A phase 3 randomized study of single-dose metronidazole vaginal gel 1.3% versus vehicle gel in participants with bacterial vaginosis demonstrated superior cure rates in the metronidazole treatment group.
Abstract
Background
Bacterial vaginosis (BV), a prevalent infection in women of reproductive age, is associated with increased risk of upper genital tract and sexually transmitted infections, and complications in pregnancy. Currently approved treatments include metronidazole, which requires once or twice daily intravaginal administration for 5 days or twice daily oral administration for 7 days. This phase 3 study determined the safety and efficacy of single-dose metronidazole vaginal gel (MVG) 1.3%.
Methods
In this double-blind, vehicle-controlled study, 651 women with clinical diagnosis of BV were randomized 1:1 to receive MVG 1.3% or vehicle vaginal gel. Primary efficacy measure was clinical cure (normal discharge, negative “whiff test,” and <20% clue cells) at day 21. Secondary measures included therapeutic cure (both clinical and bacteriological; day 21) and bacteriologic cure (Nugent score <4), clinical cure, and time to resolution of symptoms (day 7).
Results
A total of 487 participants were included in the primary analysis. Clinical and therapeutic cure rates (day 21) were higher in participants treated with MVG 1.3% compared with vehicle gel (37.2% vs. 26.6% [P = 0.010] and 16.8% vs. 7.2% [P = 0.001], respectively). Clinical and bacteriologic cure rates (day 7) were also higher in the MVG 1.3% group (46.0% vs. 20.0% [P < 0.001] and 32.7% vs. 6.3% [P < 0.001], respectively). The median time to resolution of symptoms was shorter in the MVG 1.3% (day 6) than vehicle group (not reached). No serious adverse events were reported, and incidence was similar across treatment groups.
Conclusions
Single-dose MVG 1.3% was safe and superior to vehicle gel in producing cure among women with BV.
Bacterial vaginosis (BV) is the most prevalent vaginal infection in women of reproductive age, affecting approximately 29% of women in the general population and 50% of African American women.1 BV is associated with a higher risk of preterm delivery in pregnant women and is a risk factor for acquisition of sexually transmitted diseases including HIV.2,3 Bacterial vaginosis is caused by a reduction in quantity of normally dominant, hydrogen peroxide–producing Lactobacillus species, which contribute to maintaining a healthy vaginal environment, and subsequent overgrowth of harmful, nonhydrogen peroxide–producing strains.2,3 Data indicate that BV pathogens can be transmitted sexually, and women with multiple or new sexual partners are at an increased risk for infection.4
Women with symptomatic BV typically experience abnormal vaginal discharge that is malodorous; however, most women (50%–75%) are asymptomatic.2,5 Clinical diagnosis of BV is generally based on Amsel criteria, which require the presence of 3 of the following: vaginal pH greater than 4.5, homogenous vaginal discharge, fishy odor upon addition of potassium hydroxide (KOH) to vaginal fluid (positive whiff test result), and/or presence of clue cells on saline wet mount (≥20% of total vaginal epithelial cells).6 Gram stain of vaginal discharge is considered the gold standard for BV diagnosis, but the technique requires more resources and expertise than Amsel criteria. The test quantifies the number of lactobacilli relative to BV-associated bacteria and provides a score (Nugent score) from 1 to 10 (normal flora = 1–3, intermediate BV flora = 4–6, definitive BV flora = 7–10).6
Treatment with antibiotics is indicated in women with symptomatic BV and in those with asymptomatic infection who are undergoing abortion or hysterectomy procedures.2 Metronidazole is an antimicrobial agent that has been US Food and Drug Administration (FDA)–approved since 1992 for treatment of BV. Both intravaginal and oral metronidazole formulations are available, and although both have similar efficacy, producing clinical cure rates of 70% to 80% at follow-up times of 4 weeks, intravaginal use is associated with fewer adverse effects.2,7–9 Currently approved metronidazole regimens call for twice daily administration for 7 days (oral), or once or twice daily administration for 5 days (vaginal gel 0.75%).10,11
Results of a recent dose-ranging phase 2 study demonstrated similar efficacy of a 1-day treatment with metronidazole vaginal gel (MVG) 1.3% as compared with the standard 5-day treatment with MVG 0.75%, with no safety differences.12 In the study, clinical cure rates were 37.2% and 28.6% in participants treated with single-dose MVG 1.3% and 5-day MVG 0.75%, respectively. Because a shorter treatment regimen may be favorable to patients, this phase 3 study was initiated to further evaluate the safety and efficacy of single-dose MVG 1.3% versus vehicle vaginal gel for treatment of BV.
