metabolites through de novo fatty acid synthesis in a plastid.
Mutations with a lethal phenotype are shown in red. Metabolites monitored in the present study are shown in red circles.
Biosynthesis of wax esters generally comprises three distinctive stages. Precursors required for initiation of the process are synthesized via de novo fatty acid synthesis, so this part of wax ester biosynthesis is shared with other lipid biosynthetic pathways4,5. The second stage of the enzymatic process is conversion of an activated fatty acid to a fatty alcohol by the function of a fatty acyl reductase (FAR) enzyme. This reaction varies among organisms and can be performed by a single enzyme, or by two separate enzymatic reactions3,21,22,23,24. The final stage is the esterification by a wax synthase (WS) of fatty alcohols with fatty acids to form wax esters23,25. The composition of wax esters depends on the specific catalytic enzymes (FARs and WSs) and the availability of acyl-ACP substrates26. In an earlier study, we demonstrated the feasibility of producing different qualities and quantities of wax esters in chloroplast organelles of Nicotiana benthamiana leaves by a combination of several genes encoding enzymes for wax ester biosynthesis27. Arabidopsis FAR6 (AtFAR6) showed a high substrate preference for C16:0-ACP in the production of primary fatty alcohols, while Marinobacter FAR (MaFAR) was able to use both C16:0-ACP and C18:0-ACP at a similar rate27. In addition, PES2 (phytyl ester synthase2) in chloroplasts displayed high activity in esterification of primary alcohols and fatty acids for wax ester production in N. benthamiana.
Aiming at an increase in substrates for wax ester biosynthesis, we hypothesized that the pool of C16:0-ACP could be increased by transient inhibition of KASII activity. The increased C16:0-ACP pool could then be used by one of the alcohol-forming enzymes (AtFAR6 in particular, or MaFAR), thereby resulting in an elevated wax ester content. To investigate the role of KASII in production of wax ester quantity and quality in chloroplasts of N. benthamiana, we made three different RNAi constructs against KASII genes in N. benthamiana and infiltrated leaves to test whether the inhibition could lead to an increase in C16:0 levels. Our aim was to demonstrate that modulation of KASII expression is possible in plant vegetative tissues. This provides a rational experimental approach for increasing levels of C16:0 and decreasing levels of C18:0 in the metabolic engineering of wax ester synthesis for improvement of the specific composition and quality of wax esters for industrial purposes.
Results
Gene constructs and expression levels of KASII after transient RNAi silencing
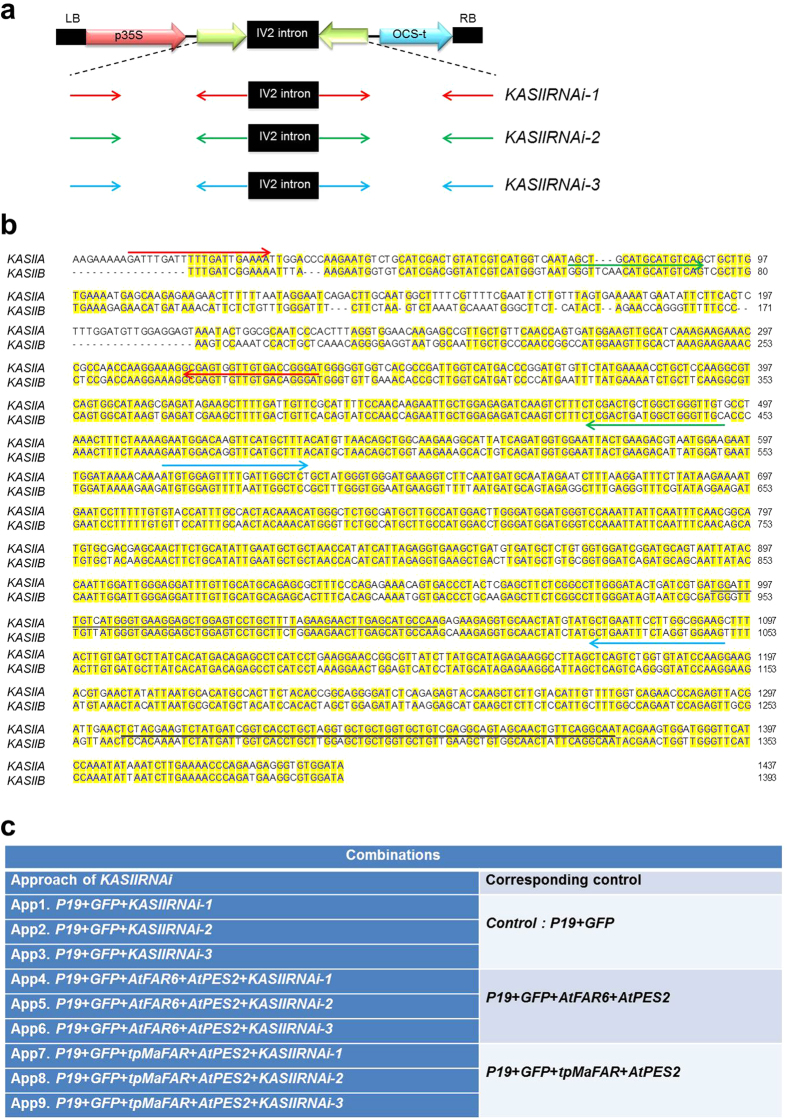
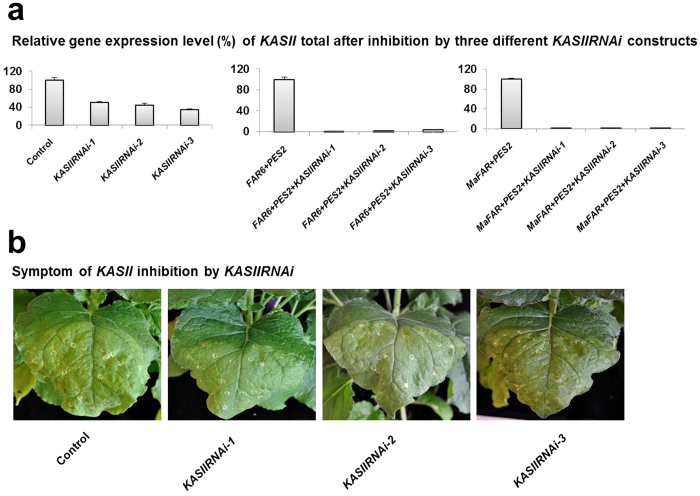
