Abstract
Aneurysmal subarachnoid hemorrhage (SAH) is a worldwide health burden with high fatality and permanent disability rates. The overall prognosis depends on the volume of the initial bleed, rebleeding, and degree of delayed cerebral ischemia (DCI). Cardiac manifestations and neurogenic pulmonary edema indicate the severity of SAH. The International Subarachnoid Aneurysm Trial (ISAT) reported a favorable neurological outcome with the endovascular coiling procedure compared with surgical clipping at the end of 1 year. The ISAT trial recruits were primarily neurologically good grade patients with smaller anterior circulation aneurysms, and therefore the results cannot be reliably extrapolated to larger aneurysms, posterior circulation aneurysms, patients presenting with complex aneurysm morphology, and poor neurological grades. The role of hypothermia is not proven to be neuroprotective according to a large randomized controlled trial, Intraoperative Hypothermia for Aneurysms Surgery Trial (IHAST II), which recruited patients with good neurological grades. Patients in this trial were subjected to slow cooling and inadequate cooling time and were rewarmed rapidly. This methodology would have reduced the beneficial effects of hypothermia. Adenosine is found to be beneficial for transient induced hypotension in 2 retrospective analyses, without increasing the risk for cardiac and neurological morbidity. The neurological benefit of pharmacological neuroprotection and neuromonitoring is not proven in patients undergoing clipping of aneurysms. DCI is an important cause of morbidity and mortality following SAH, and the pathophysiology is likely multifactorial and not yet understood. At present, oral nimodipine has an established role in the management of DCI, along with maintenance of euvolemia and induced hypertension. Following SAH, hypernatremia, although less common than hyponatremia, is a predictor of poor neurological outcome.
Key Words: subarachnoid hemorrhage, intracranial aneurysm, delayed cerebral ischemia
SUBARACHNOID HEMORRHAGE (SAH) IS A WORLDWIDE HEALTH BURDEN WITH HIGH RATES OF FATALITY AND PERMANENT DISABILITY
The estimated worldwide incidence of SAH is 9/100,000 persons/y with regional variation.1 One systematic review found the incidence to be lower in South and Central America (4.2/100,000 persons/y) and higher in Japan (22.7/100,000 persons/y) and Finland (19.7/100,000 persons/y).1 Interestingly, the prevalence of intracranial aneurysm is not found to be higher in Japan or Finland,2 but the risk for rupture is higher.3 The incidence is also reported lower in China (2.0 //100,000 persons/y).4 SAH accounts for only 5% of all strokes,5 but it has high mortality and permanent disability rates. A retrospective cohort study in 2 large Norwegian populations between 1984 and 2007 reported a 30-day case fatality rate of 36%.6 A nationwide Danish study reported a similar 30-day mortality at 38%.7 World Health Organization Multinational Monitoring Trends and Determinants in Cardiovascular Disease (WHO MONICA stroke study), a large observational study on 11 populations, in Europe and China, reported a 30-day case fatality rate of 42%.4 A review of data from the Swedish hospital discharge and cause of death registry from 1987 to 2002 on 18,443 patients with SAH showed a 28-day case fatality rate of 31.7%.8 Another study from Australia and New Zealand placed the 28-day case fatality rate at 39%.9 The risk for permanent disability is high among survivors, and the dependency rate is approximately 50%.10–12
The incidence of new cases of SAH in the United States is about 30,000/y or 10/100,000 persons/y.13,14 Ruptured cerebral aneurysms account for 75% to 85% of SAH for nontraumatic SAH.15,16 One systematic review of prospective studies estimated prevalence at 3.6% to 6.0% on the basis of autopsy and angiographic studies.17 Most of the aneurysms were small (<1 cm) with an annual risk for rupture of 0.7%.17 A recent systematic review placed the prevalence rate at 3.2%.2
RISK FACTORS FOR DEVELOPMENT AND RUPTURE OF INTRACRANIAL ANEURYSMS
Risk Factors for Development of Aneurysms
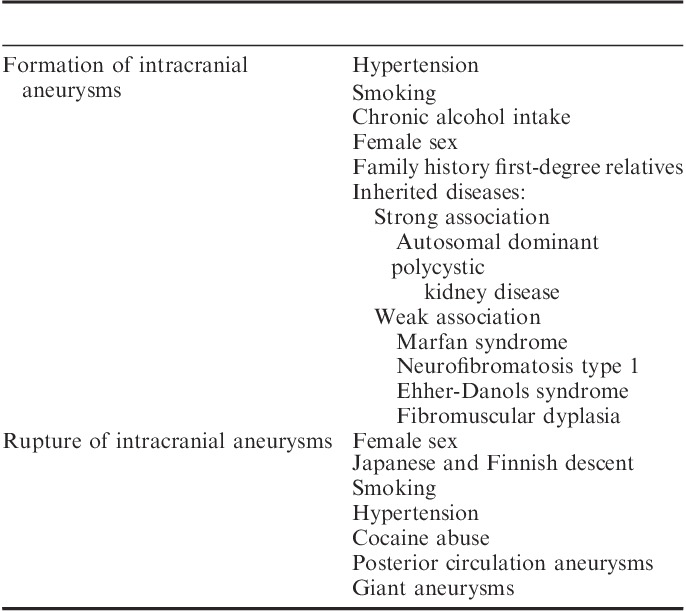
The important risk factors for the development of cerebral aneurysms are hypertension, smoking, chronic alcohol use,18–20 family history of intracranial aneurysms in first-degree relatives2,21–23 and female sex.1,2,5,8,9,12,23,24 In the United States, the incidence of aneurysmal subarachnoid hemorrhage (aSAH) is higher in the African American and Hispanic population compared with whites.23–25 In New Zealand, the incidence of aSAH is found to be higher in native Maroi and Pacific Islanders than in the white population.9 Autosomal dominant polycystic kidney disease is an inherited systemic disorder that is strongly associated with intracranial aneurysms. Autosomal dominant polycystic kidney disease has a prevalence rate 2 to 4 times higher than the general population.2,14,17,26,27 Other conditions such as Marfan syndrome, Ehlers-Danlos syndrome type IV, neurofibromatosis type 1, and fibromuscular dysplasia are weakly associated with intracranial aneurysms (Table 1).14,22,27–29
TABLE 1.
Risk Factors for the Formation of and Rupture of Intracranial Aneurysms
Risk Factors for Rupture of Intracranial Aneurysms
Both the location and type of aneurysm are important considerations in describing the risk for rupture. Most aneurysms occur in the anterior circulation of Circle of Willis, whereas aneurysms of the posterior circulation of the vertebral and basilar systems account for only 12% of intracranial aneurysms, according to the prospective component of the International Study of Unruptured Intracranial Aneurysms (ISUIA) involving 4060 patients with unruptured intracranial aneurysms.30 About 35% of the patients had >1 aneurysm in this study. Saccular berry aneurysms account for 90% of the total aneurysm morphology and their rupture is the most common cause of SAH. Fusiform aneurysms account for the remaining 10%; their most common location is posterior circulation.31 Atherosclerosis and dissection are suggested as possible mechanisms for formation of fusiform aneurysms.32 The risk factors for aneurysm rupture are female sex,3,14,22 Japanese or Finnish descent,3 size and location of aneurysms,3,20,30,33 hypertension,33–35 smoking,3,23 older patients,3,33,36 and cocaine abuse.23,37
The retrospective component of ISUIA identified the risk for rupture to be 0.05% a year for aneurysms <1 cm, whereas aneurysms >1 cm and posterior circulation aneurysms carried a higher risk for rupture.20 The prospective arm of the ISUIA trial revealed a 5-year cumulative risk for rupture of 0% for aneurysms <7 mm in anterior circulation and 2.5% for posterior circulation aneurysms of similar size.30 The risk for rupture is higher for giant aneurysms with a size >2.5 cm. Giant aneurysms in the anterior circulation have a 40% risk, whereas those in the posterior circulation carry a 50% risk for rupture.30 In 2007, a meta-analysis also reported a higher incidence of rupture for posterior circulation aneurysms compared with the anterior circulation of Circle of Willis.3 The posterior circulation aneurysms most likely to rupture are those located at the basilar tip, the posterior cerebral artery, the vertebrobasilar distribution, and the origin of the posterior communicating artery.3,38 However, a recent large Japanese cohort study showed similar incidence of rupture for anterior and posterior circulations. In addition, the authors reported a higher incidence of rupture for anterior communicating artery and posterior communicating artery aneurysms. This study found an overall annual rate of rupture of 0.95%39 (Fig. 1).
FIGURE 1.
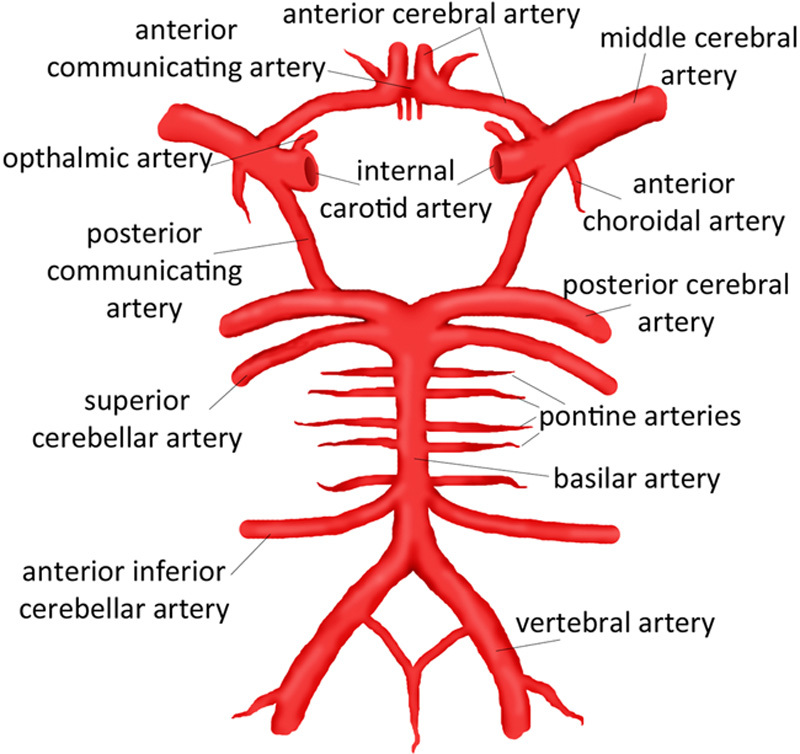
Circle of Willis. Source: Chiu et al.286 Reprinted with permission from Children’s Hospital of Wisconsin. https://www.chw.org/display/PPF/DocID/48513/Nav/1/router.asp.
CLINICAL PRESENTATION, ASSESSMENT OF SEVERITY, AND PREOPERATIVE PREPARATION
Clinical Presentation
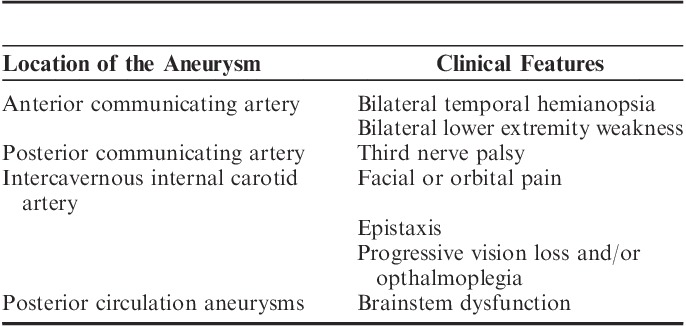
Most aneurysms remain undetected during one’s lifetime or until rupture. An aneurysm is often an incidental finding during investigations for other causes of intracranial pathology.40 Aneurysms may present in several ways: headache,23,41 bilateral temporal hemianopsia and bilateral lower extremity weakness (anterior communicating artery aneurysm),23,41,42 unilateral third nerve palsy (posterior communicating artery aneurysm),41 facial or orbital pain, epistaxis, progressive vision loss and/or opthalmoplegia (intercavernous internal carotid artery),43 and symptoms of brainstem dysfunction (posterior circulation aneurysms).14 The most common presenting feature of an aneurysm is SAH. SAH most commonly presents as a severe headache most often described by the patient as “This is the worst headache of my life.”44,45 An SAH headache is most often associated with nausea, vomiting, neck rigidity, and photophobia.23 As many as 30% to 40% of the patients may present with a sentinel headache; a warning headache occurring a few weeks before the major bleed possibly due to a warning leak.46–49 Depending on the severity of SAH, the patient may present with drowsiness, confusion, focal neurological deficits, hemiparesis, and even coma (Table 2).
TABLE 2.
Clinical Features of Intracranial Aneurysms Based on Location
Assessment of Severity
The severity of SAH is clinically assessed and graded using either the Hunt and Hess classification or the World Federation of Neurosurgeons Scale (WFNS). The prognostic advantage of one scale over the other is uncertain, these scales have limitations due to intraobserver and interobserver variability.50 The WFNS scale, widely used, is composed of focal neurological deficits and the Glasgow Coma Scale. Higher grades on both scales are associated with the worst outcomes. Neurological status should also be assessed using the Glasgow Coma Scale, which has a prognostic value and less observer variability.50 Mortality is commonly caused by neurological injury resulting from the initial bleeding and rebleeding and from delayed cerebral ischemia (DCI). Mortality is a function of the volume of initial hemorrhage and initial neurological status following SAH.51,52 Elderly patients and patients with coexisting medical conditions are at higher risk for mortality.51 The clinical goal is to prevent rebleeding and DCI.
Noncontrast head computed tomography (CT) is the initial imaging modality of choice.53,54 The presence of SAH requires further evaluation with CT angiography (CTA) or cerebral angiography.54 Noncontrast CT followed by CTA provides diagnosis in aSAH in 99% of the cases.55,56 In the presence of a normal noncontrast CT scan, lumbar puncture remains necessary to avoid potential misdiagnosis.13,54,57 The Fisher scale is used to classify the appearance of SAH on a CT scan. This scale is based on the amount of blood in the subarachnoid space on a cranial CT scan and is a predictor of cerebral vasospasm, DCI, and possibly the overall patient outcome.58–60
Cardiac Manifestations
Patients following subarachnoid bleed are likely to show a wide variety of electrocardiographic abnormalities including ST and T wave changes, suggestive of myocardial ischemia and QT prolongation and U waves. In addition, SAH may present with supraventricular and ventricular arrhythmias, elevated troponin levels, and myocardial dysfunction without coronary vasospasm.61–63 The exact mechanism is not known. The likely cause is sympathetic activation along with parasympathetic dysfunction resulting in inflammation of cardiac myocytes.63 The degree of troponin elevation is associated with an increased risk for cardiovascular complications and vasospasm-induced DCI and poor neurological outcome.61,62 An exploratory analysis of 413 patients from the CONSCIOUS-1 (Clazosentan to Overcome Neurological iSChemia and Infarct OccUring after Subarachnoid Hemorrhage) database showed an association between QT prolongation, tachycardia, and development of angiographic vasospasm.64 Thus these cardiac changes primarily reflect the severity of neurological injury, but are reversible in the majority of cases and are likely to resolve.65,66 However, patients with severe myocardial dysfunction may need inotropic support and rarely temporary intra-aortic balloon counterpulsation.