MATERIALS AND METHODS
This was a phase 3, multicenter, randomized, double-blind, vehicle-controlled study evaluating the safety and efficacy of single-dose MVG 1.3% compared with single-dose vehicle vaginal gel. Gels were approximately 5 g, formulated to pH 4.0, and contained identical ingredients with the exception of metronidazole in vehicle gels. Study participants were evaluated at 3 time points: an initial screening (D0) to determine study eligibility, follow-up at D7 to ensure compliance with the protocol and assess therapeutic response, and test-of-cure (TOC) visit at D21.
At D0 visit, participants were screened for study entry with medical history, pelvic examination, and laboratory testing. Eligible participants were randomized 1:1 to receive MVG 1.3% or vehicle vaginal gel. Study drug was applied intravaginally by the participant once at bedtime (D0) using a prefilled applicator. At D7 follow-up and D21 TOC visits, pelvic examination and laboratory testing were conducted to determine therapeutic response. Laboratory tests included 10% KOH amine (whiff) test, pH, Gram stain for Nugent scoring, and saline microscopy to determine the presence of clue cells, fungal elements, and Trichomonas vaginalis.
Females at least 18 years of age and in good general health were eligible for study participation. Participants were required to have a negative pregnancy test result and abstain from vaginal intercourse for the first 7 days of the study. Use of intravaginal products was prohibited for the entire duration of the study, and participants had to refrain from ingesting alcohol for 24 hours after treatment application. All participants had a clinical diagnosis of BV, determined by all of the following: (1) off-white, thin, homogenous discharge; (2) proportion of clue cells ≥20% of total epithelial cells; (3) vaginal fluid pH ≥4.7; and (4) positive 10% KOH whiff test result.
Participants with an infectious cause of vulvovaginitis other than BV (e.g., candidiasis, T. vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, or active genital herpes) or who had received systemic or intravaginal antifungal, antibacterial, or antiparasitic drugs; disulfram; or systemic corticosteroids within 14 days of randomization were excluded from the study. Participants receiving concurrent therapy with warfarin, undergoing treatment to cervical intraepithelial neoplasia or cervical carcinoma, or with a primary or secondary immunodeficiency were also excluded.
The primary efficacy measure was the proportion of participants achieving a clinical cure at D21, defined as follows: (1) a return to normal physiological discharge as assessed by the investigator, (2) negative 10% KOH whiff test result, and (3) clue cells less than 20% of total epithelial cells in saline wet mount. Secondary efficacy measures included the proportion of participants achieving a therapeutic cure at D21, defined as participants with both a clinical and bacteriologic cure (the latter defined as a Nugent score <4), the proportion of participants with bacteriologic cure at D21, and the proportion of participants with bacteriologic and clinical cures at D7.
Time to resolution of symptoms at D7 was based on daily diary entries made by the participant through D7. Participants were required to record responses to the following questions: “Did you experience leakage of the study drug?” and “Are your symptoms completely gone today?” Participants also completed a Treatment Satisfaction Questionnaire for Medication at D7, which documented the participants' view of the effectiveness, adverse effects, convenience, and global satisfaction with the treatments.
Data from 3 patient populations were used in the efficacy measures. The modified intent-to-treat (MITT) population was defined at the initiation of the study (April 2012) and included all participants who were negative for N. gonorrhoeae and C. trachomatis and had a Nugent score of 4 or higher at first visit, and whose D7 and D21 vaginal discharge was assessed as “present” or “absent.” Because some investigators interpreted present to describe either an abnormal or normal discharge, the study protocol was amended in July 2012 to change the assessment to “Has the original discharge characteristic of BV returned to a normal physiological discharge?” Under the new amendment, a new patient population was defined as a primary modified intent-to-treat (PMITT) population, which included the subset of the MITT population that was evaluated using the amended question. Data from the PMITT population were used for the primary analysis, and data from the MITT population were used for sensitivity testing of the primary and secondary outcomes.
The per-protocol (PP) population included participants from the PMITT population who satisfied all inclusion and exclusion criteria, began study medication within 2 days of randomization, were compliant with study medication, used no antimicrobial drugs or intravaginal products during the study, and had the Nugent score result obtained between D21 and D30. Data were analyzed using this population because there was a 10% difference in the number of participants between the PMITT and PP populations and were used to support results of the primary efficacy measure.