Blast analysis at solgenomics (www.solgenomics.net) using DNA sequences of Arabidopsis thaliana KASII corresponding to GenBank accession AY081285 identified two putative KASII genes in the N. benthamiana genome. In order to test whether simultaneous inhibition of the two KASII genes was possible, three different RNAi constructs were made towards different regions of KASII using intron-spliced hairpin technology28. These constructs were denoted KASIIRNAi-1, -2 and -3 (Fig. 2a,b). They were infiltrated without or with our previous wax ester production system27 (including AtFAR6 + AtPES2 or MaFAR + AtPES2; Fig. 2c), where p19 + GFP infiltrated tissues were used as controls, and also co-infiltrated in each combination. Five days after infiltration, down-regulation of the two KASII genes (counted as KASII total) in the three KASIIRNAi experiments was assessed by quantitative real-time PCR (Fig. 3a). The results showed severe inhibition of KASII in each combination (Fig. 3a). Upon infiltration, the control leaves were affected, resulting in visible symptoms (slight bleaching in the infiltrated area and infiltration wound spots), but there was no difference compared with any additional KASIIRNAi gene constructs (Fig. 3b). These data show that the agro-infiltration strategy could be used for inhibition of KASII in studies of wax ester metabolic engineering in N. benthamiana.
Figure 2. Constructions of KASIIRNAi-1, KASIIRNAi -2, and KASIIRNAi -3 and experimental design of agro-infiltration in Nicotiana benthamiana leaves.
(a) Schematic drawing of the three KASIIRNAi constructs used to create an hairpin RNAi. The sequences of sense and antisense regions of individual constructs were inserted on opposite sites flanking the IV2 intron in the expression vector pGEMIV23Z41. The RNAi construct thus created was then inserted into Ti-plasmid pART27. OCS-t: Octopine synthase terminator; LB and RB: left and right T-DNA border, respectively. (b) Gene sequences of KASIIA and KASIIB in N. benthamiana genome. The areas (primers) for construction are highlighted in red for KASIIRNAi-1, green for KASIIRNAi-2, and blue for KASIIRNAi-3. Black and grey bars indicate the area of gene sequences used for detection of transcript levels of KASII gene in N. benthamiana by quantitative PCR. (c) Gene constructs and different combinations used for infiltration and transient expression in N. benthamiana leaf tissues.
Figure 3. Transcript levels and appearance of plant tissues of N. benthamiana after agro-infiltration.
(a) Quantitative PCR analysis of individual genes used in each combination and controls in N. benthamiana leaf tissue. Individual gene expression levels were normalized to the expression of Actin (ACT) gene. Relative gene expression level shown as %. Relative mean values from three independent biological experiments, analyzed in technical triplicates. (b) Photos of leaf tissues of N. benthamiana five days post infiltration. The corresponding control is described in Fig. 2c. Similar symptoms (bleaching in the infiltrated area with infiltration wound spots) were observed in control leaves and leaves with additional KASIIRNAi gene constructs.
