Supplemental Digital Content is Available in the Text.
This randomized trial observed a survival difference between patients randomized to the ABThera versus Barker's vacuum pack after abbreviated laparotomy. As this difference did not seem to be mediated by improved peritoneal fluid drainage, fascial closure rates, or markers of systemic inflammation, it should be confirmed by a multicenter trial.
Keywords: abbreviated laparotomy, abdominal injury, inflammation, negative pressure peritoneal therapy, randomized controlled trial
Abstract
Objective:
To determine whether active negative pressure peritoneal therapy with the ABThera temporary abdominal closure device reduces systemic inflammation after abbreviated laparotomy.
Background:
Excessive systemic inflammation after abdominal injury or intra-abdominal sepsis is associated with poor outcomes.
Methods:
We conducted a single-center, randomized controlled trial. Forty-five adults with abdominal injury (46.7%) or intra-abdominal sepsis (52.3%) were randomly allocated to the ABThera (n = 23) or Barker's vacuum pack (n = 22). On study days 1, 2, 3, 7, and 28, blood and peritoneal fluid were collected. The primary endpoint was the difference in the plasma concentration of interleukin-6 (IL-6) 24 and 48 hours after temporary abdominal closure application.
Results:
There was a significantly lower peritoneal fluid drainage from the ABThera at 48 hours after randomization. Despite this, there was no difference in plasma concentration of IL-6 at baseline versus 24 (P = 0.52) or 48 hours (P = 0.82) between the groups. There was also no significant intergroup difference in the plasma concentrations of IL-1β, −8, −10, or −12 p70 or tumor necrosis factor α between these time points. The cumulative incidence of primary fascial closure at 90 days was similar between groups (hazard ratio, 1.6; 95% confidence interval, 0.82–3.0; P = 0.17). However, 90-day mortality was improved in the ABThera group (hazard ratio, 0.32; 95% confidence interval, 0.11–0.93; P = 0.04).
Conclusions:
This trial observed a survival difference between patients randomized to the ABThera versus Barker's vacuum pack that did not seem to be mediated by an improvement in peritoneal fluid drainage, fascial closure rates, or markers of systemic inflammation.
Trial Registration:
ClinicalTrials.gov identifier NCT01355094.
Abbreviated laparotomy with planned reoperation is frequently recommended to manage patients with severe abdominal injury and intra-abdominal sepsis.1–4 At the conclusion of abbreviated laparotomy, a temporary abdominal closure (TAC) dressing is applied.5–7 TAC dressings provide visceral coverage and ideally drain peritoneal fluid, are associated with a low risk of recurrent abdominal compartment syndrome, and increase probability of primary fascial closure (ie, definitive abdominal wall closure during the index hospitalization).1,2
Studies have recently suggested that TAC dressings that employ constant negative pressure to the peritoneal cavity after abbreviated laparotomy may reduce peritoneal and systemic inflammation.8–10 A study randomized animals with intra-abdominal sepsis to negative pressure peritoneal therapy versus passive drainage of the peritoneal cavity and observed reduced levels of systemic proinflammatory cytokines and improved cardiac, pulmonary, and renal function after 36 hours.8 A subsequent multicenter prospective cohort study reported that use of the ABThera Open Abdomen Negative Pressure Therapy device (Kinetic Concepts Inc., San Antonio, TX) after abbreviated laparotomy was associated with improved survival and primary fascial closure rates among those with intra-abdominal injury or sepsis when compared with a device that provided potentially less efficient negative pressure peritoneal therapy, the Barker's vacuum pack.11 As the survival curves diverged with time, the authors questioned whether improved mortality was attributable to enhanced removal of cytokine-rich peritoneal fluid.11
We performed a randomized controlled trial (RCT) to determine whether the ABThera reduces the extent of the systemic inflammatory response after abbreviated laparotomy for abdominal injury or intra-abdominal sepsis when compared with the Barker's vacuum pack. Our study hypothesis was that the ABThera would improve peritoneal fluid drainage and removal of intraperitoneal proinflammatory cytokines and reduce the extent of the systemic inflammatory response when compared with the Barker's vacuum pack. The primary endpoint was the difference in the plasma concentration of the proinflammatory cytokine interleukin-6 (IL-6) among patients randomized to the ABThera versus Barker's vacuum pack. Secondary endpoints included clinical efficacy and safety outcomes, including survival and primary fascial closure rates.