For D7 assessments, only those participants evaluated using the postamendment question “Has the original discharge characteristic of BV returned to a normal physiological discharge?” were included in the analyses (considered the “D7 analysis population”). Fewer participants were included in this analysis than those in the D21 analysis (PMITT population), as the new criterion was applied after the study had already been initiated.
All statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC), and significance was determined using a 2-sided type I error rate of 5% (α = 0.05). For proportion variables, data were summarized by treatment group along with 95% exact confidence intervals (CIs). Two-sided 95% CIs were estimated for cure rate differences based on binomial distribution. Missing cure values were imputed as failures. For time to resolution of symptoms, median, 95% CI, and log-rank P value were calculated using Kaplan-Meier methods.
RESULTS
In this phase 3 double-blind, vehicle-controlled study, a total of 651 participants were randomized to receive MVG 1.3% or vehicle vaginal gel (Fig. 1). Discontinuations were reported for 70 participants, primarily due to participant request (not related to an adverse event [AE], 19 participants), loss to follow-up (19 participants), or exclusionary posttreatment laboratory results (positive for N. gonorrhoeae and C. trachomatis, 18 participants).
Figure 1.
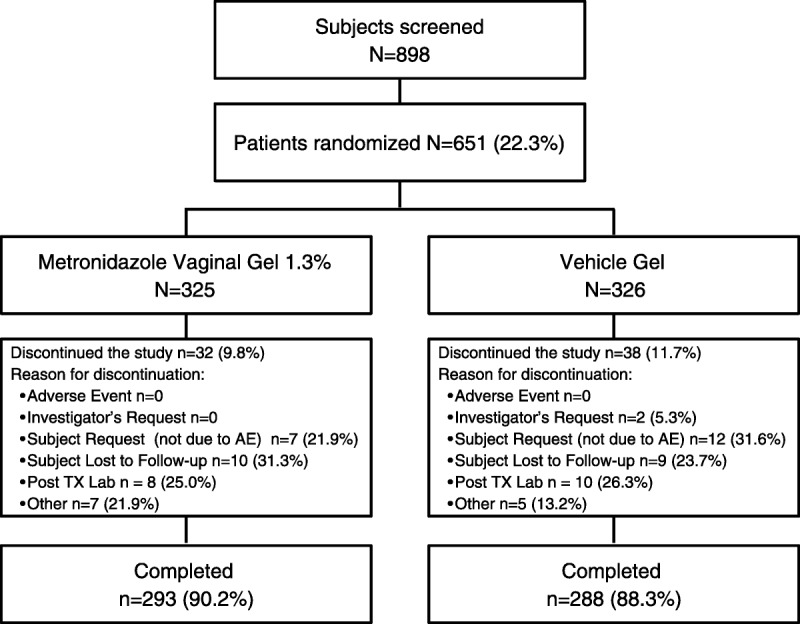
Patient disposition. Post TX Lab, postrandomization positive N. gonorrhoeae and C. trachomatis test results.
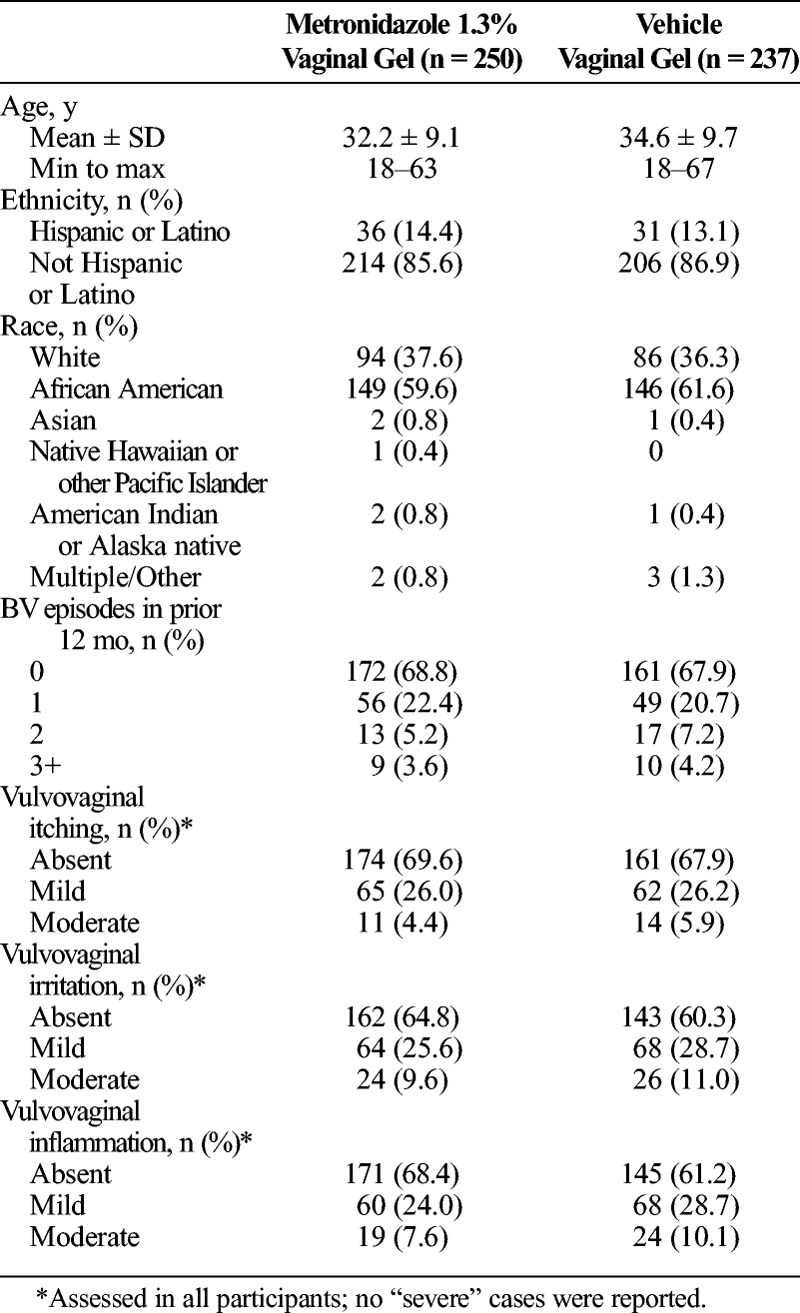
The primary analysis (PMITT) population included 487 participants, 250 randomized to receive MVG 1.3% and 237 randomized to receive vehicle gel, with 164 participants excluded because they were not evaluated at D21 for vaginal discharge using the criterion “Has the original BV discharge returned to a normal physiologic discharge?” (Table 1). The PP population, consisting of 418 participants from the PMITT population (214 receiving MVG 1.3%, 204 receiving vehicle gel), was used in the primary end point analysis. The additional 69 participants excluded from the PP population did not apply study medication within 2 days of randomization, used prohibited products, or did not have a TOC Nugent score result between D21 and D30. The MITT population consisted of 577 participants, 292 in the MVG 1.3% group and 285 in the vehicle group, and was used in ad hoc analyses to support the primary and secondary efficacy assessments. As shown in Table 2, baseline demographics and patient characteristics were similar between the treatment groups in the PMITT population.
TABLE 1.
Summary of Reasons Leading to Exclusion From Analysis Population
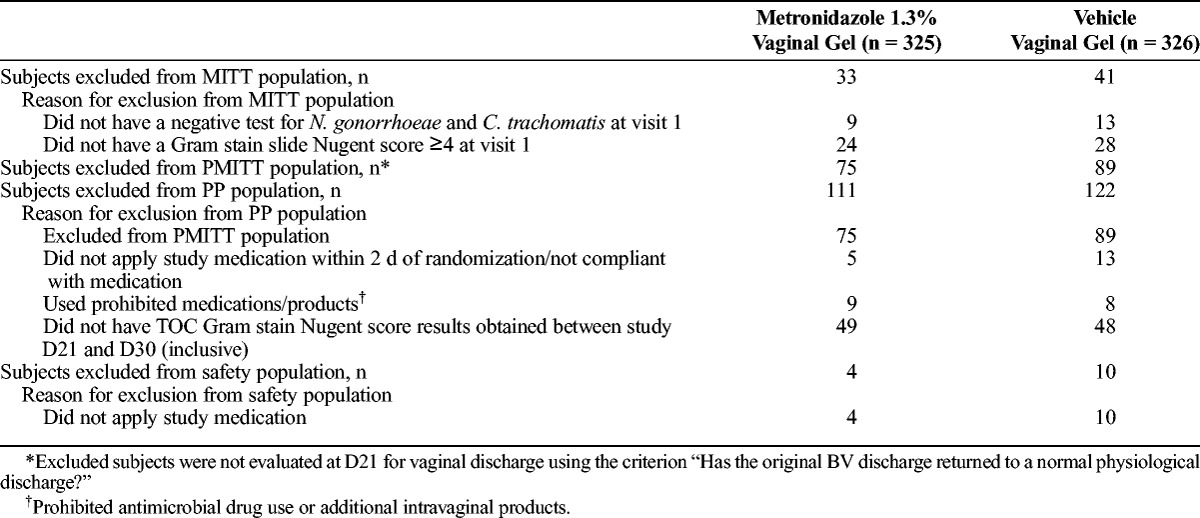
TABLE 2.