Increased wax ester levels by inhibition of KASII
We further investigated the consequences of KASII inhibition in our wax ester production system by examining total wax ester levels and compositions. Five days after infiltration, the production of wax esters was apparent after separation of total lipid extracts on thin layer chromatography (TLC) plates, while very low amounts of wax esters were observed in the controls (data not shown). The total amount of wax esters in the controls was small and infiltration of individual KASII RNAi constructs did not significantly change these levels (Fig. 4a, left panel).
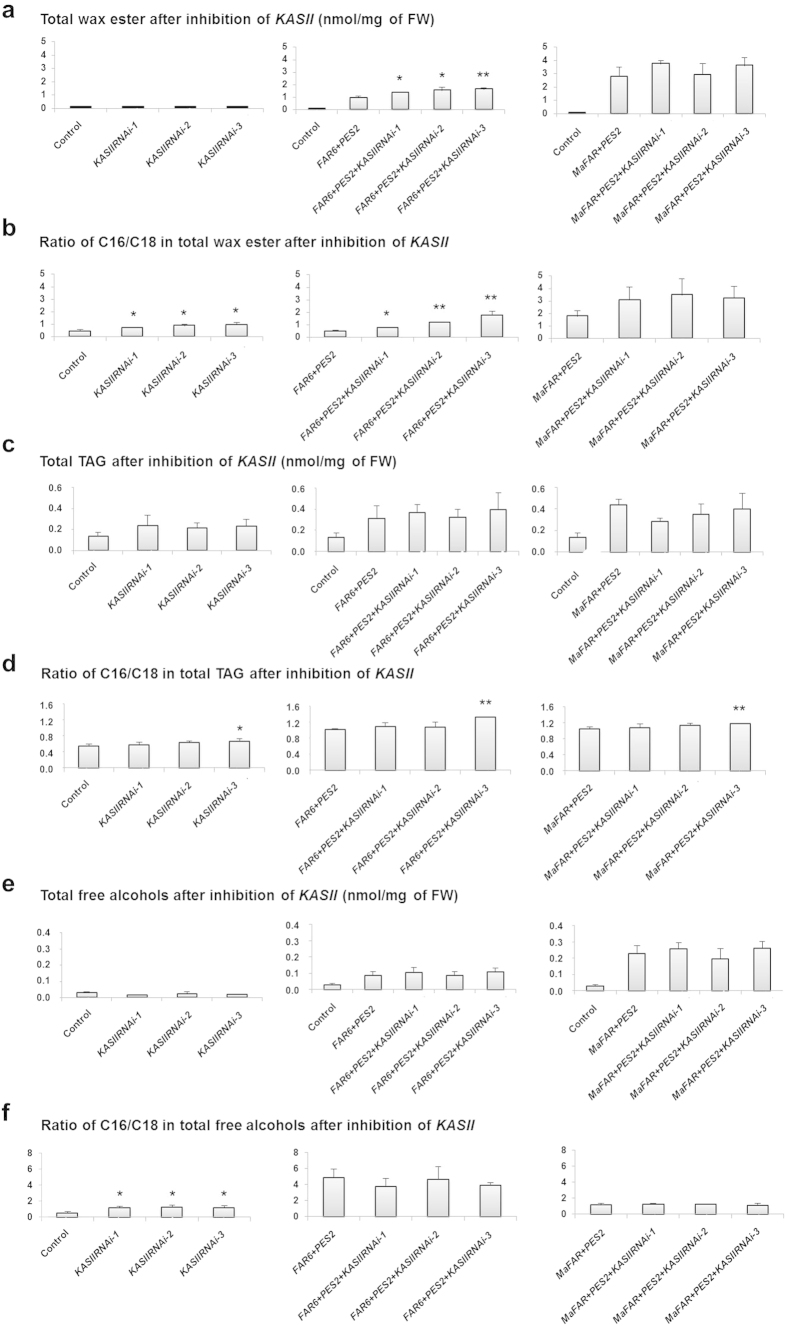
Figure 4. Metabolite levels after KASIIRNAi inhibition in N. benthamiana leaves.
(a) Total wax ester content in leaves. (b) Total C16/C18 fatty acid ratio in wax esters. (c) Total triacylglycerol (TAG) production in different combinations. (d) Total C16/C18 fatty acid ratio in TAGs. (e) Total free alcohol found in Nicotiana benthamiana leaf extracts. (f) Total C16/C18 fatty alcohol ratio in free alcohols. All data presented are mean (with SD) of biological triplicates. Statistical analyses for each RNAi sample were performed against the corresponding control. Only statistically significant increases are indicated, (* P < 0.05, **P < 0.01). The corresponding control is described in Fig. 2c.