Neurogenic Pulmonary Edema (NPE)
NPE is thought to be due to a massive sympathetic discharge resulting from neurological injury and is associated with reduced global and segmental left ventricular systolic function.67 NPE reflects the severity of the subarachnoid bleed and is associated with poor outcome.68,69
Hypertension
Elevated blood pressure (BP) following aSAH is associated with higher mortality.70,71 However, aggressive management of hypertension following aSAH is always a matter of concern, given that a higher BP is required to maintain cerebral perfusion pressure (CPP), in the presence of elevated intracranial pressure (ICP).15 Sympathetic activation following SAH is an important cause of hypertension. Elevated ICP is not the solitary cause. Sustained elevation of BP increases the risk for aneurysmal rupture and rebleeding. A recent large randomized controlled trial (RCT), INTEnsive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-2), with 2794 participants reported that systolic BP reduction to <140 mm Hg within an hour of intracerebral hemorrhage compared with <180 mm Hg systolic had similar mortality and severe disability rate at the end of 90 days.72 The IntraCerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial (ICH ADAPT) involving 75 patients did not show reduction in perfusion in the vulnerable penumbra around the hematoma in patients in whom the BP was lowered to 150 mm Hg systolic compared with 180 mm Hg systolic in the control group.73 The perfusion was measured within 2 hours of postrandomization using CT perfusion imaging. The results of these trials can be applied to patients with aSAH. On the basis of these results it can be concluded that BP can be reduced safely to 140 mm Hg systolic to decrease the risk for rebleeding without increasing the risk for ischemic complications. Another phase III trial, Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II, is currently underway.74 This trial compares aggressive BP reduction with intravenous (IV) nicardipine to systolic 140 mm Hg with the control group in which BP reduction is limited to systolic 180 mm Hg. The outcomes compared are death and disability rates at the end of 90 days. The BP reduction is done within 3 hours of randomization. This trial will provide further clarification to the issue of aggressive BP management.
Labetalol, esmolol, and nicardipine are commonly used agents to reduce hypertension in patients with aSAH.23,70 Vasodilators such as nitroglycerine, hydralazine, and sodium nitroprusside should be avoided because vasodilatation may increase cerebral blood flow and is likely to worsen ICP.70 Nicardipine provides better BP control compared with labetalol in patients with aSAH.75 However, one should note that vasodilators were used to reduce the BP in the INTERACT-2 trial without worsening neurological outcome.
Hyperglycemia
Patients with SAH who develop hyperglycemia are at increased risk for poor clinical outcome.76–78 Hyperglycemia was found to be associated with increased length of ICU stay, and increased risk of death and disability in one retrospective cohort study.78 Analysis of data from 1000 patients in the Intraoperative Hypothermia for Aneurysms Surgery Trial (IHAST II) revealed that patients with SAH, secondary to ruptured aneurysm, who underwent clipping, and whose blood sugar levels were >129 mg/dL, had long-term cognitive dysfunction. Patients with blood sugar levels >152 mg/dL had deficits in gross neurological function.79 Glycemic control with aggressive hyperglycemia management is associated with improved outcome, but further studies are required to confirm these results.80 Hyperglycemia should be treated in the perioperative period; however, there are insufficient data available to make a recommendation on target blood sugar levels.
EARLY VERSUS LATE INTERVENTION?
Early therapy with either surgical clipping or neuroradiologic intervention with endovascular coiling will prevent rebleeding and will enable safe and effective management of vasospasm. However, early surgery on an edematous brain makes the surgical task difficult. In the past, early intervention was advocated for better neurological grades, now surgical intervention is done for higher grades as well. An 11-year database of 230 consecutive ruptured aneurysms from an Australian center found that either early surgical clipping or endovascular coiling within 24 hours was associated with improved clinical outcome.81 Another meta-analysis also found that early intervention within 72 hours is associated with improved clinical outcome and that the degree of improvement is a function of the clinical condition on admission.82 An observational study from the Netherlands involving 8 centers and 1500 patients did not show a difference in outcome with either early or late intervention to secure the aneurysm, in good grade patients.83 However, patients with poor neurological grades at admission fared better with early intervention. An analysis of data from a Nationwide Inpatient Sample on 32,048 US patients with aSAH from 2005 to 2008 revealed that patients treated early (within 48 h of hospital admission) with coiling or surgery are more likely to be discharged with little or no disability.84
Whenever a delay is anticipated in definitive treatment, a short course (<72 h) of antifibrinolytic agents such as tranexemic acid and aminocaprionic acid prevents rebleeding without increasing the risk for DCI.23,70,71,85–90 However, delayed and prolonged therapy with antifibrinolytic agents is not recommended, as it may increase the risk for thrombotic events and DCI.23 The role of antifibrinolytic agents in terms of overall patient outcome is yet to be demonstrated in RCTs.85,87,88,90
CLIP VERSUS COIL?
Ruptured Aneurysms
The International Subarachnoid Aneurysm Trial (ISAT),91,92 a prospective international multicenter RCT involving 42 centers, located mainly in the United Kingdom (77% of the recruits) and Europe, used death or dependency at 1 year as the primary outcome measure. In this trial 9559 patients with SAH were screened and 2143 patients were included; 1070 patients were assigned to the surgical clipping group and 1073 were included in the coiling group. Patients with good neurological grades (WFNS I-III) were included in this study. The ISAT results reported in 2002 showed that the primary outcome measure (death or dependency rate) was better with the coiling group (23.5%) compared with the surgical clipping group (30.9%). However, the rate of rebleeding at the end of 1 year was higher in the coiling group (2.6%) compared with the surgical clipping group (1%). A 5-year follow-up of results from the ISAT trial in 2009 also showed a reduced death rate in the coiling group compared with the clipping group (11% vs. 14%).93 In addition, the percentage of survivors who were independent did not differ between the groups. Thus at the end of 5 years the advantage of coiling in death or dependency rate decreased from 7.4% to 3%. This 5-year follow-up also confirmed an increased risk for recurrent bleeding in the coiling group compared with the surgical clipping group. The risk for seizure at the end of the first and fifth year was lower in the endovascular coiling group compared with the neurosurgical clipping group.91,93 The difference in outcome for patients younger than 40 years of age in the ISAT trial is similar for both the coiling and the clipping group.94 Surgical clipping provides better protection from recurrent SAH in younger patients. Inadequate primary occlusion of an aneurysm is the primary cause of recurrent bleeding in the coiling group.95 Intramural thrombus and wide neck increases the risk for inadequate occlusion during coiling. Dissolution of thrombus and subsequent aneurysm growth leads to late reopening and increases the risk for potential future rupture.96
Before a definitive conclusion of the superiority of endovascular coiling over surgical clipping, ISAT design limitations should be considered.93,97,98 Only 22.4% of the total patients screened were recruited in the trial (2143 of 9559). Ninety-seven percent of the recruits had anterior circulation aneurysms and only 3% had posterior circulation aneurysms. The middle cerebral artery aneurysms (MCA) were also underrepresented (<15%) in the trial because they were considered unsuitable for coiling at the time of randomization. Ninety-two percent of the aneurysms were <1 cm in diameter and are likely to have a better outcome.20,30 Although the selection criteria were designed to include WFNS grades I-III, 88% of the recruits belonged to grade I-II. Because of these limitations in the selection process, the ISAT results can be reliably applied to small, anterior circulation aneurysms with good neurological grades. These results cannot be extrapolated to patients with larger aneurysms, posterior circulation aneurysms, WFNS grade III-IV patients, and patients with complex aneurysm morphology. The average time for coiling was 1.1 days compared with 1.5 days for surgical clipping; this delay would have influenced the outcome. The experience parameter of 30 coiling procedures was set for participating physicians performing the coiling procedure and no such standard was set for the surgeon performing the surgical clipping. It was not mandatory to perform angiography to confirm the surgical clipping since this procedure would have required a change in the institutional practice of participating centers. In addition, rigorous angiographic surveillance was not required for surgical clipping patients unlike their coiling counterparts in the trial. Incomplete obliteration and residual neck remnants possibly resulted in adverse outcome in surgical patients. Patients older than 70 were excluded in the trial and the choice of technique in these patients cannot be ascertained using the results of the ISAT trial. Moreover, patients were assessed using the modified Rankin scale, a subjective assessment by patients, reported through a postal questionnaire rather than an objective assessment. Although the modified Rankin scale is a commonly used measure of outcome in clinical studies, there is the possibility of bias in patients reporting their outcome because of interobserver variability.98,99 Despite these caveats, ISAT is the first landmark study comparing the outcome measure of endovascular coiling with surgical clipping and changed practice in favor of coiling. The endovascular coiling technique is currently increasingly used in the United States according to a recent Nationwide Inpatient Sample database review on cerebral aneurysms.100
The Barrow Ruptured Aneurysm Trial (BRAT), a recent prospective RCT involving 471 patients, found improved clinical outcome for endovascular coiling at the end of 1 year.101 Crossovers from one treatment group to the other were allowed in this trial on the basis of the discretion of the treating physician. A subsequent analysis of the 349 patients who were available for evaluation at the end of 3 years did not confirm this overall benefit. The 3-year data showed that a significant number of patients assigned to the coiling group were crossed over to the surgical clipping group (38%). No significant difference in outcome was found in anterior circulation aneurysms, but the coiling group had a better outcome for aneurysms located at the posterior circulation (anterior circulation n=339, posterior circulation n=69).102 Because of crossovers, secondary to study design, the reported significance of benefit of coiling in a small group of posterior circulation aneurysms is questionable.
To address the issues raised in the aftermath of ISAT trial, ISAT II, a multicenter international trial involving 50 centers, began in 2012 and is currently recruiting patients and is estimated to complete in 2024. The study design also includes aneurysms >1 cm in diameter, posterior circulation aneurysms, patients with WFNS grade 3-4, and patients over the age of 70, which were not studied in the original ISAT trial.103 Noninvasive angiographic surveillance is planned for both the coiling and the clipping group (such follow-up was not mandatory for the clipping group in the original ISAT trial). However, the primary outcome measure is similar to the original ISAT trial, which also includes assessment of dependency using the modified Rankin scale.
Unruptured Aneurysms
A retrospective study of unruptured aneurysms used data from 429 US hospitals in 18 states covering 58% of the US population. During the years 1998 to 2000, there were 2535 unruptured aneurysms evaluated. The results of this study favored endovascular coiling over surgical clipping.104 There are no randomized trials comparing the clipping and coiling technique for unruptured aneurysms. The unruptured aneurysms are likely to be smaller and they are likely to be located in anterior circulation. The results of the ISAT trial can be predictably extrapolated to unruptured aneurysms, as the ISAT recruits predominantly had anterior circulation aneurysms <1 cm in diameter and 87% of the recruits were neurological good grade patients (WFNS scale 1 and II). The role of conservative management for unruptured small aneurysms, particularly of anterior circulation, which otherwise have low risk for rupture, is not known. An RCT comparing conservative management, coiling, and clipping is not feasible and likely to face recruitment problems and would meet the same endpoint as the Trial on Endovascular Aneurysm Management (TEAM trial), which was stopped after recruiting only 80 patients.105 The TEAM trial was designed to compare the role of conservative management and endovascular coiling for unruptured intracranial aneurysms.
Choice of Technique for Management of Intracranial Aneurysms
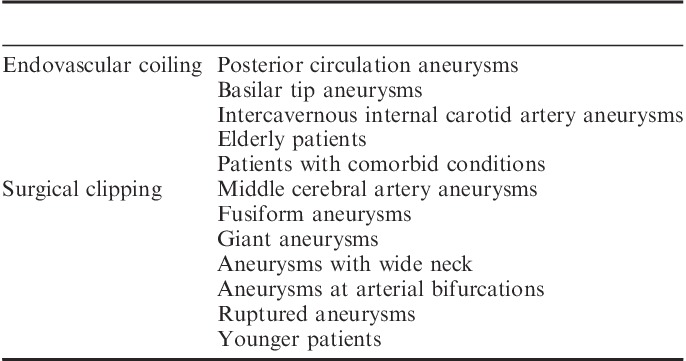
The choice of technique for non-ISAT type of aneurysms is yet to be studied in the ISAT II trial. Certain aneurysms are not suitable for endovascular coiling, such as giant aneurysms, wide neck aneurysms (a neck to dome ratio >0.5), fusiform aneurysms, and aneurysms at certain anatomic locations, such as arterial bifurcation.23 However, aneurysms located in the vertebrobasilar distribution can be easily accessed by an endovascular approach compared with surgical clipping.13,23,106 In particular, basilar tip aneurysms are not amenable to surgery because of deep location and difficulty in proximal control. These aneurysms can be safely treated with an endovascular approach, with or without stent use.107–109 Cavernous sinus internal carotid aneurysms (ICA) are the most difficult aneurysms to treat, because of their close proximity to venous structures and cranial nerves. Surgical access is difficult for aneurysms at this location.110 Aneurysms located at the intercavernous ICA can be managed with balloon occlusion of the parent vessel and coiling.111,112 MCA aneurysms are difficult to treat by coil embolization and are better suited for surgical clipping; however, recent evidence suggests that these aneurysms can be safely treated with coiling embolization.113–118 Ruptured aneurysms with mass effect are better suited for surgical therapy as it enables the surgeon to evacuate a hematoma and thereby decreases the incidence of vasospasm.23 In younger patients, surgical clipping is preferred as it provides better protection from future SAH.94 Coiling is suitable for older patients and patients with comorbid conditions (Table 3).119
TABLE 3.
Choice of Technique for Intracranial Aneurysms
ENDOVASCULAR FLOW DIVERSION
This is a relatively new endovascular technique whereby an endovascular device with a porous, tubular tight mesh and high metal ratio is deployed across the neck of the aneurysm to redirect the flow from the aneurysm to the parent vessel. This flow diversion eventually leads to intra-aneurysmal thrombosis; subsequently, reendothialization of the arterial wall intima covers the stent.120,121,123 This results in reconstruction and remodeling of the parent artery and occludes the aneurysm/parent vessel interface. Thus a combination of flow diversion hemodynamics, thrombosis, inflammation, and endothelial regrowth results in endoluminal reconstruction and overall aneurysm obliteration.120,121,123 Larger aneurysms may require a combined technique of flow diversion and coiling. The advantage of the endovascular flow diversion technique is that coiling is not required and thereby aneurysmal perforation secondary to coiling can be avoided. After the deployment of endovascular flow-diverter devices, patients require dual antiplatelet therapy.120,121,123 The duration of the antiplatelet therapy is currently debated. The combination of aspirin 325 mg and clopidogrel 75 mg is used for 6 months in most of the centers.120,123 The pipeline embolization device (PED), silk flow diverter (SFD), Surpass flow diverter (SURPASS), and the flow redirectional endoluminal device (FRED) system are the 4 flow-diverter devices CE marked in Europe for clinical use and available in other parts of the world. PED is the only device approved by the US Food and Drug Administration. SURPASS and FRED are new-generation devices, and have recently obtained CE marking. Currently limited data are available on their use.