Baseline Demographics and Characteristics by Treatment in the PMITT Population
Evaluation of the primary efficacy measure indicated that a significantly higher proportion of participants receiving MVG 1.3% demonstrated clinical cure at D21 as compared with participants receiving vehicle vaginal gel (37.2% vs. 26.6%, respectively; P = 0.010; Table 3). Analyses in the PP and MITT populations were consistent with results obtained in the PMITT population. In the PP population, 40.7% of participants in the MVG 1.3% group demonstrated clinical cure (vs. 29.9% in the vehicle group; P = 0.007), and in the MITT population, 37.0% of participants in the MVG 1.3% group demonstrated clinical cure (vs. 26.7% in the vehicle group; P = 0.007).
TABLE 3.
Cure Rates at the TOC (D21) Visit in the PMITT Population
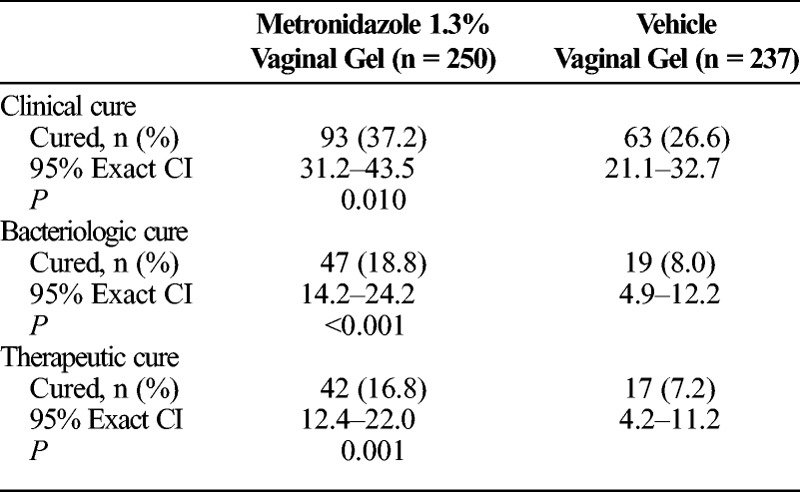
Bacteriologic and therapeutic cure rates at D21 were also significantly higher among participants treated with MVG 1.3% (18.8% and 16.8%, respectively) than with vehicle vaginal gel (8% and 7.2%, respectively; Table 3). Ad hoc analyses in the MITT population further confirmed the responses observed in the PMITT population, as 19.5% vs. 7.7% (P < 0.001) of participants receiving MVG 1.3% vs. vehicle gel achieved bacteriologic cure and 16.8% vs. 6.3% (P < 0.001) of participants receiving MVG 1.3% vs. vehicle gel achieved therapeutic cure.
At the D7 time point, 46% of participants treated with MVG 1.3% demonstrated clinical cure (vs. 20% of vehicle-treated participants; P < 0.001) and 32.7% achieved bacteriologic cure (vs. 6.3% of vehicle-treated participants; P < 0.001). Time to resolution of symptoms, assessed based on daily diary responses recorded by the participants through D7 of treatment, was significantly lower in participants treated with MVG 1.3% versus those treated with vehicle gel. In the MVG 1.3% group, median time to resolution was 6 days, whereas the median could not be calculated in the vehicle gel group, as less than half of the participants had resolution of symptoms by D7 (MITT population: 95% CI, 4.0–7.0; P < 0.001).