In the experiments with AtFAR6-containing combinations, inhibition of KASII expression significantly increased wax ester production levels, but there was no significant increase for the combinations with MaFAR. The highest wax ester level in the combinations with AtFAR6 was found for the combination AtFAR6 + PES2 + RNAi-3, with an amount of 1.62 nmol/mg per fresh weight (FW), corresponding to about 0.9% of leaf dry weight (DW) (Fig. 4a, middle panel). In general, all combinations with MaFAR produced a higher amount of wax esters compared with combinations with AtFAR6 (Fig. 4a, right panel).
Increased wax ester levels in relation to elevated C16/C18 ratio
The composition of wax esters was examined in order to monitor changes induced by KASII inhibition. We observed a significant increase in C16/C18 ratio in the small amount of wax esters in the control tissues where KASIIRNAi were expressed on their own without the wax ester synthesis capacity added (Fig. 4b, left panel).
Increased C16/C18 ratio was also observed when AtFAR6 was utilized to supply fatty alcohols (Fig. 4, middle panel). The increase in wax ester production levels in these combinations was associated with increased C16/C18 ratio (Fig. 4b vs. Fig. 4a, middle panels). When AtFAR6 was co-expressed in the combinations with KASIIRNAi, C16:0 fatty acids were increased by as much as 72% in the combination AtFAR6 + PES2 + RNAi-3 (Supplementary Table S1) compared with the corresponding control (AtFAR6 + PES2), and the total wax ester levels were increased by 73% in this specific combination (Fig. 4b, middle panel). KASII silencing did not significantly affect the C16/C18 ratio upon expression of MaFAR combinations (Fig. 4b, right panel).
Triacylglycerol (TAG) and free alcohols after KASII inhibition
De novo fatty acid synthesis is a shared pathway in the production of other lipid molecules, and we expected that any release of fatty acids from the plastid could be used for TAG assembly. We thus further investigated TAG accumulation and composition in all gene combinations used in the transient expression system. While total TAG amounts were not changed significantly in any of the combinations (Fig. 4c), the C16/C18 ratio was significantly increased when KASIIRNAi-3 was expressed with or without wax ester biosynthesis genes (Fig. 4d). However, this increase was not observed in the case of KASIIRNAi-1 and -2.
The free fatty alcohols that were not utilized in wax ester synthesis in the infiltrated leaves were also analyzed in each gene combination and in the controls. In general, the total free alcohol level was not changed significantly by KASII inhibition, and was higher in MaFAR-infiltrated leaves than in AtFAR6-infiltrated leaves (Fig. 4e). The C16:0-OH/C18:0-OH ratio in control tissues was significantly changed upon infiltration of all three KASIIRNAi constructs (Fig. 4f, left panel), while the ratio was not significantly changed upon KASII inhibition in the combinations compared with the controls (Fig. 4f, middle and right panels). Free fatty alcohols were mainly composed of 16:0-OH and 18:0-OH species, as were the wax esters. The 16:0-OH species dominated in the AtFAR6-infiltrated leaves, while equal amounts of C16:0-OH and C18:0-OH were observed in the MaFAR-infiltrated combinations (Supplementary Table S1).
Increased C16/C18 ratio in total lipid extracts of leaf tissue after KASII inhibition
To further investigate the effects of KASII inhibition on the fatty acid composition of total lipids related to KASII activity (i.e. C16/C18 ratio) in leaf tissue, we examined fatty acid composition in total leaf lipid extracts. Interestingly, the C16/C18 ratio in the total lipid samples was significantly increased after KASII inhibition, regardless of presence of AtFAR6, MaFAR, or PES2 (Fig. 5). Furthermore, a higher C16/C18 ratio was observed in samples when additional wax ester biosynthesis genes were included. The change in the KASIIRNAi-3 sample without addition of AtFAR6, MaFAR or PES2 further demonstrates the role of KASII in determination of fatty acid composition of other lipids in total lipids as the lipid compounds examined, i.e., wax esters and free fatty alcohols were present in trace amounts in the KASIIRNAi-3 sample (Fig. 4).
Figure 5. Fatty acid composition of total lipids from leaf tissues.