METHODS
Study Design
The Intra-Peritoneal Vacuum Trial was a single-center, parallel-group RCT that intraoperatively allocated adults to the ABThera versus Barker's vacuum pack in a 1:1 ratio after abbreviated laparotomy. Trial methods were prespecified in a previously published protocol10 that was registered online at ClinicalTrials.gov (identifier NCT01355094) and approved by our local research ethics board.
Study Setting
The study was set at the Foothills Medical Centre in Calgary, Alberta, Canada. This tertiary care, level 1 trauma center provides trauma and emergency surgical services to southern Alberta, southwest British Columbia, and southeast Saskatchewan. After abbreviated laparotomy, critically ill patients are cared for in a 30-bed, closed intensive care unit (ICU).
Study Participants
Enrollment occurred in the operating room after the decision was made to perform abbreviated laparotomy.10 Abbreviated laparotomy was defined as emergent laparotomy for hemorrhage or sepsis source control, followed by TAC and planned relaparotomy. An open abdomen was defined as that requiring a TAC because of the skin and fascia not being closed after laparotomy.3 To identify eligible patients who were not recruited, research coordinators screened all ICU admissions daily. We excluded patients younger than 18 years and those who were pregnant or had received intraperitoneal chemotherapy. Delayed, informed consent was obtained from all patients.
Allocation Concealment and Randomization
To ensure allocation concealment, randomization sequences were generated by a computerized random treatment generator hosted on a dedicated trial Web site (http://peritonealvac.com). After a patient was deemed eligible, an operating room team member accessed the Web site, entered demographic information, and announced the assigned allocation. Variable block size randomization was utilized.
Study Interventions
The ABThera and Barker's vacuum pack were applied according to manufacturer's recommendations and international guidelines, respectively (see our published protocol10 for details). Although the time to reoperation and TAC dressing change was decided by attending surgeons, guidelines suggest relaparotomy between 24 and 72 hours.2 If abdominal fascial closure was not felt safe or possible at the first or subsequent reoperations, surgeons were free to change the allocated TAC to another dressing.
Peritoneal Fluid and Blood Collection and Laboratory Analyses
Methods describing the collection and analysis of peritoneal fluid and blood samples were previously reported in detail.10 Before TAC application, 4 mL of peritoneal fluid and 16 mL of blood were collected. These collections were repeated at 24 and 48 hours. Plasma and peritoneal fluid concentrations of IL-1β, -6, -8, and -12, and tumor necrosis factor–α (TNF-α) were determined using Luminex technology (Bio-Rad, EMBD Millipore, Mississauga, ON) in the Snyder Translational Laboratory in Critical Care Medicine at the University of Calgary by an investigator blinded to the treatment allocation status of the patient.
Study Endpoints
The primary endpoint was the difference in the plasma concentration of the proinflammatory cytokine IL-6 at 24 and 48 hours after TAC application between patients randomized to the ABThera versus Barker's vacuum pack in those who completed at least 24 hours of the allocated therapy. Secondary endpoints included study feasibility (number of patients enrolled/number of eligible candidates) and the differential effects of these dressings on peritoneal and plasma concentrations of IL-1β, −8, and −12, and TNF-α at 24 and 48 hours; peritoneal fluid drainage volumes; postoperative fluid balance; and sequential organ failure assessment (SOFA) scores and partial pressure of arterial oxygen/fraction of inspired oxygen (Pao2/Fio2) ratios. Secondary clinical endpoints included (1) 90-day survival; (2) 90-day primary fascial closure rate and the number of days alive without an open abdomen within 30 days of hospital admission; (3) mechanical ventilation-, ICU-, and hospital-free days within 30 days of hospital admission; and (4) risk of renal replacement therapy (RRT) and the number of days alive and free of RRT within 30 days of hospital admission. We defined primary fascial closure as a fascia-to-fascia abdominal wall closure within the index hospitalization. Safety outcomes included enterocutaneous/atmospheric fistula formation and risk of intra-abdominal hypertension/abdominal compartment syndrome.