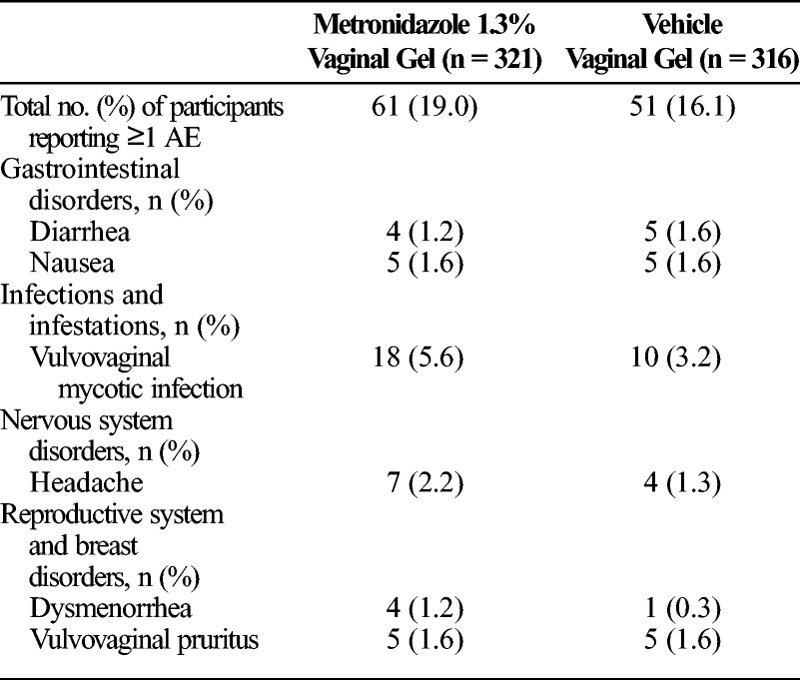
Safety assessments were performed in 637 participants, 321 of whom were treated with MVG 1.3% and 316 of whom were treated with vehicle gel. The analysis excluded 14 participants who did not apply any study medication. Overall, the incidence of AEs was similar between the treatment groups, with 19% of participants in the MVG 1.3% group and 16.1% of participants in the vehicle gel group reporting AEs (Table 4). The most commonly reported AEs in the MVG 1.3% treatment group were vulvovaginal mycotic infection (5.6%) and headache (2.2%). In participants treated with vehicle gel, vulvovaginal mycotic infection (3.2%), nausea (1.6%), diarrhea (1.6%), and vulvovaginal pruritus (1.6%) were the most frequently reported AEs. No serious AEs occurred in the study, and no participants discontinued the study due to an AE.
TABLE 4.
AEs Reported in ≥1% of Participants (Safety Population)
DISCUSSION
Bacterial vaginosis is a common condition affecting millions of women annually and is characterized by an altered vaginal flora, with loss of protective Lactobacillus bacterial species and acquisition of pathogenic BV-associated species.13,14 Women with BV are at an increased risk for acquiring sexually transmitted infections, including HIV, herpes simplex virus type 2, gonorrhea, chlamydia, and trichomoniasis, and may be predisposed to development of precancerous lesions.2 Current treatment with intravaginal preparations such as MVG 0.75% can produce high cure rates, but requires once or twice daily use for 5 or 7 days.10,11 A new hydrogel formulation consisting of a higher concentration of metronidazole (1.3%) was developed to provide a convenient, single-dose regimen that would have similar efficacy as the multiple day regimens.
In the current phase 3 trial, a total of 651 participants were randomized to receive MVG 1.3% or vehicle vaginal gel. Comparable demographics and baseline disease characteristics were observed between the groups. The study met its primary end point, demonstrating superior efficacy of MVG 1.3% over vehicle gel (P = 0.010) at D21 TOC visit in the PMITT patient population, with results confirmed in the MITT and PP populations. The study also met its secondary end points, with MVG 1.3% proving to be statistically significantly more effective than vehicle gel in producing bacteriologic (P < 0.001) and therapeutic (P = 0.001) cures at D21. Furthermore, responses were more rapid in participants using single-dose MVG 1.3%, as a significantly higher proportion of these participants achieved clinical and bacteriologic cures at D7 (P < 0.001), and median time to resolution of symptoms was D6.
The MVG 1.3% treatment regimen was well tolerated, with most AEs consistent with those previously reported for approved metronidazole regimens. The AEs reported in at least 1% of participants receiving MVG 1.3% included vulvovaginal mycotic infection (5.6%), headache (2.2%), vulvovaginal pruritus (1.6%), nausea (1.6%), dysmenorrhea (1.2%), and diarrhea (1.2%). The incidence of AEs was similar between the treatment groups, and no serious AEs were reported.