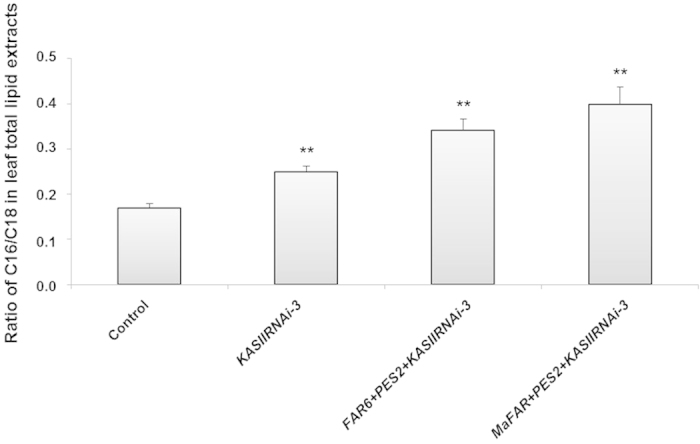
The C16/C18 fatty acid ratio in total lipids related to KASII activities is shown. All data presented are mean (with SD) of biological triplicates. Statistical analyses for each RNAi sample were performed against the control. Statistically significant increases are indicated (**P < 0.01). The control is described in Fig. 2c.
Discussion
Transient gene expression systems can be used to rapidly assess simultaneous effects of several genes on the quality and quantity of a specific product. The results presented here provide evidence that hairpin-RNAi targeting the KASII genes in Nicotiana benthamiana leaf tissues makes more palmitic acid available for metabolism of complex products and, as a consequence, elevates the C16/C18 ratio in wax esters. These results provide new insights into the use of transient approaches to manipulate fatty acid synthesis for both quality and quantity, with emphasis on enhancing production of oleochemicals in leaves of N. benthamiana. Complete inhibition of the KASII genes can lead to lethality in stable transformation systems. It has been shown that in Arabidopsis transformants, strong seed-specific hairpin-RNAi reduction of FAB1 (alias KASII) expression is lethal, while lines with less severe reduction of FAB1 expression show normal embryo development and increased palmitic acid levels15. We showed here that transient expression of any KASIIRNAi gene did not affect leaf health differently from the control five days after infiltration (Fig. 3b). To avoid detrimental effects on plant vigor in future applications using KASII inhibition in stable transformation, we suggest that genotypes with an appropriate degree of silencing be selected among transformants or that a developmentally regulated or inducible promoter be used. Inhibition efficiency of KASIIRNAi was apparently higher when co-expressed with additional genes FAR and PES (Fig. 3a). P19 has been found to form a homo-dimer which binds siRNAs29,30. According to Fig. 2C of Naim et al.31, co-infiltration with p19 enhances expression of GFP, but it also makes hpGFP inhibition of GFP less efficient. Therefore a possible explanation could be that when KASIIRNAi is expressed on its own together with p19, inhibition is less efficient than if additional products such as FAR and PES are expressed. There could thus be a titration effect towards p19.
From this study we conclude that composition and quality of wax esters can be modified by transient KASIIRNAi inhibition. Through metabolic engineering, the fatty acid composition of total lipids other than wax esters can also be altered via this approach. Moreover, quantity of wax esters can be improved by using certain enzymes (e.g., AtFAR6) that prefer palmitic components. To our knowledge, the present work is the first report on metabolic engineering of wax ester compositions by transient silencing of the KASII genes. In future development work to ensure higher substrate supply and further enhance production of wax esters, it would be of great interest to study inhibition of acyl-ACP thioesterases10,15,16,32 and/or stearoyl-ACP (SAD)15,16,17,33 desaturase activity in our wax ester production system27 (see also Fig. 1).
Methods
Molecular cloning of genes
The construction procedure of binary vectors containing the genes in wax ester biosynthesis, AtFAR6, MaFAR, and AtPES2 driven by the constitutive CaMV-35 S promoter, was performed as described previously27. The constructs containing the tomato bushy stunt virus p19 viral silencing suppressor and GFP34 genes under control of the CaMV-35S promoter were kindly provided by Dr. Craig C. Wood (CSIRO, Australia). In order to get the sequences of β-ketoacyl-ACP synthase II genes in the Nicotiana benthamiana genome, DNA sequences of Arabidopsis thaliana corresponding to GenBank accession AY081285 were blasted on http://solgenomics.net against N. benthamiana genome. The blasting results revealed two genes in N. benthamiana genome with 81% identity (GenBank ID#NbS00033926g0004.1), and 80% identity (GenBank ID#NbS00001834g0011.1), respectively, to Arabidopsis KASII. The RNAi constructs against different coding regions (denoted KASIIRNAi-1, KASIIRNAi-2, and KASIIRNAi-3) were cloned as inverted repeat hairpin constructs. The DNA sequences of sense and antisense regions (325 bp for KASIIRNAi-1, 396 bp for KASIIRNAi-2, and 483 bp for KASIIRNAi-3) were amplified by PCR using the wild-type cDNA of N. benthamiana as a template. The primers used for cloning of individual constructs are listed in Supplementary Table S2. The amplified PCR fragments were sub-cloned into chemically competent E. coli TOP10 cells using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA, USA). The expression cassette in the binary vector was digested with BamHI and XbaI for the sense region and SacI and KpnI for the antisense regions, and then inserted between the corresponding sites of expression vector pGEMIV23Z, which includes the IV2 intron35 sequences. The final construct was digested with XbaI and SalI (Fermentas), and then inserted into the corresponding sites of expression vector pART2736, resulting in plasmids p35S-KASIIRNAi-1, p35S-KASIIRNAi-2, and p35S-KASIIRNAi-3 (Fig. 2). Final constructs were verified by DNA sequencing (Macrogen Europe, Amsterdam, the Netherlands). All constructs used in this study were transformed into Agrobacterium tumefaciens strain GV3101 using the freeze-thaw method.