Sample Size
As clinical data are lacking concerning the effect of negative pressure peritoneal therapy on the inflammatory response after abbreviated laparotomy, estimates were unavailable for sample size determination. We therefore obtained funding to randomize 45 patients.
Statistical Methods
We summarized data using proportions, medians with interquartile ranges (IQRs) and means (with standard deviations). These statistics were compared using Fisher's exact, Wilcoxon rank sum, and unequal variance t tests, respectively.
We used mixed-effects models with a subject-specific random intercept to compare plasma cytokine concentrations, SOFA scores, and Pao2/Fio2 ratios between baseline and 24 and 48 hours.12 Models included variables for group assignment, time postrandomization, baseline values of these measures (to adjust for any differences between groups), and an interaction between time postrandomization and group assignment. Plasma cytokine concentrations were log-transformed. As the enrollment peritoneal fluid samples were often collected after the peritoneum was irrigated with saline, only 24- and 48-hour peritoneal fluid cytokine concentrations were compared using mixed-effects models. Peritoneal fluid concentrations of IL-1β, −8, −10, −12 p70, and TNF-α were log-transformed before being entered into models.
Cox proportional hazards models were used to calculate hazard ratios (HRs) for mortality. We also used a time-dependent Cox model to determine whether baseline plasma concentrations of IL-6 predicted mortality within 72 hours. We confirmed the assumption of proportional hazards by plotting log-minus-log survival plots. We used a Fine and Gray regression model to calculate the HR for primary fascial closure between the groups while accounting for the competing risk of death.13
We conducted prespecified subgroup analyses that stratified by patient type (abdominal injury or intra-abdominal sepsis). An exploratory post hoc analysis adjusted our reported HRs for any observed imbalances between groups. Although clinical efficacy and safety data were analyzed according to intention-to-treat methods, mediator data were examined using per-protocol principles. Per-protocol was defined a priori as an allocated TAC dressing having been in place for at least 24 hours.10 All tests were 2-sided, and those with a P value of less than 0.05 were considered significant. Stata MP version 13.1 (StataCorp LP, College Station, TX) was used for statistical analyses.
RESULTS
Patients
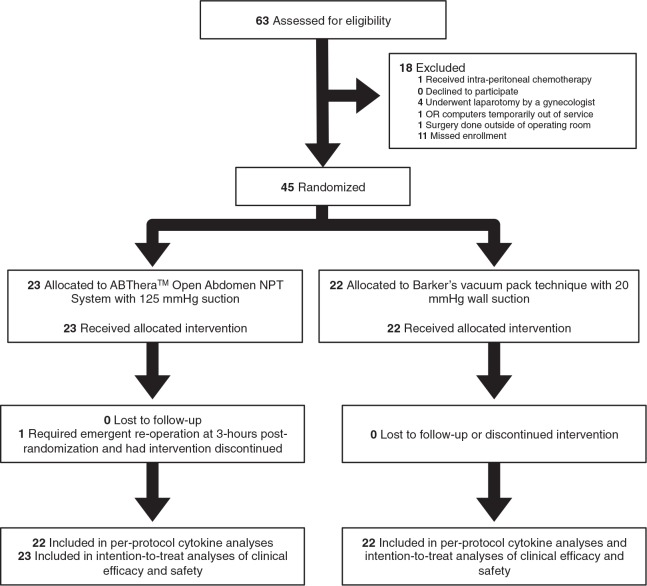
Patients were recruited between September 29, 2011 and December 9, 2012. Among 63 eligible patients, 45 (71.4%) were randomized (Fig. 1). All patients were followed for 90 days. There were no exclusions after randomization. One trauma patient underwent emergent reoperation for ongoing intra-abdominal hemorrhage 3 hours postrandomization, and his allocated ABThera dressing was exchanged for a Bogota bag. Aside from this 1 patient, the intention-to-treat and per-protocol populations were identical. There were no other protocol violations. The trial ended on January 3, 2013 when the last randomized patient died.