Although the cure rates in the current study were lower than those reported in previous studies of metronidazole, the criteria used to determine cure vary widely across studies. For example, studies of MVG 0.75% administered twice daily for 5 days versus placebo reported clinical cure rates ranging from 78% to 91% in the MVG 0.75% group at follow-up times of 4 to 16 days and 28 to 32 days (vs. 17%–50% in the placebo group).15,16 In Hillier et al.,15 cure was defined as 20% or less clue cells plus absence of at least 2 additional Amsel signs, whereas Livengood et al.16 defined cure as less than 20% clue cells plus absence of at least 1 additional Amsel sign. In addition to differences in application of Amsel criteria, microbiologic cure based on Nugent scoring has been variably defined, as either resolution of BV (e.g., Nugent score <7) or return to normal vaginal microflora (e.g., Nugent score <4).12,17,18 The criteria for cure are further impacted by differences in time points of assessment, which ranged from 1 to 5 weeks across studies.17,19,20 Adopting more standardized methods for establishing cure may help in identifying agents that can produce more sustained cure rates in women with BV.21
The initial design of this phase 3 study was based on an FDA guidance document drafted in 1998, which defined clinical cure as the absence of all four Amsel criteria, and therapeutic cure as clinical cure plus a Nugent score lower than 4, at D21-30 after the initiation of treatment.22 Based on subsequent review of the phase 3 study design by the FDA under a special protocol assessment in 2012, the definition of clinical cure was modified to exclude Amsel's pH criteria. A recent phase 2 study investigating efficacy of single, 3-, and 5-day doses of MVG 1.3% as compared with the standard 5-day regimen with MVG 0.75% used the more stringent FDA guidance to assess clinical cure.12 In the phase 2 study, clinical cure rates at the TOC visit (D21–30) in participants receiving single-dose MVG 1.3% were 30.5% and 37.2% in the ITT and PP populations, respectively.12 Despite the exlusion of pH in the criteria for clinical cure in the current phase 3 study, clinical cure rates on D21 in the MVG 1.3% group were similar to those found in the phase 2 study (37.2% and 40.7% in the PMITT and PP populations, respectively).
The phase 2 study was a multicenter, randomized, investigator-blinded trial designed to test the higher concentration metronidazole (1.3%) as a single treatment versus the 5-day MVG 0.75% regimen.12 The results indicated similar efficacy between the groups at the D21–30 assessment, with clinical and therapeutic cure rates of 37.2% and 30.2%, respectively, in the 1-day MVG 1.3% group and 28.6% and 20.4%, respectively, in the 5-day MVG 0.75% group.12 In addition, participants indicated the highest acceptability for the single-dose application based on ease of application, convenience, and treatment satisfaction.
The convenience of a single-dose use was also evident based on treatment satisfaction questionnaires administered to participants in the phase 3 trial, with responses equivalent between the treatment groups. However, responses regarding the effectiveness of and global satisfaction with treatment were significantly higher in the MVG 1.3% treatment group compared with the vehicle gel group. Together with the phase 2 trial, the current well-controlled phase 3 study provides additional confirmation that single-dose MVG 1.3% is safe and more effective for treatment of BV than vehicle gel, with all primary and secondary end points met, and safety similar to placebo. Overall, cure rates in both study arms were lower than have previously been observed with standard of care treatment. Nevertheless, MVG 1.3% may be an option for those patients who do not tolerate oral metronidazole or tinidazole. Further supportive studies comparing 1-day MVG 1.3% with active product are required to ensure that a reasonable treatment alternative is available for women desiring single-dose therapy.
Footnotes
Conflicts of interest: Jane Schwebke is or has been a compensated consultant to Hologic (ongoing [O]), Akesis (O), Symbionix (O), StarPharma (O), and BD Diagnostics (past); has grants/grants pending paid to her institution from Hologic (O), Akesis (current [C]), Symbionix (C), StarPharma (C), BD Diagnostics (O), Biohelix (C), and Cepheid (O); and has received payment from Hologic (C) for development of educational presentations. Jeanne Marrazzo has received a grant paid to her institution from Watson/Actavis plc (past). Andrew Beelen was an employee of Activis plc at the time the manuscript was originally drafted and owns stock/stock options in Actavis plc. Jack Sobel has no conflicts of interest to declare.
Funding support: This study was sponsored by Medicis Pharmaceutical Corporation, a division of Valeant Pharmaceuticals. Writing and editorial assistance were provided by Scientific Connexions, funded by Actavis Laboratories UT (clinical research group is Actavis Laboratories UT).