Plant materials and transient gene expression in N. benthamiana leaves
Nicotiana benthamiana plants were grown at 25 °C in a 16 h light:8 h dark regime in a phytotron. Light intensity was 320 μmol/m2/s and relative humidity (RH) was 60%. The plants were grown in 21 × 16 cm pots and watered regularly with 1% of NPK macronutrients37. The second-from-top leaves of 6-week-old plants were used for agro-infiltration.
Transient expression of genes in N. benthamiana leaves by the agro-infiltration method was performed as described previously27,34,38 with small modifications. Agrobacterium tumefaciens cultures containing the gene(s) of interest were mixed so that the final OD600 of each culture was equal to 0.2 prior to infiltration. The different combinations of the genes and controls are listed in Fig. 2c. In every combination, a p19 construct for silencing effect and GFP construct for identifying the infiltrated areas were included. After infiltration, N. benthamiana plants were grown for a further five days39,40, infiltrated areas were exposed to UV light, and GFP-expressing regions were excised, freeze-dried, weighed, and stored at −80 °C for further analyses.
Fatty alcohol and lipid analysis by TLC and gas chromatography (GC)
The leaf areas that showed fluorescence under UV light were excised, homogenized in 3.75 ml methanol/chloroform (2:1 v/v) and 1 ml 0.15 M acetic acid, and mixed with chloroform for extraction as described41. Total lipid extracts corresponding to 100 mg fresh weight of leaves were applied to TLC (silica 60, Merck) separation. Free fatty alcohols were separated with hexane:diethylether:acetic acid (85:15:1, v/v/v) as mobile phase. The alcohol spots were located on the plates by co-migration with the heptadecanol (C17:0-OH) standard sprayed with water. Free alcohols were eluted from the silica material with methanol and extracted with chloroform as described previously27. Total lipid extracts corresponding to 20 mg fresh weight of leaves were used for analysis of total fatty acid composition. The leaf extracts were dried under nitrogen flow, dissolved in 500 μl n-hexane, methylated, and analyzed by GC as described below. The extracted fatty alcohols, wax esters, TAGs, and fatty acids in total leaf lipids were analyzed by GC as described previously27. As internal standard, added prior to lipid extraction, 5 nmol of heptadecanoyl heptadecanoate was used for wax esters, 5 nmol of heptadecanol for free alcohols, and 5 nmol of triheptadecanoin for TAGs and total leaf fatty acids.
RNA isolation and gene expression analysis
Total RNA from the collected leaf materials was extracted by grinding approximately 100 mg of fresh leaf tissue in liquid nitrogen and applying the Spectrum™ Plant Total RNA Kit (Sigma-Aldrich, St. Louis, MO, US). All samples were treated with DNase I (Sigma-Aldrich, St. Louis, MO, US) to remove any residues of DNA contamination. The first-strand cDNA was performed using the qScript cDNA Synthesis kit following the manufacturer’s instructions (Quanta Biosciences, Gaithersburg, MD, USA), and 1 μg of total RNA was used as a template in 20 μl reaction volume. Quantitative RT-PCR (qRT-PCR) reactions (20 μl) included SYBR Green PCR master mix (Applied Biosystems, Life Technologies Europe BV, Stockholm, Sweden), supplemented with 5 μM primer (Supplementary Table S2) and 1 μl cDNA as template, which was diluted up to 200 μl after synthesis.
Primers for qRT-PCR were designed using the tool of Roche Applied Sciences. qRT-PCR primers were analyzed by blasting against the N. benthamiana genome sequences on the solgenomics network website (http://solgenomics.net/). Reaction mixtures without cDNA were used as a negative control. PCR was performed as follows: 10 min at 50 °C, 5 min at 95 °C, 40 cycles of 10 s at 95 °C and 30 s at 60 °C, and finally 1 min at 95 °C, in 96-well optical reaction plates (Applied Biosystems, Life Technologies Europe BV, Stockholm, Sweden). The specificity of the reactions and the amplicon identities were verified by melting curve analysis. The data were analyzed by the comparative CT method42 with PCR efficiency correction, which was determined based on the slope of standard curves. The gene expression level by qRT-PCR was normalized using the gene Actin (ACT) gene43 and in some cases the fold-differences in the transcript levels, and mean standard error was calculated as described previously42. Primer combinations and reactions were shown to be specific for the purposes of the experiment and therefore no signal was found in the control tissues.