The recognized problems with this technique are intraprocedural aneurysmal perforation or rupture, ischemic stroke, perianeurysmal cerebral edema, early and delayed distal intraparenchymal hemorrhages (IPH), perforator infarction, side branch occlusion, delayed aneurysm rupture, and late parent artery stenosis/occlusion from neointimal overgrowth.120–129 The perforator infarction is more common in posterior circulation compared with anterior circulation.122 Ischemic stroke is thought to be due to stent occlusion from thrombus formation and thromboembolism.120,122 The neointimal overgrowth may cause parent artery stenosis/occlusion and late cerebral infarction.123 The exact mechanism of IPH is not known. The proposed mechanisms are hemorrhagic reperfusion of the ischemic stroke, hemodynamic alteration from flow-diverter placement, and dual antiplatelet therapy.120,122,123 The intra-aneurysmal thrombosis results in an inflammatory process and extension of such an inflammatory process around the aneurysm is the most likely mechanism of perianeurysmal cerebral edema.120,123 Such edema may result in headache and worsening of presenting compressive signs and symptoms.123 The early SAH can occur due to intraprocedural rupture. The flow diversion technique does not occlude the aneurysm immediately and requires a latency period. Rupture can occur during this latency period.120 The late SAH is caused by aneurysmal rupture due to inadequate occlusion. In addition, there are other theories to explain the delayed aneurysmal rupture. The inflammatory process resulting from intra-aneurysmal thrombosis may contribute to weakening and breakdown of the aneurysmal wall and subsequently rupture causing delayed SAH.120,123 The proximal migration of the flow-diverter device may redirect the flow from the parent vessel to the aneurysmal sac resulting in its rupture.120 A meta-analysis involving 29 studies with 1654 aneurysms treated with PED and SFD reported the total occlusion rate as 76% at 6 months.122 The periprocedural mortality and morbidity rate was 4% and 5%, respectively. The overall incidence of ischemic stroke was 6% and the rate of IPH was 3%.122 Another meta-analysis involving 1018 aneurysms treated with SFD and PED reported the total occlusion rate of the aneurysms at 9 months as 76.2%.124 The reported early neurological morbidity was 7.3% and late neurological morbidity was 2.3%.
The currently available data are primarily derived from prospective and retrospective studies involving unruptured aneurysms. The current ongoing trials involving flow-diverter devices have excluded patients with ruptured aneurysms. The ISAT II trial is the only ongoing RCT comparing clinical outcomes for patients with SAH randomized to either endovascular management or surgical management.103 Within the endovascular study arm the physician may choose the coil type and supplement this with balloon-remodeling, stents, or flow diverters. Considering the uncertain safety profile of the flow diverter, a low enrollment of patients receiving this device is anticipated. The requirement of a latency period for obliteration and inadequate occlusion leaves the already ruptured aneurysm unprotected. In addition, the use of dual antiplatelet therapy is likely to potentiate the risk for hemorrhage. Because of these concerns, the use of flow diverters should be avoided in treating patients with aSAH.
ANESTHETIC MANAGEMENT OF SURGICAL CLIPPING
Depending on the severity of SAH, cerebrovascular reactivity and cerebral autoregulation are likely to be impaired in these patients. Patients with impaired cerebral autoregulation are at risk for delayed cerebral infarction following SAH.130,131 In these patients, cerebral perfusion depends directly on the mean arterial pressure. The anesthetic goal is to maintain the transmural pressure gradient (TMPG) across the wall of the aneurysm and to maintain CPP. A sudden increase in BP coupled with a sudden decrease in ICP can cause sudden changes in the TMPG across the wall of the aneurysm, increasing the risk for rupture. If the patient has a lumbar drain, the optimal time for release of the drain is after opening the dura, which will prevent a sudden drop in ICP and change in the TMPG. Smooth induction and maintenance of anesthesia is desired. Hypotension or hypertension at the time of induction, intubation, surgical pinning, incision, and opening the dura should be avoided. An arterial line usually placed before induction and wide-bore venous access is preferred.
Choice of Anesthetic Technique: Inhalational Versus IV Anesthesia?
Anesthesia can be maintained with inhalational agents ≤1 minimum alveolar concentration (MAC), using narcotics such as fentanyl, sufentanil, or remifentanil, with adequate neuromuscular blockade using nondepolarizing agents. The difference in the cerebral vasodilator effect of modern inhalational agents such as desflurane, isoflurane, and sevoflurane is not clinically relevant when used at 1 MAC or lower.132,133 The cerebral vasodilatory effect is dose dependent, halothane>desflurane>isoflurane>sevoflurane134,135; however, from a postoperative neurological recovery point of view, desflurane>sevoflurane>isoflurane and this property is dependent on their blood gas solubility coefficients.136,137 Alternatively, a propofol-based total IV anesthesia (TIVA) technique can also be used. This technique is likely to reduce the cerebral blood flow and ICP.138,139 However, its superiority over inhalational agents in terms of brain relaxation and neurological recovery is not proven when used at 1 MAC or lower.140–142
The studies comparing IV with inhalational technique did not compare the long-term neurological outcome. Alternatively, a combination of IV and inhalational techniques can be used. There is no advantage to using remifentanil with inhalational or propofol-based TIVA compared with fentanyl with a similar technique in terms of neurological recovery.141 A recent retrospective analysis of Japanese Diagnosis Procedure Combination Database revealed that remifentanil use is associated with lower in-house mortality following clipping of ICA aneurysms.143 The exact cause of such an observation with remifentanil use is not known, but could possibly be due to a reduced surgical stress response in the remifentanil group. An RCT reported reduced stress response in the remifentanil group undergoing supratentorial tumor resection.141 Urinary catecholamines and cortisol levels were measured as markers of stress response in this trial, which were found to be lower in the remifentanil group compared with the fentanyl group. However, these beneficial observations with remifentanil use need to be further studied in large randomized trials with long-term neurological outcome and mortality as a primary outcome measure.
One should note that the RCTs comparing IV and inhalational technique examined patients presenting for supratentorial tumor resection with good neurological condition. However, data are lacking in patients with poor neurological condition with critically elevated ICP. In such patients the above data comparing IV and inhalational agents cannot be predictably extrapolated to make an effective conclusion. Patients with raised ICP, at the upper portion of the intracranial volume-ICP curve, are likely to benefit from a TIVA technique. Deepening of anesthesia during anticipated surgical stimulation (for example surgical pinning) should be done with IV agents rather than volatile agents over 1 MAC. If patients are monitored using evoked potentials, TIVA is preferred, because volatile agents have a significant effect on evoked potentials. Moreover, large randomized trials are required to look into the long-term outcome before a definite conclusion can be made with regard to a choice of technique between IV and inhalational technique.
ICP Management
Elevated ICP should be managed along with simple measures such as head end elevation and prevention of jugular venous compression. Clinicians should maintain a low normal arterial carbon dioxide (PaCO2 of 35 mm Hg) and ensure adequate anesthetic depth; ICP reduction may require administration of IV mannitol or hypertonic saline (HTS) and slow controlled cerebrospinal fluid (CSF) drainage. Sudden drop of ICP with CSF drainage is to be avoided as it may lead to sudden change in TMPG and possible aneurysm rupture.
Role of Hyperventilation?
Hyperventilation produces brain relaxation and improves surgical conditions.144 There are insufficient data with regard to the benefits and risks in terms of neurological outcome with hyperventilation therapy in neurosurgical patients. One RCT reported adverse neurological outcome in severe head injury patients following prolonged hyperventilation.145 Hyperventilation results in cerebral vasoconstriction with reduction in cerebral perfusion and cerebral tissue oxygen content as per small studies in traumatic brain injury (TBI) patients. These studies did not look into the neurological outcome.146–149 The effects of hyperventilation comparing normocarbia in terms of neurological outcome need to be studied in a large RCT. Hyperventilation should only be used when all other measures have failed to control the ICP and should be terminated when the indication for its use ceases.150–152
Mannitol Versus HTS?
Mannitol 20% is a commonly used agent to reduce ICP in a dose range of 0.25 to 1 g/kg body weight, with 0.5 g/kg being the most common. Mannitol should be infused intravenously over a period of 10 to 15 minutes to prevent a transient increase in ICP.151 There are no randomized trials comparing the efficacy of HTS over mannitol in patients with aSAH. Two RCTs proved the benefit of HTS over mannitol in terms of brain relaxation in patients undergoing supratentorial craniotomy.153,154 However, the ICU days and hospital days were similar in both groups in one of these studies,153 and the long-term neurological outcomes were not studied in either trial.153,154 A meta-analysis and systemic review from TBI reported better control of elevated ICP with the use of HTS compared with mannitol.155 However, the authors cited the limitations of this meta-analysis as lack of patient numbers, limited RCTs, and inconsistent methodology.
HTS administration requires a central venous access because infusion through a peripheral IV line is likely to result in thrombophlebitis. HTS administration may result in hypernatremia, but its association with the development of acute kidney disease and central pontine myelinolysis is not established in the normonatremic patient.156 Until the superiority of HTS is demonstrated in terms of neurological outcome (long-term benefit in particular) in a large RCT, the use of HTS or mannitol is acceptable during intracranial aneurysm clipping for the control of ICP.
Neuromonitoring
Neuromonitoring techniques such as electroencephalography (EEG), somatosensory evoked potentials (SSEP), and brainstem auditory evoked potentials (BAEP) are used for aneurysm surgery, but their role in patient outcome is uncertain. In a recent analysis of 691 consecutive patients, who underwent aneurysm clipping, the overall accuracy of SSEP changes in predicting postoperative stroke rate was reported as a positive predictive value of 30% and a negative predictive value of 94%, with a sensitivity of 25% and a specificity of 95%.157 SSEP monitoring is useful for aneurysm surgery involving anterior circulation158; a combination of SSEP and BAEP monitoring is useful for aneurysms of posterior circulation.159,160 Transcranial or direct cortical stimulation, motor evoked potential (MEP) monitoring identifies the ischemia of the motor cortex and its pathway secondary to the occlusion of the perforating arteries of middle cerebral and basilar arteries.161–164 The role of neuromonitoring in terms of neurological outcome needs to be studied in a large placebo controlled trial. Until then, neuromonitoring techniques should be given a consideration when there is a possibility of the application of a temporary clip or if induced hypotension is used to facilitate surgical access. These techniques may identify an improperly applied permanent clip.
Induced Hypothermia
A large multicenter prospective, randomized partially blinded trial, IHAST II determined that mild induced hypothermia (33°C) is not associated with improved neurological outcome in patients with good neurological grades in patients undergoing surgical clipping of intracranial aneurysms.165 Ninety-five percent of the recruits belonged to WFNS grades 1 and 2. Because of the limitations of the IHAST II, the role of induced hypothermia cannot be completely ruled out as a neuroprotective strategy.165–167 The patients in this study were cooled slowly over a period of 5 hours. Target temperature was only reached at or just before clipping. This slow phase of cooling is likely to deny protection from initial ischemic insults from placement of surgical retractors. Cooling time was limited to a mean operating time of 5 to 6 hours. Insufficient cooling might be the cause of the lack of beneficial effect of hypothermia. Rapid rewarming in hypothermia may have contributed to the lack of benefit of induced hypothermia. The outcome measure was based upon the Glasgow Outcome Score (GOS), which is a crude method of outcome assessment and should have been supplemented with the modified Rankin scale for better assessment. In addition, the GOS cannot detect subtotal cognitive beneficial effects. Moreover, even though 1001 patients were recruited in the trial, the study is underpowered to detect a change of one point on GOS. Also, the role of hypothermia has not been studied in patients with poor neurological grades.168 Because of the above considerations, the role of hypothermia cannot be completely negated in patients with aSAH on the basis of the results of IHAST II trial alone.
Role of Albumin for Neuroprotection
The Albumin in Subarachnoid Hemorrhage (ALISAH), a multicenter pilot study with 48 patients, reported the beneficial neuroprotective effect of 25% albumin when administered daily for 7 days.169 Neurological outcome was measured at the end of 90 days. In severe TBI patients, the Saline versus Albumin Fluid Evaluation (SAFE) study revealed increased mortality at the end of 2 years when patients were resuscitated with 4% albumin compared with 0.9% saline.170 The cause of the increase in mortality with the 4% albumin resuscitation group is not known, but increased ICP secondary to 4% albumin administration is a likely explanation.171 One should note that 4% albumin is used in the SAFE study in contrast to 25% hyperoncotic solution in the ALISAH study. A large placebo controlled RCT is needed to confirm the benefit of the ALISAH trial. Until such confirmation one should be cautious with the use of albumin as a neuroprotective agent in light of the results of the SAFE study 2-year follow-up.
Temporary Clip
Surgeon may apply a temporary clip to the feeding vessel to produce localized hypotension to reduce the vascularity of the aneurysm. This facilitates dissection around the neck of the aneurysm enabling the surgeon to place a permanent clip in difficult cases. The application of a temporary clip also reduces the incidence of intraoperative aneurysm rupture.172 After the temporary clip is placed, mean arterial pressure should be maintained or elevated to improve perfusion through collateral circulation while avoiding hypotensive episodes. The safe duration of temporary clipping is not entirely known.173,174 However, temporary clipping for an MCA aneurysm should preferably be limited to <10 minutes.173 A duration >20 minutes of temporary clipping on any intracranial artery puts the patient at risk for ischemia.175,176 Patients with poor neurological grades, elderly patients, those with delayed surgery (4 to 10 d after SAH), or patients with episodes of multiple clipping increase the risk for ischemia and stroke.177
During temporary clipping, burst suppression on EEG using propofol is commonly practiced; however, a beneficial clinical outcome of this practice is not demonstrated, according to the subset analysis of IHAST II in which patients received supplemental drug for neuroprotection when a temporary clip was applied.178 Intraoperative hypothermia did not affect the outcome of patients who had a temporary occlusion.178
Induced Hypotension
A surgeon may request a brief period of induced hypotension to gain surgical access to the base of the aneurysm. The traditional methods of induced hypotension using nitroprusside and nitroglycerine are likely to result in prolonged hypotension, which may increase the risk for cerebral ischemia. A retrospective data analysis from 2 centers in the United States showed that adenosine can be safely used to produce transient flow arrest without adverse cardiac or neurological outcome in patients with low risk for coronary artery disease.179–182 A mean duration of 45 seconds of circulatory arrest can be achieved with 0.3 to 0.4 mg/kg of adenosine.179 However, one should be prepared to manage prolonged asystolic arrest. Pacer pads should be applied for external pacing and equipment should be kept ready for transvenous pacing.
A brief period of induced hypotension with adenosine is particularly useful for paraclinoid aneurysms, basilar tip aneurysms, and large anterior communicating artery aneurysms in which proximal control using a temporary clip is not feasible. In the event of unintentional aneurysmal rupture, rapid onset of adenosine-induced hypotension will aid in quick control of hemorrhage and will facilitate in securing the aneurysm with a permanent clip.