FIGURE 1.
CONSORT flow diagram. NPT indicates negative pressure therapy.
The baseline characteristics of patients allocated to the ABThera versus Barker's vacuum pack group are presented in Table 1. Aside from a higher Charlson Comorbidity Index (P = 0.04) in the ABThera group, there were no significant differences in baseline patient characteristics. Although the median arterial lactate concentration was 3.6 in the ABThera group versus 6.3 in the Barker's vacuum pack group, this difference did not reach statistical significance (P = 0.06). The indication for abbreviated laparotomy was abdominal injury in 46.7% of patients. Injuries were penetrating in 6 (60.0%) patients in the ABThera group versus 2 (18.2%) in the Barker's vacuum pack group (P = 0.08). Median Injury Severity and Acute Physiology and Chronic Health Evaluation-II scores at randomization were 29 and 24. The median time from TAC dressing application to first reoperation or death was 42 (IQR, 32–54) hours in the ABThera group versus 44 (IQR, 22–48) hours in the Barker's vacuum pack group (P = 0.29). With the exception of 1 patient in the Barker's vacuum pack group (who instead had an ABThera dressing applied), all patients whose fascia was not closed at reoperation had their originally allocated TAC dressing reapplied.
TABLE 1. Baseline Characteristics of the Study Patients.
| Barker's Vacuum | |||
|---|---|---|---|
| Variable | ABThera (n = 23) | Pack (n = 22) | P |
| Age, median (IQR), yr | 56 (39–71) | 56 (33–68) | 0.83 |
| Male sex, n (%) | 19 (82.6) | 18 (81.8) | >0.99 |
| Abdominal injury, n% | 10 (43.5) | 11 (50.0) | 0.77 |
| Penetrating, n/total n (%) | 6/10 (60.0) | 2/11 (18.2) | 0.08 |
| Mechanism of injury, no./total no. (%) | |||
| Motor vehicle collision | 2/10 (20.0) | 6/11 (54.5) | 0.14 |
| Pedestrian vs motor vehicle | 0/10 (0) | 1/11 (9.1) | 1.0 |
| Gunshot wound | 3/10 (30.0) | 2/11 (18.2) | 0.64 |
| Stab wound | 3/10 (30.0) | 0/11 (0) | 0.09 |
| Other | 2/10 (20.0) | 2/11 (18.2) | 1.0 |
| Injury Severity Scale score, median (IQR)* | 23 (18–34) | 34 (22–34) | 0.32 |
| Revised Trauma score, median (IQR)† | 5.4 (1.8–7.8) | 5.3 (1.0–6.4) | 0.45 |
| Abbreviated Injury Scale scoring, mean ± SD‡ | |||
| Head and neck | 4.0 ± 0 | 3.25 ± 0.5 | 0.10 |
| Thorax | 2.9 ± 1.0 | 3.7 ± 0.9 | 0.08 |
| Abdomen | 3.6 ± 0.7 | 3.1 ± 1.2 | 0.20 |
| Extremities/pelvis | 3.0 ± 0 | 2.667 ± 0.5 | 0.50 |
| APACHE-II score, mean ± SD§ | 22.5 ± 8.9 | 26.6 ± 11.9 | 0.20 |
| SOFA score, mean ± SD¶ | 7.7 ± 3.9 | 9.4 (4.7) | 0.19 |
| Charlson Comorbidity Index score, median (IQR)‖ | 3 (1–6) | 2 (0–3) | 0.04 |
| Worst physiologic measurements before randomization, median (IQR) | |||