REFERENCES
- 1. Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109: 114– 120. [DOI] [PubMed] [Google Scholar]
- 2. Sobel JD. Bacterial vaginosis. UpToDate® Web site. July 29, 2014. Available at: http://www.uptodate.com/contents/bacterialvaginosis?source=search_result&search=bacterial+vaginosis+sobel&selectedTitle=1%7E72. Accessed August 27, 2014.
- 3. Marrazzo JM. Interpreting the epidemiology and natural history of bacterial vaginosis: Are we still confused? Anaerobe 2011; 17: 186– 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: A systematic review and meta-analysis. Clin Infect Dis 2008; 47: 1426– 1435. [DOI] [PubMed] [Google Scholar]
- 5. Klebanoff MA, Schwebke JR, Zhang J, et al. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol 2004; 104: 267– 272. [DOI] [PubMed] [Google Scholar]
- 6. Sha BE, Chen HY, Wang QJ, et al. Utility of Amsel criteria, Nugent score and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus–infected women. J Clin Microbiol 2005; 43: 4607– 4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joesoef MR, Schmid GP, Hillier SL. Bacterial vaginosis: Review of treatment options and potential clinical indications for therapy. Clin Infect Dis 1999; 28(suppl 1): S57– S65. [DOI] [PubMed] [Google Scholar]
- 8. Hanson JM, McGregor JA, Hillier SL, et al. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. J Reprod Med 2000; 45: 889– 896. [PubMed] [Google Scholar]
- 9. Brandt M, Abels C, May T, et al. Intravaginally applied metronidazole is as effective as orally applied in the treatment of bacterial vaginosis, but exhibits significantly less side effects. Eur J Obstet Gynecol Reprod Biol 2008; 141: 158– 162. [DOI] [PubMed] [Google Scholar]
- 10.MetroGel [package insert]. Scottsdale, AZ: Medicis Pharmaceuticals. Revised December 2011. [Google Scholar]
- 11. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2014 (DRAFT). Available at: http://www.cdc.gov/std/treatment/2014/2014-std-guidelines-peer-reviewers-08-20-2014.pdf. Accessed August 20, 2014. [DOI] [PubMed]
- 12. Chavoustie SE, Jacobs M, Reisman HA, et al. Metronidazole vaginal gel 1.3% in the treatment of bacterial vaginosis: A dose-ranging study. J Low Genit Tract Dis 2015; 19: 129– 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005; 353: 1899– 1911. [DOI] [PubMed] [Google Scholar]
- 14. Fredricks DN, Fiedler TL, Thomas KK, et al. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol 2007; 45: 3270– 3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hillier SL, Lipinski C, Briselden AM, et al. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol 1993; 81: 963– 967. [PubMed] [Google Scholar]
- 16. Livengood CH, 3rd, McGregor JA, Soper DE, et al. Bacterial vaginosis: Efficacy and safety of intravaginal metronidazole treatment. Am J Obstet Gynecol 1994; 170: 759– 764. [DOI] [PubMed] [Google Scholar]
- 17. Verstraelen H, Verhelst R. Bacterial vaginosis: An update on diagnosis and treatment. Expert Rev Anti Infect Ther 2009; 7: 1109– 1124. [DOI] [PubMed] [Google Scholar]
- 18. Leite SR, Amorim MM, Sereno PF, et al. Randomized clinical trial comparing the efficacy of the vaginal use of metronidazole with a Brazilian pepper tree (Schinus) extract for the treatment of bacterial vaginosis. Braz J Med Biol Res 2011; 44: 245– 252. [DOI] [PubMed] [Google Scholar]
- 19. Ferris DG, Litaker MS, Woodward L, et al. Treatment of bacterial vaginosis: A comparison of oral metronidazole, metronidazole vaginal gel, and clindamycin vaginal cream. J Fam Pract 1995; 41: 443– 449. [PubMed] [Google Scholar]
- 20. Soper DE, Livengood C, Sheehan KL, et al. Safety and efficacy comparison of two dosing regimens of MetroGel-Vaginal(R) in the treatment of bacterial vaginosis: A multicenter, randomized, single-blind, parallel comparison. Prim Care Update Ob Gyns 1998; 5: 150. [DOI] [PubMed] [Google Scholar]
- 21. Marrazzo JM, Martin DH, Watts DH, et al. Bacterial vaginosis: Identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID November 19–20, 2008. Sex Transm Dis 2010; 37: 732– 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidance for industry: Bacterial vaginosis – developing antimicrobial drugs for treatment. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; July 1998. [Google Scholar]