The use of adenosine should be avoided in patients with severe reactive airway disease, severe coronary artery stenosis, and preexisting cardiac conduction abnormalities.183 In addition, one should be cautious with the use of adenosine in patients who are on dipyridamole, carbamazepine, digoxin, and verapamil. These medications are likely to prolong the duration of action of adenosine. Patients on methylxanthines require larger doses because of antagonistic effects.
Confirmation of Surgical Clipping
The near-infrared indocyanine green videoangiography technique is increasingly used in many neurological centers for assessment of adequate permanent clip placement. A recent retrospective analysis of 295 consecutive surgical clipping procedures using this technique resulted in clip modification in 15% of the patients.184 However, small neck remnants <2 mm wide and small residual aneurysms were missed in 10% of the patients. The combination of near-infrared indocyanine green videoangiography along with intraoperative angiography is likely to improve the success of surgical clipping. Postoperative digital subtraction angiography should be used as a confirmatory method for adequate occlusion.185
At the conclusion of the surgery extubation should be considered in patients with good neurological grades. The most common postoperative problems include DCI, rebleeding, hydrocephalus, and fluid and electrolyte abnormalities.
ANESTHESIA FOR COILING EMBOLIZATION
The requirements and principles of anesthetic management for this procedure are similar to surgical clipping; CPP and TMPG should be maintained. In addition, the procedure requires a still patient during critical periods. Other concerns include the working environment outside the operating room, exposure to radiation, and the need for anticoagulation. An arterial line is usually inserted along with a wide-bore venous access. The procedure is usually performed using general anesthesia with endotracheal intubation and muscle relaxation. However, a recent retrospective analysis reported that the procedure can be safely performed with midazolam and fentanyl in cooperative, good grade patients following SAH, who are alert and able to maintain their airway.186 Alternatively, the procedure can be performed using dexmeditomidine, an alpha 2 agonist, which provides sedation and analgesia with minimal respiratory depression.187 Goals during monitored anesthesia care include anxiolysis, analgesia, maintenance of verbal contact, and patient immobility. The reasons for patient discomfort include injection of contrast resulting in burning sensation and headache due to traction on cerebral vessels.
A parallel can be drawn with the patients presenting for endovascular management of ischemic stroke. The Society for Neuroscience in Anesthesiology and Critical Care (SNACC) consensus statement opinioned that local anesthesia with anxiolytics can be used in neurologically cooperative patients for anterior circulation stroke who are able to protect the airway.188 This recommendation lacked evidence from randomized trials. Similarly, endovascular coiling can be done for good grade ruptured aneurysms or unruptured aneurysms with local anesthesia with anxiolytics. Neurological monitoring on an awake patient is an added advantage; but the advantage of such monitoring in long-term neurological outcome is not known. RCTs are needed to compare the outcome of local anesthesia with general anesthesia in neurologically good grade patients.
Possible procedure-specific complications include aneurysm perforation and cerebral ischemia or infarction secondary to thromboembolism, arterial dissection, catheter or coil misplacement, and vasospasm.189–192 Perforation of the aneurysm during coiling embolization can be identified by extravasation and delay in transit of the contrast medium.191 When perforation is diagnosed, the procedure should be completed with further coil deposition, if it is deemed safe, anticoagulation should then be reversed.191 Control of hemostasis may necessitate the use of proximal balloon inflation.193 This intervention risks thromboembolism following the reversal of anticoagulation. Neurosurgical intervention may be required for open craniotomy for decompression and to secure the aneurysm. In the event of vascular occlusion during coiling secondary to thromboembolism or vasospasm, mean arterial pressure should be increased to maintain collateral circulation. Treatment with heparin and antiplatelet agents may be required as well. Catheter-induced vasospasm may require intra-arterial vasodilator therapy; nimodipine, a calcium channel blocker, is the common agent used.
DCI
DCI is a serious complication of SAH and is associated with adverse neurological outcome and may occur with or without vasospasm. The term DCI is preferred over vasospasm because cerebral ischemia can occur following SAH without angiographically detected vasospasm.
Pathophysiology of DCI
The incidence of symptomatic vasospasm, DCI and long-term neurological outcome is comparable in both the surgical clipping and coiling groups.194–198 Smoking has been identified as a risk factor for the development of DCI.199 The mechanism of DCI is not definitely known, but likely to be multifactorial with severity a function of the degree of initial hemorrhage. The suggested mechanisms are loss of blood-brain integrity from early brain injury, initial cerebral edema, loss of cerebral autoregulation, cortical spreading, depression, and microthrombosis.200,201 Cortical spreading depression is a depolarization wave in the gray matter that propagates across the brain at 2 to 5 mm/min and depresses the spontaneous and evoked EEG activity. The spreading of clusters of these slow waves results in severe vasoconstriction, impairment of brain ion homeostasis, and recurrent tissue ischemia.201–204 Microthrombosis is a result of activation of the coagulation cascade following initial hemorrhage.201
Biomarkers for DCI
There are currently no established biomarkers to predict the development of DCI or to monitor its progression. Systemic inflammatory response syndrome following SAH is associated with an increased incidence of delayed ischemic neurological deficit, cerebral infarction, and poor neurological outcome, according to exploratory analysis of data from 413 patients of CONSCIOUS-1 database.205 Systemic inflammatory markers like C-reactive protein and IL-6 levels are elevated early following SAH; higher levels are associated with poor neurological outcome.206–208 Thus elevation of systemic and CSF inflammatory markers may play a role in monitoring the development of DCI, but further studies are required to substantiate this possibility.208,209
Management of DCI
DCI is usually managed with nimodipine, maintenance of normal circulating blood volume, and induced hypertension.70,71 Nimodipine is a calcium antagonist and its oral administration is useful in the management of vasospasm and DCI.210–212 On the basis of current evidence nimodipine’s IV use is not recommended.210 High-dose IV nicardipine (0.15 mg/kg/h for 14 d) reduces symptomatic vasospasm, but does not improve neurological outcome at 3 months as per the results of a multicenter prospective double-blind RCT.213 The routine use of IV nicardipine is not indicated.213 The value of HHH (induced Hypertension, Hypervolemia, and Hemodilution) therapy is not validated and needs to be studied in RCTs.214,215 Hypertension is the more important component of HHH therapy and increases cerebral blood flow and brain tissue oxygen levels and reverses neurological symptoms.216–220 There are currently insufficient data to confirm the usefulness of hypervolemic therapy.216,218–221 Hypervolemic therapy results in a decrease in brain tissue oxygenation, fluid overload, and is associated with deleterious cardiac and pulmonary events. Isovolumic and hypervolemic hemodilution increases global cerebral blood flow, but is associated with decreased oxygen delivery.219–222 Balloon angioplasty for distal vasospasm, refractory to medical management, is shown to be beneficial in smaller retrospective analyses.223,224 Intra-arterial papaverine relieves the vasospasm effectively, but its effects are transient lasting for about 3 hours, requiring multiple papaverine infusions.225–227 Elevated ICP is a frequent complication of intra-arterial papaverine therapy with adverse neurological outcome.225,228,229 Papaverine preserved with chlorobutanol may result in selective permanent gray matter changes.230 The possible cause of this change is unclear, but could be the preservative, papaverine, or both. Other less frequently reported complications include monocular blindness, brainstem dysfunction leading to hemodynamic and respiratory compromise, and seizures.225 Precipitation of papaverine during infusion can result in arterial embolism. Because of these complications intra-arterial papaverine should be avoided. Transluminal angioplasty and injection of intra-arterial nimodipine proximal to a vasospastic vessel improves clinical outcome as observed in small case studies.231–236 Balloon angioplasty along with intra-arterial nicardipine is also found to be useful in the management of refractory vasospasm.227,237 Intra-arterial milrinone has also shown benefit in small case studies.237–239 The beneficial effect of intra-arterial verapamil has been reported as per a small case series.240,241 The effects of intra-arterial vasodilator therapy needs to be studied in a large prospective randomized trial. A multicenter RCT, Intra-arterial Vasospasm Trial (IVT), comparing the effects of intra-arterial nicardipine, milrinone, verapamil, and a combination therapy with nicardipine, verapamil, and nitroglycerine is currently underway.242 Transluminal balloon angioplasty and intra-arterial vasodilator therapy should be considered for the management of symptomatic vasospasm.70,71,243 Prophylactic intra-arterial vasodilator therapy is not indicated before development of angiographic vasospasm.70,71
Other Pharmacological Agents for the Management of DCI
The Magnesium in Aneurysmal Subarachnoid Hemorrhage (MASH) trial, a phase II RCT found that IV magnesium, an arteriolar dilator, improved neurological outcome.244 This finding was confirmed in other prospective RCTs.245–247 However, the Magnesium in Aneurysmal Subarachnoid Hemorrhage (MASH-2) trial,248 a phase III trial, did not confirm these beneficial effects on clinical outcome.248 Similarly, the Intravenous Magnesium for Subarachnoid Hemorrhage (IMASH) trial,249 a phase III, multicenter RCT, did not support the use of IV magnesium for vasospasm management. Currently, there are insufficient data to support the use of IV magnesium to prevent DCI and to improve neurological outcome.250,251 At present, there is no evidence to include tirilazad, a nonglucocorticoid aminosteroid, in addition to nimodipine, in the management of vasospasm.252 Clazosentan, an endothelin-I antagonist, is found to be effective in the prevention and management of vasospasm and in reducing vasospasm-induced morbidity and mortality as shown in a phase II multicenter RCT (CONSCIOUS-1).253 However, a phase III randomized double-blind placebo controlled trial (CONSCIOUS-2) failed to confirm this benefit in patients who had surgical clipping.254 Similarly, a phase III trial (CONSCIOUS-3) trial did not show benefit from clazosentan in patients who had endovascular coiling and the trial was prematurely terminated.255 At present, the available data are not conclusive to recommend the use of oral statins to prevent vasospasm and DCI.256–258 The SimvaSTatin in Aneurysmal Subarachnoid Hemorrhage (STASH), a multicenter randomized placebo controlled phase 3 trial, did not identify the beneficial effects of simvastatin in aSAH patients at the end of 6 months as per the outcome data in 782 patients aged 18 to 65 with 1:1 randomization. The study group received simvastatin 40 mg within 96 hours from ictus of aSAH. The authors concluded against the routine use of simvastatin despite the lack of increased adverse events in the simvastatin group.258 The use of antiplatelet agents is not found to be beneficial in the management of DCI.259,260 Two randomized trials involving the use of low–molecular weight heparin for the prevention and management of microthrombi in DCI provided contradictory results; further large trials are needed.261,262 Intercisternal thrombolysis is beneficial in the management of DCI according to a meta-analysis and systemic review, but this review did not have randomized trials.263 Further large randomized trials are required.
Monitoring for DCI
Cerebral angiography is the technique of choice for diagnosing intracranial arterial vasospasm, but it is invasive and carries the risk of stroke due to arterial dissection, embolism, and arterial rupture.264 CTA, magnetic resonance angiography, and digital subtraction angiography are other modalities used to diagnose arterial vasospasm.265 However, it is practically impossible to use these techniques for daily monitoring in the intensive care unit. Transcranial Doppler (TCD) has the advantage of being a noninvasive technique and is portable, but has a limited role in the diagnosis and monitoring of vasospasm progression and overall neurological outcome.265–278 An increase in mean blood flow velocity (mBFV) >120 cm/s indicates vasospasm in the MCA.265,268,271,272,276 A mBFV>200 cm/s in the MCA territory has high positive predictive value for angiographic vasospasm.265,272 The progressive severity of vasospasm is indicated by an increase in mBFV, an increase in the ratio of mBFV of ipsilateral to contralateral cerebral arteries, and the reversal of diastolic blood flow during serial TCD monitoring.265,271,275,277 TCD is better for detecting and monitoring vasospasm in the ICA and the MCA distribution than in other circulations.265,271 TCD is also better in detecting proximal vasospasm than in detecting distal vasospasm.265,268 Serial TCDs are required to monitor the progression or regression of vasospasm.265
NEUROENDOCRINE ABNORMALITIES
Patients with SAH are likely to present with fluid and electrolyte abnormalities. Hyponatremia is more frequently seen than hypernatremia.278,279 Hyponatremia is due to cerebral salt wasting syndrome or syndrome of inappropriate secretion of antidiuretic hormone; both conditions may coexist. The etiology of hyponatremia is possibly multifactorial; increased levels of renin, angiotensin-II, natriuretic peptide, arginine vasopressin, hypoaldosteroinism and an increase in sympathetic tone are likely causes.280,281 Patients with cerebral salt wasting syndrome typically show a triad of hyponatremia, hypovolemia, and high random urinary sodium concentration (>50 mmol/L) (Table 4).282
TABLE 4.
Differential Diagnosis Between SIADH, CSWS, and DI
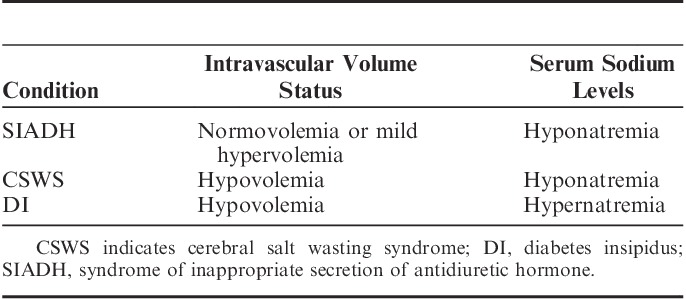
Patients with syndrome of inappropriate secretion of antidiuretic hormone are likely to show normovolemia or mild hypervolemia. Hypernatremia may also be found in patients with SAH: it is most likely to be iatrogenic secondary to mannitol or HTS infusion.281 Rarely, hypernatremia is due to diabetes insipidus, which is most likely due to hypothalamic ischemia. Multifactorial causes of hypothalamic ischemia include: decreased CPP, elevated ICP with a reduction in CPP, vasospastic involvement of anterior cerebral artery, and anterior communicating artery distribution. Hyponatremia is associated with longer hospital stay, but is not associated with increased mortality.278 However, hypernatremia after SAH is independently associated with adverse outcomes and death.278,283 Partial hypopituitarism is also seen in these patients, again it is possibly due to pituitary-hypothalamic ischemia secondary to various possible causes including: vasospasm, decreased CPP, and elevated ICP, resulting in reduced CPP.284,285
SUMMARY
The aSAH is a major health problem in the United States with an incidence of 10/100,000 persons/y. The common mode of presentation of an intracranial hemorrhage is SAH with an acute onset of headache. Patients with good neurological grades at the time of admission have a favorable outcome. NPE and cardiac involvement indicate the severity of SAH. Noncontrast CT scan is a useful initial screening tool, but lumbar puncture is still needed to avoid potential misdiagnosis. Coiling embolization is increasingly used, following the publication of the ISAT results. Surgical clipping is still favored for younger patients, giant aneurysms, fusiform aneurysms, aneurysms located at arterial bifurcations, and MCA aneurysms. Endovascular flow diversion is a new technique, which avoids coiling, but its role is yet to be established in clinical practice.