| Systolic blood pressure, mm Hg | 90 (80–108) | 84 (60–91) | 0.27 |
| Temperature (injured patients), °C | 36 (35.8–36.3) | 35.3 (33.5–36) | 0.15 |
| Temperature (sepsis patients), °C | 36.2 (36–38.1) | 37.6 (36.6–38.6) | 0.13 |
| pH | 7.2 (7.1–7.3) | 7.2 (7.1–7.2) | 0.15 |
| Lactate, mmol/L | 3.6 (1.8–6.6) | 6.3 (2.3–10.3) | 0.06 |
| Base deficit, mmol/L | 10 (7–17) | 12 (9.5–17.5) | 0.40 |
| INR | 1.5 (1.1–1.8) | 1.5 (21.2–1.7) | 0.84 |
| Fluid administration before randomization, median (IQR) | |||
| PRBC, units (n = 21 injured patients) | 10 (3–20) | 12 (6–22) | 0.30 |
| FFP, units (n = 21 injured patients) | 2.5 (2–8) | 3 (0–6) | 0.54 |
| PRBC/FFP ratio (n = 21 injured patients) | 4:1 | 4:1 | |
| Crystalloid, L | 2.5 (1–3.3) | 3.2 (2–4.5) | 0.21 |
| Patient location before OR admission, n (%) | |||
| Emergency department | 12 (52.2) | 15 (68.2) | 0.73 |
| Hospital ward | 5 (21.7) | 2 (9.1) | 0.41 |
| Intensive care unit | 6 (26.1) | 5 (22.7) | 1.0 |
| Vasopressors required before randomization, n (%) | 16 (69.6) | 16 (76.2) | 0.44 |
| Hours from injury to laparotomy, median (IQR) | 2 (2–5) | 5 (2–7) | 0.38 |
| Hours from sepsis diagnosis to laparotomy, median (IQR) | 10 (5–12) | 10 (5–10) | 0.74 |
*Values for the Injury Severity score ranged from 0 to 75. Higher values indicate more severe injury.
†Values for the Revised Trauma score range from 0 to 7.84. Higher values indicate greater survival probability.
‡Scores on the Abbreviated Injury Scale range from 1 to 6. Higher values indicate more severe injury.
§Scores on the APACHE-II scale range from 0 to 71. Higher values indicate greater illness severity.
¶Scores on the SOFA scale range from 0 to 24. Higher values indicate greater illness severity.
‖Scores on the Charlson Comorbidity Index range from 0 to 6. Higher scores indicate a lower survival probability.
APACHE indicates Acute Physiology and Chronic Health Evaluation; FFP, fresh frozen plasma; INR, international normalized ratio; OR, operating room; PRBC, packed red blood cells; SOFA, Sequential Organ Failure Assessment.
Primary Outcome
Figure 2 summarizes plasma concentrations of IL-6 at baseline and 24 and 48 hours. Median plasma concentrations of IL-6 were significantly lower in the ABThera (637.4 pg/mL; IQR, 144.0–3123.8 pg/mL) versus Barker's vacuum pack (2388.0 pg/mL; IQR, 881.4–4820.1 pg/mL) group at randomization (P = 0.03). With regard to the primary outcome, there was no significant difference in the plasma concentrations of IL-6 at baseline versus 24 (P = 0.52) or 48 hours (P = 0.82) between groups. When stratified by indication for abbreviated laparotomy, there remained to be no significant difference in plasma IL-6 between groups (Table 2).
FIGURE 2.