Statistical analysis
One-way ANOVA was used for statistical analysis.
Additional Information
How to cite this article: Aslan, S. et al. Transient silencing of the KASII genes is feasible in Nicotiana benthamiana for metabolic engineering of wax ester composition. Sci. Rep. 5, 11213; doi: 10.1038/srep11213 (2015).
Supplementary Material
Acknowledgments
The authors are grateful to Sten Stymne for essential support and to Sarosh Bejai and Yunkai Jin at Plant Biology, SLU, Uppsala, for advice on qPCR and statistical analyses, respectively. This work was supported by the EU FP7 project “ICON”, the Swedish Research Council Formas, and the Swedish Government Agency for Innovation Systems, VINNOVA.
Footnotes
Author Contributions S.A. performed all experiments and drafted the manuscript. P.H. helped design the experiments and revised the manuscript. P.D. and F.S. gave critic discussions and revised the manuscript. C.S. coordinated the work and restructured and revised the manuscript.
References
- Jetter R. & Kunst L. Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J. 54, 670–683, (2008). [DOI] [PubMed] [Google Scholar]
- Li W. et al. Green waxes, adhesives and lubricants. Philos. Trans. A Math. Phys. Eng. Sci. 368, 4869–4890 (2010). [DOI] [PubMed] [Google Scholar]
- Lardizabal K. D. et al. Purification of a jojoba embryo wax synthase, cloning of its cDNA, and production of high levels of wax in seeds of transgenic Arabidopsis. Plant Physiol. 122, 645–655 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- PostBeittenmiller D. Biochemistry and molecular biology of wax production in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 405–430 (1996). [DOI] [PubMed] [Google Scholar]
- Samuels L., Kunst L. & Jetter R. Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 59, 683–707 (2008). [DOI] [PubMed] [Google Scholar]
- Fixter L. M., Nagi M. N., McCormack J. G. & Fewson C. A. Structure, Distribution and Function of Wax Esters in Acinetobacter calcoaceticus. J Gen. Microbiol. 132, 3147–3157 (1986). [Google Scholar]
- Phleger C. F. Buoyancy in marine fishes: Direct and indirect role of lipids. Am. Zool. 38, 321–330 (1998). [Google Scholar]
- Biester E. M., Hellenbrand J., Gruber J., Hamberg M. & Frentzen M. Identification of avian wax synthases. BMC Biochem. 13, 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhercke T., Wood C. C., Stymne S., Singh S. P. & Green A. G. Metabolic engineering of plant oils and waxes for use as industrial feedstocks. Plant Biotechnol. J. 11, 197–210 (2013). [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B. & Jaworski J. G. Regulation of fatty acid synthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 109–136 (1997). [DOI] [PubMed] [Google Scholar]
- Browse J. & Somerville C. Glycerolipid synthesis-biochemistry and regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 467–506 (1991). [Google Scholar]
- Carlsson A. S., LaBrie S. T., Kinney A. J., von Wettstein-Knowles P. & Browse J. A KAS2 cDNA complements the phenotypes of the Arabidopsis fab1 mutant that differs in a single residue bordering the substrate binding pocket. Plant J. 29, 761–770 (2002). [DOI] [PubMed] [Google Scholar]
- Shimakata T. & Stumpf P. K. Isolation and function of spinach leaf beta-ketoacyl- acyl-carrier-protein synthases. Proc. Natl. Acad. Sci. U.S.A. 79, 5808–5812 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz F., Kiviniemi S., Palva T. E. & Sutinen M. L. Contribution of omega-3 fatty acid desaturase and 3-ketoacyl-ACP synthase II (KASII) genes in the modulation of glycerolipid fatty acid composition during cold acclimation in birch leaves. J. Exp. Bot. 57, 897–909 (2006). [DOI] [PubMed] [Google Scholar]
- Pidkowich M. S., Nguyen H. T., Heilmann I., Ischebeck T. & Shanklin J. Modulating seed beta-ketoacyl-acyl carrier protein synthase II level converts the composition of a temperate seed oil to that of a palm-like tropical oil. Proc. Natl. Acad. Sci. U.S.A. 104, 4742–4747 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.-Y., Hammerlindl J., Forseille L., Zhang H. & Smith M. A. Simultaneous over-expressing of an acyl-ACP thioesterase (FatB) and silencing of acyl-acyl carrier protein desaturase by artificial microRNAs increases saturated fatty acid levels in Brassica napusseeds. Plant Biotech. J. 12, 624–637 (2014). [Google Scholar]