Hypertension is common following SAH, and systolic BP needs to be lowered to 140 mm Hg to prevent rebleeding based on the most recent evidence from INTERACT-2 trial of intracerebral bleeding. Early intervention following SAH to secure the aneurysm is widely preferred. IV adenosine is found to be useful for induced hypotension in 2 retrospective analyses and further data are required. The benefit of neuromonitoring and pharmacological neuroprotection during temporary clipping is not proven in terms of neurological outcome. The role of induced hypothermia cannot be completely rejected as the patients with IHAST II trial were subjected to slow cooling, inadequate cooling time, and rewarmed slowly. DCI can occur without angiographic vasospasm and is multifactorial in origin, the mechanism of which is yet to be fully understood. There are no established biomarkers to predict its occurrence and to monitor its progression. The established management of DCI includes induced hypertension, maintenance of euvolemia, and oral nimodipine. The role of magnesium, tirilazad, statins, and clazosentan is not proven in phase III randomized trials. Sodium abnormalities are common after SAH. Hyponatremia is more frequently found than hypernatremia. Hypernatremia is associated with unfavorable prognosis.
The body of evidence on the neuroanesthetic considerations for the management on aSAH is expanding. Several trials are underway that may offer results that will further enhance the practice of the anesthesiologist and thus improve the short-term and long-term outcomes for aSAH and SAH patients. This review points to the need for further large RCTs to answer some remaining questions.
ACKNOWLEDGMENTS
The author acknowledges Charles Gibson RN, MA, and Annemarie Begley, RN, BS, Research Nurses in the Department of Anesthesiology, Baystate Medical Center for their assistance with this article.
Footnotes
The author has no funding or conflicts of interest to disclose.
REFERENCES
- 1.de Rooij NK, Linn FH, van der Plas JA, et al. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. [DOI] [PubMed] [Google Scholar]
- 3.Wermer MJ, van der Schaaf IC, Algra A, et al. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007;38:1404–1410. [DOI] [PubMed] [Google Scholar]
- 4.Ingall T, Asplundh K, Mähönen M, et al. A multinational comparison of subarachnoid hemorrhage in the WHO MONICA stroke study. Stroke. 2000;31:1054–1061. [DOI] [PubMed] [Google Scholar]
- 5.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. [DOI] [PubMed] [Google Scholar]
- 6.Sandvei MS, Mathiesen EB, Vatten LJ, et al. Incidence and mortality of aneurysmal subarachnoid hemorrhage in two Norwegian cohorts, 1984-2007. Neurology. 2011;77:1833–1839. [DOI] [PubMed] [Google Scholar]
- 7.Fischer T, Johnsen SP, Pedersen L, et al. Seasonal variation in hospitalization and case fatality of subarachnoid hemorrhage—a nationwide Danish study on 9,367 patients. Neuroepidemiology. 2005;24:32–37. [DOI] [PubMed] [Google Scholar]
- 8.Koffijberg H, Buskens E, Granath F, et al. Subarachnoid haemorrhage in Sweden 1987-2002: regional incidence and case fatality rates. J Neurol Neurosurg Psychiatry. 2008;79:294–299. [DOI] [PubMed] [Google Scholar]
- 9.The ACROSS Group. Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study. Stroke. 2000;31:1843–1850. [DOI] [PubMed] [Google Scholar]
- 10.Ie Roux AA, Wallace MC. Outcome and cost of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:235–246. [DOI] [PubMed] [Google Scholar]
- 11.Alleyne CH., Jr Aneurysmal subarachnoid hemorrhage: Have outcomes really improved? Neurology. 2010;74:1486–1487. [DOI] [PubMed] [Google Scholar]
- 12.Rose MJ. Aneurysmal subarachnoid hemorrhage: an update on the medical complications and treatments strategies seen in these patients. Curr Opin Anaesthesiol. 2011;24:500–507. [DOI] [PubMed] [Google Scholar]
- 13.Bederson JB, Connolly ES, Jr, Batjer HH, et al. American Heart Association: Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. [DOI] [PubMed] [Google Scholar]
- 14.Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928–939. [DOI] [PubMed] [Google Scholar]
- 15.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124Pt:2249–278. [DOI] [PubMed] [Google Scholar]
- 16.Priebe HJ. Aneurysmal subarachnoid haemorrhage and the anaesthetist. Br J Anaesth. 2007;99:102–118. [DOI] [PubMed] [Google Scholar]
- 17.Rinkel GJ, Djibuti M, Algra A, et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251–256. [DOI] [PubMed] [Google Scholar]
- 18.Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36:2773–2780. [DOI] [PubMed] [Google Scholar]
- 19.Ellamushi HE, Grieve JP, Jäger HR, et al. Risk factors for the formation of multiple intracranial aneurysms. J Neurosurg. 2001;94:728–732. [DOI] [PubMed] [Google Scholar]
- 20.The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725–1733. [DOI] [PubMed] [Google Scholar]
- 21.Ronkainen A, Hernesniemi J, Puranen M, et al. Familial intracranial aneurysms. Lancet. 1997;349:380–384. [DOI] [PubMed] [Google Scholar]
- 22.Bor AS, Rinkel GJ, Adami J, et al. Risk of subarachnoid haemorrhage according to number of affected relatives: a population based case-control study. Brain. 2008;131Pt 102662–2665. [DOI] [PubMed] [Google Scholar]
- 23.Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396. [DOI] [PubMed] [Google Scholar]
- 24.Eden SV, Meurer WJ, Sánchez BN, et al. Gender and ethnic differences in subarachnoid hemorrhage. Neurology. 2008;71:731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labovitz DL, Halim AX, Brent B, et al. Subarachnoid hemorrhage incidence among Whites, Blacks and Caribbean Hispanics: the Northern Manhattan Study. Neuroepidemiology. 2006;26:147–150. [DOI] [PubMed] [Google Scholar]
- 26.Schievink WI, Torres VE, Piepgras DG, et al. Saccular intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc of Nephrol. 1992;3:88–95. [DOI] [PubMed] [Google Scholar]
- 27.Rinkel GJ. Natural history, epidemiology and screening of unruptured intracranial aneurysms. J Neuroradiol. 2008;35:99–103. [DOI] [PubMed] [Google Scholar]
- 28.Pepin M, Schwarze U, Superti-Furga A, et al. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673–680. [DOI] [PubMed] [Google Scholar]
- 29.Schievink WI, Parisi JE, Piepgras DG, et al. Intracranial aneurysms in Marfan’s syndrome: an autopsy study. Neurosurgery. 1997;41:866–870. [DOI] [PubMed] [Google Scholar]
- 30.Wiebers DO, Whisnant JP, Huston J, III, et al. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. [DOI] [PubMed] [Google Scholar]
- 31.Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965-1992. J Neurosurg. 1997;87:141–162. [DOI] [PubMed] [Google Scholar]
- 32.Day AL, Gaposchkin CG, Yu CJ, et al. Spontaneous fusiform middle cerebral artery aneurysms: characteristics and a proposed mechanism of formation. J Neurosurg. 2003;99:228–240. [DOI] [PubMed] [Google Scholar]
- 33.Nahed BV, DiLuna ML, Morgan T, et al. Hypertension, age and location predict rupture of small intracranial aneurysms. Neurosurgery. 2005;57:676–683. [PubMed] [Google Scholar]
- 34.Juvela S. Prehemorrhage risk factors for fatal intracranial aneurysm rupture. Stroke. 2003;34:1852–1857. [DOI] [PubMed] [Google Scholar]
- 35.Taylor CL, Yuan Z, Selman WR, et al. Cerebral arterial aneurysm formation and rupture in 20,767 elderly patients: hypertension and other risk factors. J Neurosurg. 1995;83:812–819. [DOI] [PubMed] [Google Scholar]
- 36.Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2008;108:1052–1060. [DOI] [PubMed] [Google Scholar]
- 37.Nanda A, Vannemreddy PS, Polin RS, et al. Intracranial aneurysms and cocaine abuse: analysis of prognostic indicators. Neurosurgery. 2000;46:1063–1069. [DOI] [PubMed] [Google Scholar]
- 38.Loewenstein JE, Gayle SC, Duffis EJ, et al. The natural history and treatment options for unruptured intracranial aneurysms. Int J Vasc Med. 2012;2012:898052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.UCAS Japan Investigators. Morita A, Kirino T, Hashi K. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. [DOI] [PubMed] [Google Scholar]
- 40.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. [DOI] [PubMed] [Google Scholar]
- 41.Keedy A. An overview of intracranial aneurysms. McGill J Med. 2006;9:141–146. [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH, Park SK, Kim TH, et al. Anterior communicating artery aneurysm related to visual symptoms. J Korean Neurosurg Soc. 2009;46:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linskey ME, Sekhar LN, Hirsch WL, Jr, et al. Aneurysms of the intracavernous carotid artery: natural history and indications for treatment. Neurosurgery. 1990;26:933–937. [PubMed] [Google Scholar]
- 44.Perry JJ, Stiell IG, Sivilotti ML, et al. High risk clinical characteristics for subarachnoid haemorrhage in patients with acute headache: prospective cohort study. BMJ. 2010;341:c5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davenport R. Acute headache in the emergency department. J Neurol Neurosurg Psychiatry. 2002;72suppl 2ii33–ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostergaard JR. Headache as a warning symptom of impending aneurysmal subarachnoid hemorrhage. Cephalalgia. 1991;11:53–55. [DOI] [PubMed] [Google Scholar]
- 47.Verweij RD, Wijdicks EF, van Gijn J. Warning headache in aneurysmal subarachnoid hemorrhage. A case-control study. Arch Neurol. 1988;45:1019–1020. [DOI] [PubMed] [Google Scholar]
- 48.Ritz R, Reif J. Comparison of prognosis and complications after warning leaks in subarachnoidal hemorrhage-experience with 214 patients following aneurysm clipping. Neurol Res. 2005;27:620–624. [DOI] [PubMed] [Google Scholar]
- 49.Beck J, Raabe A, Szelenyi A, et al. Sentinel headache and the risk of rebleeding after aneurysmal subarachnoid hemorrhage. Stroke. 2006;37:2733–2737. [DOI] [PubMed] [Google Scholar]
- 50.Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005;2:110–118. [DOI] [PubMed] [Google Scholar]
- 51.Broderick JP, Brott TG, Duldner JE, et al. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. [DOI] [PubMed] [Google Scholar]
- 52.Kassell NF, Torner JC, Haley EC, Jr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: overall management results. J Neurosurg. 1990;73:18–36. [DOI] [PubMed] [Google Scholar]
- 53.Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29–36. [DOI] [PubMed] [Google Scholar]
- 54.Manno EM. Subarachnoid hemorrhage. Neurol Clin. 2004;22:347–366. [DOI] [PubMed] [Google Scholar]
- 55.McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med. 2010;17:444–451. [DOI] [PubMed] [Google Scholar]
- 56.Westerlaan HE, van Dijk JM, Jansen-van der Weide MC, et al. Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis—systematic review and meta-analysis. Radiology. 2011;258:134–145. [DOI] [PubMed] [Google Scholar]
- 57.Sidman R, Connolly E, Lemke T. Subarachnoid hemorrhage diagnosis: lumbar puncture is still needed when the computed tomography scan is normal. Acad Emerg Med. 1996;3:827–831. [DOI] [PubMed] [Google Scholar]
- 58.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. [DOI] [PubMed] [Google Scholar]
- 59.Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–27. [DOI] [PubMed] [Google Scholar]
- 60.Nomura Y, Kawaguchi M, Yoshitani K, et al. Retrospective analysis of predictors of cerebral vasospasm after ruptured cerebral aneurysm surgery: influence of the location of subarachnoid blood. J Anesth. 2010;24:1–6. [DOI] [PubMed] [Google Scholar]
- 61.Jeon IC, Chang CH, Choi BY, et al. Cardiac troponin 1 elevation in patients with aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2009;46:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naidech AM, Kreiter KT, Janjua N, et al. Cardiac troponin elevation, cardiovascular morbidity and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–2856. [DOI] [PubMed] [Google Scholar]
- 63.Mashaly HA, Provencio JJ. Inflammation as a link between brain injury and heart damage: the model of subarachnoid hemorrhage. Cleve Clin J Med. 2008;75suppl 2S26–S30. [DOI] [PubMed] [Google Scholar]
- 64.Ibrahim GM, Macdonald RL. Electrocardiographic changes predict angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:2102–2107. [DOI] [PubMed] [Google Scholar]
- 65.Jain R, Deveikis J, Thompson BG. Management of patients with stunned myocardium associated with subarachnoid hemorrhage. Am J Neuroradiol. 2004;25:126–129. [PMC free article] [PubMed] [Google Scholar]
- 66.Kono T, Morita H, Kuroiwa T, et al. Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol. 1994;24:636–640. [DOI] [PubMed] [Google Scholar]
- 67.Mayer SA, Fink ME, Homma S, et al. Cardiac injury associated with neurogenic pulmonary edema following subarachnoid hemorrhage. Neurology. 1994;44:815–820. [DOI] [PubMed] [Google Scholar]
- 68.Muroi C, Keller M, Pangalu A, et al. Neurogenic pulmonary edema in patients with subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2008;20:188–192. [DOI] [PubMed] [Google Scholar]
- 69.Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012;16:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, et al. on behalf of American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovasular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–1737. [DOI] [PubMed] [Google Scholar]
- 71.Diringer MN, Bleck TP, Claude Hemphill J, III, et al. Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15:211–240. [DOI] [PubMed] [Google Scholar]
- 72.Anderson CS, Heeley E, Huang Y, et al. INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. [DOI] [PubMed] [Google Scholar]
- 73.Butcher KS, Jeerakathil T, Hill M, et al. for the ICH ADAPT Investigators. The Intracerebral Haemorrhage Acutely Decreasing Arterial Pressure Trial (ICH ADAPT): Final results. International Stroke Conference Oral Abstracts. Stroke. 2013;44:A113. [DOI] [PubMed] [Google Scholar]
- 74.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15:559–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woloszyn AV, McAllen KJ, Figueroa BE, et al. Retrospective evaluation of nicardipine versus labetalol for blood pressure control in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2012;16:376–380. [DOI] [PubMed] [Google Scholar]
- 76.Kruyt ND, Biessels GJ, de Haan RJ, et al. Hyperglycemia and clinical outcome in aneurysmal subarachnoid hemorrhage: a meta-analysis. Stroke. 2009;40:e424–e430. [DOI] [PubMed] [Google Scholar]
- 77.Dorhout Mees SM, van Dijk GW, Algra A, et al. Glucose levels and outcome after subarachnoid hemorrhage. Neurology. 2003;61:1132–1133. [DOI] [PubMed] [Google Scholar]
- 78.Frontera JA, Fernandez A, Claassen J, et al. Hyperglycemia after SAH: predictors, associated complications, and impact on outcome. Stroke. 2006;37:199–203. [DOI] [PubMed] [Google Scholar]
- 79.Pasternak JJ, McGregor DG, Schroeder DR, et al. IHAST Investigators. Hyperglycemia in patients undergoing cerebral aneurysm surgery: its association with long-term gross neurologic and neuropsychological function. Mayo Clin Proc. 2008;83:406–417. [DOI] [PubMed] [Google Scholar]
- 80.Latorre JG, Chou SH, Nogueira RG, et al. Effective glycemic control with aggressive hyperglycemia management is associated with improved outcome in aneurysmal subarachnoid hemorrhage. Stroke. 2009;40:1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phillips TJ, Dowling RJ, Yan B, et al. Does treatment of ruptured intracranial aneurysms within 24 hours improve clinical outcome? Stroke. 2011;42:1936–1945. [DOI] [PubMed] [Google Scholar]
- 82.de Gans K, Nieuwkamp DJ, Rinkel GJ, et al. Timing of aneurysm surgery in subarachnoid hemorrhage: a systematic review of the literature. Neurosurgery. 2002;50:336–340. [DOI] [PubMed] [Google Scholar]
- 83.Nieuwkamp DJ, de Gans K, Algra A, et al. Timing of aneurysm surgery in subarachnoid haemorrhage—an observational study in The Netherlands. Acta Neurochir (Wein). 2005;147:815–821. [DOI] [PubMed] [Google Scholar]
- 84.Siddiq F, Chaudhry SA, Tummala RP, et al. Factors and outcomes associated with early and delayed aneurysm treatment in subarachnoid hemorrhage patients in the United States. Neurosurgery. 2012;71:670–678. [DOI] [PubMed] [Google Scholar]
- 85.Starke RM, Kim GH, Fernandez A, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysmal rebleeding after subarachnoid hemorrhage. Stroke. 2008;39:2617–2621. [DOI] [PubMed] [Google Scholar]
- 86.Hillman J, Fridriksson S, Nilsson O, et al. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97:771–778. [DOI] [PubMed] [Google Scholar]
- 87.Roos Y. Antifibrinolytic treatment in subarachnoid hemorrhage: a randomized placebo-controlled trial. STAR Study Group. Neurology. 2000;54:77–82. [DOI] [PubMed] [Google Scholar]
- 88.Chwajol M, Starke RM, Kim GH, et al. Antifibrinolytic therapy to prevent early rebleeding after subarachnoid hemorrhage. Neurocrit Care. 2008;8:418–426. [DOI] [PubMed] [Google Scholar]
- 89.Harrigan MR, Rajneesh KF, Ardelt AA, et al. Short-term antifibrinolytic therapy before aneurysm treatment in subarachnoid hemorrhage: effects on rehemorrhage, cerebral ischemia, and hydrocephalus. Neurosurgery. 2010;67:935–940. [DOI] [PubMed] [Google Scholar]
- 90.Baharoglu MI, Germans MR, Rinkel GJ, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2013;8:CD001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Molyneux AJ, Kerr RS, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267–1274. [DOI] [PubMed] [Google Scholar]
- 92.Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups and aneurysm occlusion. Lancet. 2005;366:809–817. [DOI] [PubMed] [Google Scholar]
- 93.Molyneux AJ, Kerr RS, Birks J, et al. ISAT Collaborators. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardized mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitchell P, Kerr R, Mendelow AD, et al. Could late rebleeding overturn the superiority of cranial aneurysm coil embolization over clip ligation seen in the International Subarachnoid Aneurysm Trial? J Neurosurg. 2008;108:437–442. [DOI] [PubMed] [Google Scholar]
- 95.Johnston SC, Dowd CF, Higashida RT, et al. CARAT Investigators. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke. 2008;39:120–125. [DOI] [PubMed] [Google Scholar]
- 96.van Rooij WJ, Sluzewski M. Opinion: Imaging follow-up after coiling of intracranial aneurysms. Am J Neuroradiol. 2009;30:1646–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ogilvy CS. Neurosurgical clipping versus endovascular coiling of patients with ruptured intracranial aneurysms. Stroke. 2003;34:2540–2542. [DOI] [PubMed] [Google Scholar]
- 98.Sade B, Mohr G. Critical appraisal of the International Subarachnoid Aneurysm Trial (ISAT). Neurol India. 2004;52:32–35. [PubMed] [Google Scholar]
- 99.Quinn TJ, Dawson J, Walters MR, et al. Reliability of the modified Rankins Scale: a systemic review. Stroke. 2009;40:3393–3395. [DOI] [PubMed] [Google Scholar]
- 100.Smith GA, Dagostino P, Maltenfort MG, et al. Geographic variation and regional trends in adoption of endovascular techniques for cerebral aneurysms. J Neurosurg. 2011;114:1768–1777. [DOI] [PubMed] [Google Scholar]
- 101.McDougall CG, Spetzler RF, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial. J Neurosurg. 2012;116:135–144. [DOI] [PubMed] [Google Scholar]
- 102.Spetzler RF, McDougall CG, Albuquerque FC, et al. The Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg. 2013;119:146–157. [DOI] [PubMed] [Google Scholar]
- 103.Darsaut TE, Jack AS, Kerr RS, et al. International Subarachnoid Aneurysm Trial - ISAT part II: study protocol for a randomized controlled trial. Trials. 2013;14:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Higashida RT, Lahue BJ, Torbey MT, et al. Treatment of unruptured intracranial aneurysms: a nationwide assessment of effectiveness. Am J Neuroradiol. 2007;28:146–151. [PMC free article] [PubMed] [Google Scholar]
- 105.Raymond J, Darsaut TE, Molyneux AJ. On behalf of the TEAM collaborative Group. A trial on unruptured intracranial aneurysms (the TEAM trial): results, lessons from a failure and the necessity for clinical care trials. Trials. 2011;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mordasini P, Schroth G, Guzman R, et al. Endovascular treatment of posterior circulation cerebral aneurysms by using Guglielmi detachable coils: a 10-year single-center experience with special regard to technical development. Am J Neuroradiol. 2005;26:1732–1738. [PMC free article] [PubMed] [Google Scholar]
- 107.Sato E, Konishi Y, Shimada A, et al. Applications and roles of coil embolization and/or clipping in the treatment of cerebral aneurysm. Interv Neuroradiol. 2006;12supp 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peluso JP, van Rooij WJ, Sluzewski M, et al. Coiling of basilar tip aneurysms: results of 154 consecutive patients with emphasis on recurrent hemorrhage and re-treatment during mid- and long-term follow-up. J Neurol Neurosurg Psychiatry. 2008;79:706–711. [DOI] [PubMed] [Google Scholar]
- 109.Chalouhi N, Jabbour P, Gonzalez LF, et al. Safety and efficacy of endovascular treatment of basilar tip aneurysms by coiling with and without stent assistance: a review of 235 cases. Neurosurgery. 2012;71:785–794. [DOI] [PubMed] [Google Scholar]
- 110.Diaz FG, Ohaegbulam S, Dujovny M, et al. Surgical alternatives in the treatment of cavernous sinus aneurysms. J Neurosurg. 1989;71:846–853. [DOI] [PubMed] [Google Scholar]
- 111.van der Schaaf IC, Brilstra EH, Buskens E, et al. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke. 2002;33:313–318. [DOI] [PubMed] [Google Scholar]
- 112.van Rooij WJ. Endovascular treatment of cavernous sinus aneurysms. Am J Neuroradiol. 2012;33:323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vendrell JF, Menjot N, Costalat V, et al. Endovascular treatment of 174 middle cerebral artery aneurysms: clinical outcome and radiological results at long-term follow-up. Radiology. 2009;253:191–198. [DOI] [PubMed] [Google Scholar]
- 114.Vendrell JF, Costalat V, Brunel H, et al. Stent-assisted coiling of complex middle cerebral artery aneurysms: initial and midterm results. Am J Neuroradiol. 2011;32:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doerfler A, Wanke I, Goericke SL, et al. Endovascular treatment of middle cerebral artery aneurysms with electrolytically detachable coils. Am J Neuroradiol. 2006;27:513–520. [PMC free article] [PubMed] [Google Scholar]
- 116.Quadros RS, Gallas S, Noudel R, et al. Endovascular treatment of middle cerebral artery aneurysms as first option: a single center experience of 92 aneurysms. Am J Neuroradiol. 2007;28:1567–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim BM, Kim DI, Park SI, et al. Coil embolization of unruptured middle cerebral artery aneurysms. Neurosurgery. 2011;68:346–354. [DOI] [PubMed] [Google Scholar]
- 118.Kim KH, Cha KC, Kim JS, et al. Endovascular coiling of middle cerebral artery aneurysms as an alternative to surgical clipping. J Clin Neurosci. 2013;20:520–522. [DOI] [PubMed] [Google Scholar]
- 119.Gonzalez NR, Dusick JR, Duckwiler G, et al. Endovascular coiling of intracranial aneurysms in elderly patients: report of 205 treated aneurysms. Neurosurgery. 2010;66:714–721. [DOI] [PubMed] [Google Scholar]
- 120.D’Urso PI, Lanzino G, Cloft HJ, et al. Flow diversion for intracranial aneurysms: a review. Stroke. 2011;42:2363–2368. [DOI] [PubMed] [Google Scholar]
- 121.Lanzino G. Flow diversion of intracranial aneurysms. J Neurosurg. 2013;118:405–406. [DOI] [PubMed] [Google Scholar]
- 122.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44:442–447. [DOI] [PubMed] [Google Scholar]
- 123.Alderazi YJ, Shastri D, Kass-Hout T, et al. Flow diverters for intracranial aneurysms. Stroke Res Treat. 2014;2014:415653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arrese I, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 2013;73:193–200. [DOI] [PubMed] [Google Scholar]
- 125.Jabbour P, Chalouhi N, Tjoumakaris S, et al. The Pipeline Embolization Device: learning curve and predictors of complications and aneurysm obliteration. Neurosurgery. 2013;73:113–120. [DOI] [PubMed] [Google Scholar]
- 126.Piano M, Valvassori L, Quilici L, et al. Midterm and long-term follow-up of cerebral aneurysms treated with flow diverter devices: a single-center experience. J Neurosurg. 2013;118:408–416. [DOI] [PubMed] [Google Scholar]
- 127.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013;267:858–868. [DOI] [PubMed] [Google Scholar]
- 128.Murthy SB, Shah S, Shastri A, et al. The silk flow diverter in the treatment of intracranial aneurysms. Clin Neurosci. 2014;21:203–206. [DOI] [PubMed] [Google Scholar]
- 129.Wakhloo AJ, Lylyk P, de Vries J, et al. the Surpass study group. Surpass flow diverter for endovascular treatment of intracranial aneurysms—A multicenter preliminary clinical and angiographic experience in 161 patients with 186 aneurysms. International Stroke Conference oral abstracts. Stroke. 2014;45:A120. [Google Scholar]
- 130.Jaeger M, Schuhmann MU, Soehle M, et al. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38:981–986. [DOI] [PubMed] [Google Scholar]
- 131.Jaeger M, Soehle M, Schuhmann MU, et al. Clinical significance of impaired cerebrovascular autoregulation after severe aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:2097–2101. [DOI] [PubMed] [Google Scholar]
- 132.Sponheim S, Skraastad Ø, Helseth E, et al. Effects of 0.5 and 1.0 MAC isoflurane, sevoflurane and desflurane on intracranial and cerebral perfusion pressures in children. Acta Aneaesthesiol Scand. 2003;47:932–938. [DOI] [PubMed] [Google Scholar]
- 133.Fraga M, Rama-Maceiras P, Rodi S, et al. The effects of isoflurane and desflurane on intracranial pressure, cerebral perfusion pressure, and cerebral arteriovenous oxygen content difference in normocapnic patients with supratentorial brain tumors. Anesthesiology. 2003;98:1085–1090. [DOI] [PubMed] [Google Scholar]
- 134.Holmström A, Akeson J. Desflurane induces more cerebral vasodilatation than isoflurane at the same A-line autoregressive index level. Acta Anaesthesiol Scand. 2005;49:754–758. [DOI] [PubMed] [Google Scholar]
- 135.Matta BF, Heath KJ, Tipping K, et al. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology. 1999;91:667–668. [DOI] [PubMed] [Google Scholar]
- 136.Magni G, Rosa IL, Melillo G, et al. A comparison between sevoflurane and desflurane anesthesia in patients undergoing craniotomy for supratentorial intracranial surgery. Anesth Analg. 2009;109:567–571. [DOI] [PubMed] [Google Scholar]
- 137.Gauthier A, Girard F, Boudreault D, et al. Sevoflurane provides faster recovery and postoperative neurological assessment than isoflurane in long-duration neurosurgical cases. Anesth Analg. 2002;95:1384–1388. [DOI] [PubMed] [Google Scholar]
- 138.Petersen KD, Landsfeldt U, Cold GE, et al. Intracranial pressure and cerebral hemodynamic in patients with cerebral tumors: a randomized prospective study of patients subjected to craniotomy in propofol-fentanyl, or sevoflurane-fentanyl anesthesia. Anesthesiology. 2003;98:329–336. [DOI] [PubMed] [Google Scholar]
- 139.Conti A, Lacopino DG, Fodale V, et al. Cerebral haemodynamic changes during propofol-remifentanil or sevoflurane anesthesia: transcranial Doppler study under bispectral index monitoring. Br J Anaesth. 2006;97:333–339. [DOI] [PubMed] [Google Scholar]
- 140.Lauta E, Abbinante C, Del Gaudio A, et al. Emergence times are similar with sevoflurane and total intravenous anesthesia: results of a multicenter RCT of patients scheduled for supratentorial craniotomy. J Neurosurg Anesthesiol. 2010;22:110–118. [DOI] [PubMed] [Google Scholar]
- 141.Citerio G, Pesenti A, Latini R, et al. NeuroMorfeo Study Group. A multicentre, randomised, open-label, controlled trial evaluating equivalence of inhalational and intravenous anaesthesia during elective craniotomy. Eur J Anaesthesiol. 2012;29:371–379. [DOI] [PubMed] [Google Scholar]
- 142.Magni G, Baisi F, La Rosa I, et al. No difference in emergence time and early cognitive function between sevoflurane-fentanyl and propofol-remifentanil in patients undergoing craniotomy for supratentorial intracranial surgery. J Neurosurg Anesthesiol. 2005;17:134–138. [DOI] [PubMed] [Google Scholar]
- 143.Uchida K, Yasunaga H, Sumitani M, et al. Effects of Remifentanil on in-hospital mortality and length of stay following clipping of intracranial aneurysm: a propensity score-matched analysis. J Neurosurg Anesthesiol. 2014;26:291–298. [DOI] [PubMed] [Google Scholar]
- 144.Gelb AW, Craen RA, Rao GS, et al. Does hyperventilation improve operating condition during supratentorial craniotomy? A multicenter randomized crossover trial. Anesth Analg. 2008;106:585–594. [DOI] [PubMed] [Google Scholar]
- 145.Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–739. [DOI] [PubMed] [Google Scholar]