- Cahoon E. B. & Shanklin J. Substrate-dependent mutant complementation to select fatty acid desaturase variants for metabolic engineering of plant seed oils. Proc. Natl. Acad. Sci. U.S.A. 97, 12350–12355 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. U. & Huang A. H. C. Plastid lysophosphatidyl acyltransferase is essential for embryo development in arabidopsis. Plant Physiol. 134, 1206–1216 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J., McCourt P. J. & Somerville C. R. Fatty-acid composition of leaf lipids determined after combined digestion and fatty-acid methyl-ester formation from fresh tissue. Anal. Biochem. 152, 141–145 (1986). [DOI] [PubMed] [Google Scholar]
- Wu J. R., James D. W., Dooner H. K. & Browse J. A mutant of arabidopsis deficient in the elongation of palmitic acid. Plant Physiol. 106, 143–150 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. B. & Russell D. W. Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. J. Biol. Chem. 279, 37789–37797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofvander P., Doan T. T. & Hamberg M. A prokaryotic acyl-CoA reductase performing reduction of fatty acyl-CoA to fatty alcohol. FEBS Lett. 585, 3538–3543 (2011). [DOI] [PubMed] [Google Scholar]
- Kalscheuer R. & Steinbuchel A. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 278, 8075–8082 (2003). [DOI] [PubMed] [Google Scholar]
- Metz J. G., Pollard M. R., Anderson L., Hayes T. R. & Lassner M. W. Purification of a jojoba embryo fatty acyl-coenzyme A reductase and expression of its cDNA in high erucic acid rapeseed. Plant Physiol. 122, 635–644 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. et al. Identification of the wax ester synthase/acyl-coenzyme A: diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiol. 148, 97–107 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iven T. et al. Wax ester profiling of seed oil by nano-electrospray ionization tandem mass spectrometry. Plant Methods. 9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan S. et al. Wax esters of different compositions produced via engineering of leaf chloroplast metabolism in Nicotiana benthamiana. Metab. Eng. 25, 103–112 (2014). [DOI] [PubMed] [Google Scholar]
- Helliwell C. A. & Waterhouse P. M. Constructs and methods for hairpin RNA-mediated gene silencing in plants. Methods Enzymol. 392, 24–35 (2005). [DOI] [PubMed] [Google Scholar]
- Baulcombe D. C. & Molnár A. Crystal structure of p19--a universal suppressor of RNA silencing. Trends Biochem Sci. 29, 279–281 (2004). [DOI] [PubMed] [Google Scholar]
- Ye K., Malinina L. & Patel D. J. Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature 426, 874–878 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim F. et al. Advanced engineering of lipid metabolism in Nicotiana benthamiana using a draft genome and the V2 viral silencing-suppressor protein. PLoS One 7, e52717 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A., Davies H. M. & Voelker T. A. Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell. 7, 359–371 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarhloul M. K. et al. Breeding high-stearic oilseed rape (Brassica napus) with high- and low-erucic background using optimised promoter-gene constructs. Mol. Breed. 18, 241–251 (2006). [Google Scholar]
- Wood C. C. et al. A leaf-based assay using interchangeable design principles to rapidly assemble multistep recombinant pathways. Plant Biotechnol. J. 7, 914–924 (2009). [DOI] [PubMed] [Google Scholar]
- Ahlandsberg S., Sathish P., Sun C. & Jansson C. A set of useful monocotyledon transformation vectors. Biotechnol. Lett. 23, 1871–1875 (2001). [Google Scholar]
- Gleave A. P. A versatile binary vector system with a T-DNA organizational-structure conducive to efficient integration of cloned dna into the plant genome. Plant Mol. Biol. 20, 1203–1207 (1992). [DOI] [PubMed] [Google Scholar]
- Nalawade S., Nalawade S., Liu C., Jansson C. & Sun C. Development of an efficient Tissue Culture after Crossing (TCC) system for transgenic improvement of barley as a bioenergy crop. Appl. Energ. 91, 405–411 (2012). [Google Scholar]
- Voinnet O., Rivas S., Mestre P. & Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33, 949–956 (2003). [DOI] [PubMed] [Google Scholar]
- Petrie J. R. et al. Rapid expression of transgenes driven by seed-specific constructs in leaf tissue: DHA production. Plant Methods. 6, 8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhercke T. et al. Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett. 587, 364–369, (2013). [DOI] [PubMed] [Google Scholar]
- Bligh E. G. & Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Liu D. et al. Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PloS One. 7, e46451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.