- 146.Bouma GJ, Muizelaar JP, Stringer WA, et al. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon enhanced computerized tomography. J Neurosurg. 1992;77:360–368. [DOI] [PubMed] [Google Scholar]
- 147.Sioutous PJ, Orozco JA, Carter LP, et al. Continuous regional cerebral cortical blood flow monitoring in head-injured patients. Neurosurgery. 1995;36:943–949. [DOI] [PubMed] [Google Scholar]
- 148.Marion DW, Darby J, Yonas H. Acute regional cerebral blood flow changes caused by severe head injuries. J Neurosurg. 1991;74:407–414. [DOI] [PubMed] [Google Scholar]
- 149.Coles JP, Fryer TD, Coleman MR, et al. Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35:568–578. [DOI] [PubMed] [Google Scholar]
- 150.Stocchetti N, Al Maas, Chieregato A, et al. Hyperventilation in head injury: a review. Chest. 2005;127:1812–1827. [DOI] [PubMed] [Google Scholar]
- 151.Rothenberg DM, O’Connor CJ, Tuma KE.Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL. Neurosurgical anesthesia. Neurosurgical Anesthesia, Miller’s Anesthesia. 2009:7th edPhiladelphia, Churchill-Livingstone: Elsevier; Publishers;2071. [Google Scholar]
- 152.Moore AJ, Newell DW. Tumor Neurosurgery Principles and Practice. 2006London, United Kingdom: Springer-Verlag; London Limited Publishers;88. [Google Scholar]
- 153.Wu CT, Chen LC, Kuo CP, et al. A comparison of 3% hypertonic saline and mannitol for brain relaxation during elective supratentorial brain tumor surgery. Anesth Analg. 2010;110:903–907. [DOI] [PubMed] [Google Scholar]
- 154.Demneri M, Hoxha A, Pillika K, et al. Comparison of 20% mannitol and 7.5% hypertonic saline for supratentorial craniotomy. Eur J Anesth. 2011;28:106–107. [Google Scholar]
- 155.Mortazavi MM, Romeo AK, Deep A, et al. Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis. J Neurosurg. 2012;116:210–221. [DOI] [PubMed] [Google Scholar]
- 156.White H, Cook D, Venkatesh B. The use of hypertonic saline for treating intracranial hypertension after traumatic brain injury. Anesth Analg. 2006;102:1836–1846. [DOI] [PubMed] [Google Scholar]
- 157.Wicks RT, Pradilla G, Raza SM, et al. Impact of changes in intraoperative somatosensory evoked potentials on stroke rates after clipping of intracranial aneurysms. Neurosurgery. 2012;70:1114–1124. [DOI] [PubMed] [Google Scholar]
- 158.Parenti G, Marconi F, Fiori L. Electrophysiological (EEG-SSEP) monitoring during middle cerebral aneurysm surgery. J Neurosurg Sci. 1996;40:195–205. [PubMed] [Google Scholar]
- 159.Lopéz JR, Chang SD, Steinberg GK. The use of electrophysiological monitoring in the intraoperative management of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1999;66:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Manninen PH, Patterson S, Lam AM, et al. Evoked potential monitoring during posterior fossa aneurysm surgery: a comparison of two modalities. Can J Anaesth. 1994;41:92–97. [DOI] [PubMed] [Google Scholar]
- 161.Szelényi A, Langer D, Kothbauer K, et al. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: intraoperative changes and postoperative outcome. J Neurosurg. 2006;105:675–681. [DOI] [PubMed] [Google Scholar]
- 162.Guo L, Gelb AW. The use of motor evoked potential monitoring during cerebral aneurysm surgery to predict pure motor deficits due to subcortical ischemia. Clin Neurophysiol. 2011;122:648–655. [DOI] [PubMed] [Google Scholar]
- 163.Irie T, Yoshitani K, Ohnishi Y, et al. The efficacy of motor evoked potentials on cerebral aneurysm surgery and new onset postoperative motor deficits. J Neurosurg Anesthesiol. 2010;22:247–251. [DOI] [PubMed] [Google Scholar]
- 164.Yeon JY, Seo DW, Hong SC, et al. Transcranial motor evoked potential monitoring during the surgical clipping of unruptured intracranial aneurysms. J Neurol Sci. 2010;293:29–34. [DOI] [PubMed] [Google Scholar]
- 165.Todd MM, Hindman BJ, Clarke WR, et al. Introperative Hypothermia for Aneurysm Surgery Trial (IHAST) Investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135–145. [DOI] [PubMed] [Google Scholar]
- 166.Choi R, Andres RH, Steinberg GK, et al. Intraoperative hypothermia during vascular neurosurgical procedures. Neurosurg Focus. 2009;26:E 24. [DOI] [PubMed] [Google Scholar]
- 167.Komotar RJ, Jones JE, Connolly ES., Jr Introperative hypothermia for Aneurysm surgery Trial (IHAST). Neurosurgery. 2005;56:N9–N10. [Google Scholar]
- 168.Li LR, You C, Chaudhury B. Cooling the brain during surgery for preventing death or severe disability in patients with brain aneurysms. Cochrane Summ. 2012;2:CD008445. [Google Scholar]
- 169.Suarez JI, Martin RH, Calvillo E, et al. ALISAH Investigators. The Albumin in Subarachnoid Hemorrhage (ALISAH) multicenter pilot clinical trial: safety and neurological outcomes. Stroke. 2012;43:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health. Myburgh J, Cooper DJ, Finfer S, et al. Saline or albumin for Fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884. [DOI] [PubMed] [Google Scholar]
- 171.Cooper DJ, Myburgh J, Heritier S, et al. SAFE-TBI Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Albumin resuscitation for traumatic brain injury: is intracranial hypertension the cause of increased mortality? J Neurotrauma. 2013;30:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ka-Hung Pang P, Chan K, Zhu X, et al. Use of elective temporary clips in preventing intraoperative cerebral aneurysm rupture. Ann Coll Surg Hong Kong. 2004;8920:44–48. [Google Scholar]
- 173.Lavine SD, Masri LS, Levy ML, et al. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: time limitation and advantage of brain protection. J Neurosurg. 1997;87:817–824. [DOI] [PubMed] [Google Scholar]
- 174.Schick U, Döhnert J, Meyer JJ, et al. Effects of temporary clips on somatosensory evoked potentials in aneurysm surgery. Neurocrit Care. 2005;2:141–149. [DOI] [PubMed] [Google Scholar]
- 175.Samson D, Batjer HH, Bowman G, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34:22–28. [PubMed] [Google Scholar]
- 176.Woertgen C, Rothoerl RD, Albert R, et al. Effects of temporary clipping during aneurysm surgery. Neurol Res. 2008;30:542–546. [DOI] [PubMed] [Google Scholar]
- 177.Ogilvy CS, Carter BS, Kaplan S, et al. Temporary vessel occlusion for aneurysm surgery: risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996;84:785–791. [DOI] [PubMed] [Google Scholar]
- 178.Hindman BJ, Bayman EO, Pfisterer WK, et al. IHAST Investigators. No association between intraoperative hypothermia or supplemental protective drug and neurological outcomes in patients undergoing temporary clipping during cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology. 2010;112:86–101. [DOI] [PubMed] [Google Scholar]
- 179.Bebawy JF, Gupta DK, Bendok BR, et al. Adenosine-induced flow arrest to facilitate intracranial aneurysm clip ligation: dose-response data and safety profile. Anesth Analg. 2010;110:1406–1411. [DOI] [PubMed] [Google Scholar]
- 180.Bendok BR, Gupta DK, Rahme RJ, et al. Adenosine for temporary flow arrest during intracranial aneurysm surgery: a single-center retrospective review. Neurosurgery. 2011;69:815–821. [DOI] [PubMed] [Google Scholar]
- 181.Guinn NR, McDonagh DL, Borel CO, et al. Adenosine-induced transient asystole for intracranial aneurysm surgery: a retrospective review. J Neurosurg Anesthesiol. 2011;23:35–40. [DOI] [PubMed] [Google Scholar]
- 182.Khan SA, McDonagh DL, Adogwa O, et al. Perioperative cardiac complications and 30-day mortality in patients undergoing intracranial aneurysmal surgery with adenosine-induced flow arrest: a retrospective comparative study. Neurosurgery. 2014;74:267–272. [DOI] [PubMed] [Google Scholar]
- 183.Khan AS, Nimjee SM, Guinn NN, et al. The use of adenosine in cerebral aneurysm clipping: a review. Curr Anesthesiol Rep. 2013;3:210–213. [Google Scholar]
- 184.Roessler K, Krawagna M, Dörfler A, et al. Essentials in intraoperative indocyanine green videoangiography assessment for intracranial aneurysm surgery: conclusions from 295 consecutively clipped aneurysms and review of the literature. Neurosurg Focus. 2014;36:E7. [DOI] [PubMed] [Google Scholar]
- 185.Washington CW, Zipfel GJ, Chicoine MR, et al. Comparing indocyanine green videoangiography to the gold standard of intraoperative digital subtraction angiography used in aneurysm surgery. J Neurosurg. 2013;118:420–427. [DOI] [PubMed] [Google Scholar]
- 186.Kan P, Jahshan S, Yashar P, et al. Feasibility, safety, and periprocedural complications associated with endovascular treatment of selected ruptured aneurysms under conscious sedation and local anesthesia. Neurosurgery. 2013;72:216–220. [DOI] [PubMed] [Google Scholar]
- 187.Lee CZ, Young WL. Anesthesia for endovascular neurosurgery and interventional neuroradiology. Anesthesiol Clin. 2012;30:127–147. [DOI] [PubMed] [Google Scholar]
- 188.Talke PO, Sharma D, Heyer EJ, et al. Society for Neuroscience in Anesthesiology and Critical Care Expert Consensus Statement: Anesthetic management of endovascular treatment for acute ischemic stroke: Endorsed by the Society of NeuroInterventional Surgery and the Neurocritical Care Society. J Neurosurg Anesthesiol. 2014;26:95–108. [DOI] [PubMed] [Google Scholar]
- 189.Doerfler A, Wanke I, Egelhof T, et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management and outcome. Am J Neuroradiol. 2001;22:1825–1832. [PMC free article] [PubMed] [Google Scholar]
- 190.van Rooij WJ, Sluzewski M, Beute GN, et al. Procedural complications of coiling of ruptured intracranial aneurysms: incidence and risk factors in a consecutive series of 681 patients. Am J Neuroradiol. 2006;27:1498–1501. [PMC free article] [PubMed] [Google Scholar]
- 191.Levy E, Koebbe CJ, Horowitz MB, et al. Rupture of intracranial aneurysms during endovascular coiling management and outcomes. Neurosurgery. 2001;49:807–813. [DOI] [PubMed] [Google Scholar]
- 192.McDougall CG, Halback VV, Dowd CF, et al. Causes and management of aneurysmal hemorrhage occurring during embolization with Guglielmi detachable coils. J Neurosurg. 1998;89:87–92. [DOI] [PubMed] [Google Scholar]
- 193.Layton KF, Cloft HJ, Kallmes DF. Cerebral aneurysm perforations during treatment with detachable coils. Use of remodeling balloon inflation to achieve hemostasis. Interv Neuroradiol. 2006;12:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rabinstein AA, Pichelmann MA, Friedman JA, et al. Symptomatic vasospasm and outcomes following aneurysmal subarachnoid hemorrhage: a comparison between surgical repair and endovascular coil occlusion. J Neurosurg. 2003;98:319–325. [DOI] [PubMed] [Google Scholar]
- 195.Hoh BL, Topcuoglu MA, Singhal AB, et al. Effect of clipping, craniotomy or intravascular coiling on cerebral vasospasm and patient outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2004;55:779–789. [DOI] [PubMed] [Google Scholar]
- 196.Dehdashti AR, Mermillod B, Rufenacht DA, et al. Does treatment modality of intracranial ruptured aneurysms influence the incidence of cerebral vasospasm and clinical outcome? Cerebrovasc Dis. 2004;17:53–60. [DOI] [PubMed] [Google Scholar]
- 197.Goddard AJ, Raju PP, Gholkar A. Does the method of treatment of acutely ruptured intracranial aneurysms influence the incidence and duration of cerebral vasospasm and clinical outcome? J Neurol Neurosurg Psychiatry. 2004;75:868–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.de Oliveira JG, Beck J, Ulrich C, et al. Comparison between clipping and coiling on the incidence of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurosurg Rev. 2007;30:22–31. [DOI] [PubMed] [Google Scholar]
- 199.deRooji NK, Rinkel GJ, Dankbaar JW, et al. Delayed cerebral ischemia after subarachnoid hemorrhage; a systemic review of clinical, laboratory, and radiological predictors. Stroke. 2013;44:43–54. [DOI] [PubMed] [Google Scholar]
- 200.Budohoski KP, Czosnyka M, Smielewski P, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43:3230–3237. [DOI] [PubMed] [Google Scholar]
- 201.Rowland MJ, Hadjipavlou G, Kelly M, et al. Delayed cerebral ischemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth. 2012;109:315–329. [DOI] [PubMed] [Google Scholar]
- 202.Woitzik J, Dreier JP, Hecht N, et al. COSBID study group. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2012;32:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lauritzen M, Dreier JP, Fabricius M, et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Drier JP, Major S, Manning A, et al. COSBID study group. Cortical spreading ischaemia is a novel process involved in ischemic damage in patients with aneurysmal subarachnoid hemorrhage. Brain. 2009;132:1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tam AK, Ilodigwe D, Mocco J, et al. Impact of systemic inflammatory response on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: exploratory analysis of CONSCIOUS-1 database. Neurocrit Care. 2010;13:182–189. [DOI] [PubMed] [Google Scholar]
- 206.Fountas KN, Tasiou A, Kapsalaki EZ, et al. Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Clinical article. Neurosurg Focus. 2009;26:E22. [DOI] [PubMed] [Google Scholar]
- 207.Sarrafzadeh A, Schlenk F, Gericke C, et al. Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2010;13:339–346. [DOI] [PubMed] [Google Scholar]
- 208.Muroi C, Hugelshofer M, Seule M, et al. Correlation among systemic inflammatory parameter, occurrence of delayed neurological deficits, and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2013;72:367–375. [DOI] [PubMed] [Google Scholar]
- 209.McMahon CJ, Hopkins S, Vail A, et al. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. Neurointerv Surg. 2013;5:512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dorhout Mees SM, Rinkel GJE, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:514–515. [Google Scholar]
- 211.Velat GJ, Kimball MM, Mocco JD, et al. Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analysis in the literature. World Neurosurg. 2011;76:446–454. [DOI] [PubMed] [Google Scholar]
- 212.Barker FG, II, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a meta-analysis. J Neurosurg. 1996;84:405–414. [DOI] [PubMed] [Google Scholar]
- 213.Haley EC, Jr, Kassell NF, Torner JC. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J Neurosurg. 1993;78:537–547. [DOI] [PubMed] [Google Scholar]
- 214.Lee KH, Lukovits T, Friedman JA. “Triple-H” therapy for cerebral vasospasm following subarachnoid hemorrhage. Neurocrit Care. 2006;4:68–76. [DOI] [PubMed] [Google Scholar]
- 215.Sen J, Belli A, Albon H, et al. Triple-H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2003;2:614–621. [DOI] [PubMed] [Google Scholar]
- 216.Muench E, Horn P, Bauhuf C, et al. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Crit Care Med. 2007;35:1844–1851. [DOI] [PubMed] [Google Scholar]
- 217.Harrigan MR. Hypertension may be the most important component of hyperdynamic therapy in cerebral vasospasm. Crit Care. 2010;14:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dankbaar JW, Slooter AJ, Rinkel GJ, et al. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: a systemic review. Crit Care. 2010;14:R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Treggiari MM. Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Hemodynamic management of subarachnoid hemorrhage. Neurocrit Care. 2011;15:329–335. [DOI] [PubMed] [Google Scholar]
- 220.Treggiari MM, Deem S. Which H is the most important in triple-H therapy for cerebral vasospasm? Curr Opin Crit Care. 2009;15:83–86. [DOI] [PubMed] [Google Scholar]
- 221.Rinkel GJE, Feigin VL, Algra A, et al. Hypervolemia in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36:1104–1105.15790944 [Google Scholar]
- 222.Chittiboina P, Conrad S, McCarthy P, et al. The evolving role of hemodilution in treatment of cerebral vasospasm: a historical perspective. World Neurosurg. 2011;75:660–664. [DOI] [PubMed] [Google Scholar]
- 223.Santillan A, Knopman J, Zink W, et al. Transluminal balloon angioplasty for symptomatic distal vasospasm refractory to medical therapy in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2011;69:95–102. [DOI] [PubMed] [Google Scholar]
- 224.Eskridge JM, McAuliffe W, Song JK, et al. Balloon angioplasty for the treatment of vasospasm: results of first 50 cases. Neurosurgery. 1998;42:510–517. [DOI] [PubMed] [Google Scholar]
- 225.Athar MK, Levine JM. Treatment options for cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurotherapeutics. 2012;9:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu JK, Couldwell WT. Intra-arterial papaverine infusions for the treatment of cerebral vasospasm induced by aneurysmsal subarachnoid hemorrhage. Neurocrit Care. 2005;2:124–132. [DOI] [PubMed] [Google Scholar]
- 227.Hoh BL, Ogilvy CS. Endovascular treatment of cerebral vasospasm: transluminal balloon angioplasty, intra-arterial papaverine and intra-arterial nicardipine. Neurosurg Clin N Am. 2005;16:501–516. [DOI] [PubMed] [Google Scholar]
- 228.McAuliffe W, Townsend M, Eskridge JM, et al. Intracranial pressure changes induced during papaverine infusion for treatment of vasospasm. J Neurosurg. 1995;83:430–434. [DOI] [PubMed] [Google Scholar]
- 229.Cross DT, III, Moran CJ, Angtuaco EE, et al. Intracranial pressure monitoring during intraarterial papaverine infusion for cerebral vasospasm. Am J Neuroradiol. 1998;19:1319–1323. [PMC free article] [PubMed] [Google Scholar]
- 230.Smith WS, Dowd CF, Johnston SC, et al. Neurotoxicity of intra-arterial papaverine preserved with chlorobutanol used for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:2518–2522. [DOI] [PubMed] [Google Scholar]
- 231.Biondi A, Ricciardi GK, Puybasset L, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. Am J Neuroradiol. 2004;25:1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 232.Cho WS, Kang HS, Kim JE, et al. Intra-arterial nimodipine infusion for cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Interv Neuroradiol. 2011;17:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Musahl C, Henkes H, Vajda Z, et al. Continuous local intra-arterial nimodipine administration in severe symptomatic vasospasm after subarachnoid hemorrhage. Neurosurgery. 2011;68:1541–1547. [DOI] [PubMed] [Google Scholar]
- 234.Oran I, Cinar C. Continuous intra-arterial infusion of nimodipine during embolization of cerebral aneurysms associated with vasospasm. Am J Neuroradiol. 2008;29:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wolf S, Martin H, Landscheidt JF, et al. Continuous selective intraarterial infusion of nimodipine for therapy of refractory cerebral vasospasm. Neurocrit Care. 2010;12:346–351. [DOI] [PubMed] [Google Scholar]
- 236.Hui C, Lau KP. Efficacy of intra-arterial nimodipine in the treatment of cerebral vasospasm complicating subarachnoid hemorrhage. Clin Radiol. 2005;60:1030–1036. [DOI] [PubMed] [Google Scholar]
- 237.Schmidt U, Bittner E, Pivi S, et al. Hemodynamic management and outcome of patients treated for cerebral vasospasm with intraarterial nicardipine and/or milrinone. Anesth Analg. 2010;110:895–902. [DOI] [PubMed] [Google Scholar]
- 238.Fraticelli AT, Cholley BP, Losser MR, et al. Milrinone for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:893–898. [DOI] [PubMed] [Google Scholar]
- 239.Shankar JJ, dos Santos MP, Deus-Silva L, et al. Angiographic evaluation of the effect of intra-arterial milrinone therapy in patients with vasospasm from aneurysmal subarachnoid hemorrhage. Neuroradiology. 2011;53:123–128. [DOI] [PubMed] [Google Scholar]
- 240.Keuskamp J, Murali R, Chao KH. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;108:458–463. [DOI] [PubMed] [Google Scholar]
- 241.Sehy JV, Holloway WE, Lin SP, et al. Improvement in angiographic cerebral vasospasm after intra-arterial verapamil administration. Am J Neuroradiol. 2010;31:1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.The University of Texas Health Science Center, Houston. Intrarterial Vasospasm Trial (IVT) A Multi-Center Randomized Study. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2014. Available at: http://clinicaltrials.gov/show clinicalTrails.gov identifier NCT01996436.
- 243.Abruzzo T, Moran C, Blackham KA, et al. Invasive interventional management of post-hemorrhagic cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurointerv Surg. 2012;4:169–177. [DOI] [PubMed] [Google Scholar]
- 244.van den Bergh WM, Algra A, van Kooten F, et al. MASH Study Group. Magnesium sulfate in aneurysmal subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2005;36:1011–1015. [DOI] [PubMed] [Google Scholar]
- 245.Westermaier T, Stetter C, Vince GH, et al. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, clinical study. Crit Care Med. 2010;38:1284–1290. [DOI] [PubMed] [Google Scholar]
- 246.Stippler M, Crago E, Levy El, et al. Magnesium infusion for vasospasm prophylaxis after subarachnoid hemorrhage. J Neurosurg. 2006;105:723–729. [DOI] [PubMed] [Google Scholar]
- 247.Muroi C, Terzic A, Fortunati M, et al. Magnesium sulfate in the management of patients with aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, dose-adapted trial. Surg Neurol. 2008;69:33–39. [DOI] [PubMed] [Google Scholar]
- 248.Dorhout Mees SM, Algra A, Vandertop WP, et al. MASH-2 Study Group. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomized placebo-controlled trial. Lancet. 2012;380:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wong GK, Poon WS, Chan MT, et al. IMASH Investigators. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke. 2010;41:921–926. [DOI] [PubMed] [Google Scholar]
- 250.Wong GK, Boet R, Poon WS, et al. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage: an updated systemic review and meta-analysis. Crit Care. 2011;15:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhao XD, Zhou YT, Zhang X, et al. A meta-analysis of treating subarachnoid hemorrhage with magnesium sulfate. J Clin Neurosci. 2009;16:1394–1397. [DOI] [PubMed] [Google Scholar]
- 252.Zhang S, Wang L, Liu M, et al. Tirilazad for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2010;17:CD006778. [DOI] [PubMed] [Google Scholar]
- 253.Macdonald RL, Kassell NF, Mayer S, et al. CONSCIOUS-1 Investigators. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. [DOI] [PubMed] [Google Scholar]
- 254.Macdonald RL, Higashida RT, Keller E, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomized, double blind, placebo-controlled phase 3 trial (CONSCIOUS -2). Lancet Neurol. 2011;10:618–625. [DOI] [PubMed] [Google Scholar]
- 255.Clazosentan in Aneurysmal Subarachnoid Hemorrhage (CONSCIOUS-3). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2009- 2012 Available at: http://clinicaltrials.gov/show clinicalTrails.gov identifierNCT00940095.
- 256.Kramer AH, Fletcher JJ. Statins in the management of aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurocrit Care. 2010;12:285–296. [DOI] [PubMed] [Google Scholar]
- 257.Vergouwen MD, de Haan RJ, Vermeulen M, et al. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke. 2010;41:e47–e52. [DOI] [PubMed] [Google Scholar]
- 258.Kirkpatrick PJ, Turner CL, Smith C, et al. for the STASH Collaborators. Simvastatin in aneurysmal subarachnoid hemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. 2014;13:666–675. [DOI] [PubMed] [Google Scholar]
- 259.van den Bergh WM, Algra A, Dorhout Mees SM, et al. MASH Study Group. Randomized controlled trial of acetylsalicylic acid in aneurysmal subarachnoid hemorrhage: the MASH Study. Stroke. 2006;37:2326–2330. [DOI] [PubMed] [Google Scholar]
- 260.Dorhout Mees SM, van den Berg WM, Algra A, et al. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007;17:CD 006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wurm G, Tomancok B, Nussbaumer K, et al. Reduction of ischemic sequelae following spontaneous subarachnoid hemorrhage: a double-blind, randomized comparison of enoxaparin versus placebo. Clin Neurol Neurosurg. 2004;106:97–103. [DOI] [PubMed] [Google Scholar]
- 262.Siironen J, Juvela S, Varis J, et al. No effect of enoxaparin on outcome of aneurysmal subarachnoid hemorrhage: a randomized double-blind placebo-controlled clinical trial. J Neurosurg. 2003;99:953–959. [DOI] [PubMed] [Google Scholar]
- 263.Amin-Hanjani S, Ogilvy CS, Barker FG., II Does intracisternal thrombolysis prevent vasospasm after aneurysmal subarachnoid hemorrhage? A meta-analysis. Neurosurgery. 2004;54:326–335. [DOI] [PubMed] [Google Scholar]
- 264.Heiserman JE. MR angiography for the diagnosis of vasospasm after subarachnoid hemorrhage. Is it accurate? Is it safe? Am J Neuroradiol. 2000;21:1571–1572. [PMC free article] [PubMed] [Google Scholar]
- 265.Mills JN, Mehta V, Russin J, et al. Advanced imaging modalities in the detection of cerebral vasospasm. Neurol Res Int. 2013;2013:415960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rigamonti A, Ackery A, Baker AJ. Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anaesth. 2008;55:112–123. [DOI] [PubMed] [Google Scholar]
- 267.Lysakowski C, Walder B, Costanza MC, et al. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systemic review. Stroke. 2001;32:2292–2298. [DOI] [PubMed] [Google Scholar]
- 268.Saqqur M, Zygun D, Demchuk A. Role of transcranial Doppler in neurocritical care. Crit Care Med. 2007;35supplS216–S223. [DOI] [PubMed] [Google Scholar]
- 269.Marshall SA, Nyquist P, Ziai WC. The role of transcranial Doppler ultrasonography in the diagnosis and management of vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:291–303. [DOI] [PubMed] [Google Scholar]
- 270.Miller CM, Palestrant D, Schievink WI, et al. Prolonged transcranial Doppler monitoring after aneurysmal subarachnoid hemorrhage fails to adequately to predict ischemic risk. Neurocrit Care. 2011;15:387–392. [DOI] [PubMed] [Google Scholar]
- 271.Suarez JI, Al Qureshi, Yahia AB, et al. Symptomatic vaospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30:1348–1355. [DOI] [PubMed] [Google Scholar]
- 272.Vora YY, Suarez-Almazor M, Strinke DE, et al. Role of transcranial doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44:1237–1248. [PubMed] [Google Scholar]
- 273.Fontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–1968. [DOI] [PubMed] [Google Scholar]
- 274.Wong JK, Poon WS. Clinical, transcranial Doppler ultrasound, radiological features and, prognostic significance of delayed cerebral ischemia. Acta Neurochir Suppl. 2013;115:9–11. [DOI] [PubMed] [Google Scholar]
- 275.Nakae R, Yokota H, Yoshioda D, et al. Transcranial Doppler ultrasonography for diagnosis of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: mean blood flow velocity ratio of the ipsilateral and contralateral middle cerebral arteries. Neurosurgery. 2011;69:876–883. [DOI] [PubMed] [Google Scholar]
- 276.Newell DW, Winn HR. Transcranial Doppler in cerebral vasospasm. Neurosurg Clin N Am. 1990;1:319–328. [PubMed] [Google Scholar]
- 277.Sharma VK, Pereira AW, Chan BP. Vasospasm followed by diastolic flow reversal in the intracranial arteries after subarachnoid haemorrhage. J Clin Neurosci. 2007;14:389–391. [DOI] [PubMed] [Google Scholar]
- 278.Qureshi Al, Suri MF, Sung GY, et al. Prognostic significance of hypernatremia and hyponatremia among patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;50:749–756. [DOI] [PubMed] [Google Scholar]
- 279.Sherlock M, O’Sullivan E, Agha A, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid hemorrhage. Clin Endocrinol (Oxf). 2006;64:250–254. [DOI] [PubMed] [Google Scholar]
- 280.Audibert G, Steinmann G, de Talanc N, et al. Endocrine response after severe subarachnoid hemorrhage related to sodium and blood volume regulation. Anesth Analg. 2009;108:1922–1928. [DOI] [PubMed] [Google Scholar]
- 281.Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:325–338. [DOI] [PubMed] [Google Scholar]
- 282.Palmer BF. Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endocrinol Metab. 2003;14:182–187. [DOI] [PubMed] [Google Scholar]
- 283.Fisher LA, Ko N, Miss J, et al. Hypernatremia predicts adverse cardiovascular and neurological outcomes after SAH. Neurocrit Care. 2006;5:180–185. [DOI] [PubMed] [Google Scholar]
- 284.Kreitschmann-Andermahr I. Subarachnoid hemorrhage as a cause of hypopitutarism. Pituitary. 2005;8:219–225. [DOI] [PubMed] [Google Scholar]
- 285.Kreitschmann-Andermahr I, Hoff C, Niggemeier S, et al. Pituitary deficiency following aneurysmal subarachnoid hemorrhage. J Neurol Neurosurg Psychiatry. 2003;74:1133–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chiu Y, Drolet B, Seefeldt M, et al. Abnormalities of the Head and Neck Arteries (Cerebrovascular Abnormalities). In: PHACE Syndrome Handbook: A Guide for Parents and Physicians, Children's Hospital of Wisconsin. Milwaukee, WI. Available at: https://www.chw.org/display/PPF/DocID/48513/Nav/1/router.asp. [Google Scholar]