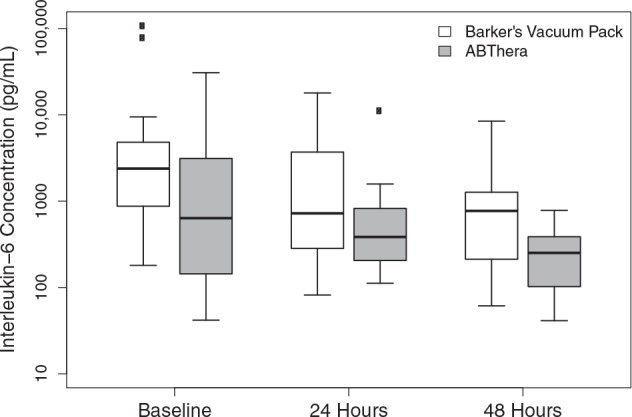
Plasma interleukin-6 concentrations after abbreviated laparotomy.
TABLE 2. Plasma Interleukin-6 Concentrations Between Baseline and 24 and 48 hours by Treatment Group Among Patients With Abdominal Injury or Intra-abdominal Sepsis.
| Patient Type | Treatment Group | Baseline (pg/mL) | 24 hr (pg/mL) | P Value for Difference at 24 hr Between Groups* | 48 hr (pg/mL) | P Value for Difference at 48 hr Between Groups* |
|---|---|---|---|---|---|---|
| Abdominal injury patients | ABThera | 679.0 (210.1–2935.96) | 573.4 (352.3–827.8) | 0.51 | 288.8 (177.6–461.6) | >0.99 |
| Barker's vacuum pack | 1854.1 (771.9–2982.1) | 430.0 (194.4–756.1) | 747.6 (212.6–797.0) | |||
| Intra-abdominal sepsis patients | ABThera | 553.9 (102.8–3123.8) | 315.5 (152.3–678.6) | 0.66 | 200.1 (51.6–339.7) | 0.89 |
| Barker's vacuum pack | 3518.0 (2254.4–5488.7) | 1430.8 (680.3–4938.5) | 813.0 (172.6–3387.5) |
Values in Table summarizing plasma cytokine concentrations represent medians (with associated interquartile ranges). Tests of hypotheses and P values were estimated using mixed-effects regression models.
*Test of hypothesis comparing whether peritoneal fluid or plasma cytokine concentrations are significantly different at 24 or 48 hr versus baseline between patients randomized to the ABThera versus Barker's vacuum pack.
Secondary Outcomes
Peritoneal Fluid Drainage and Postoperative Fluid Balance
Resuscitation volumes and fluid balances were similar between groups. Although there was no significant difference in the volume of drained peritoneal fluid during the first 24 hours of therapy, there was a significantly lower peritoneal fluid drainage from the ABThera versus Barker's vacuum pack at 48 hours after randomization (see Table in Supplemental Digital Content 1, available at http://links.lww.com/SLA/A701).
Peritoneal Fluid and Plasma Cytokine Concentrations
In both groups, peritoneal fluid concentrations of IL-6 and IL-8 were substantially higher than other cytokines at all time points (see Table in Supplemental Digital Content 2, available at http://links.lww.com/SLA/A702). There was no significant difference in the peritoneal fluid or plasma concentrations of IL-6, -1β, -8, -10, or -12 p70 or TNF-α between 24 and 48 hours among patients randomized to the ABThera versus Barker's vacuum pack (see Tables in Supplemental Digital Content 2, available at http://links.lww.com/SLA/A702, and Supplemental Digital Content 3, available at http://links.lww.com/SLA/A703). There was, however, an increased clearance of plasma IL-12 p70 among the subgroup of patients with abdominal injury at 48 hours who were fitted with the ABThera (P = 0.02; see Table in Supplemental Digital Content 4, available at http://links.lww.com/SLA/A704).
SOFA Scores and Pao2/Fio2 Ratios
There was no significant difference in SOFA scores between baseline and 24 (P = 0.78) or 48 hours (P = 0.71) between patients randomized to the ABThera versus Barker's vacuum pack. There was also no difference between groups in Pao2/Fio2 ratios at baseline versus 24 (P = 0.20) or 48 hours (P = 0.09).
Clinical Efficacy and Safety
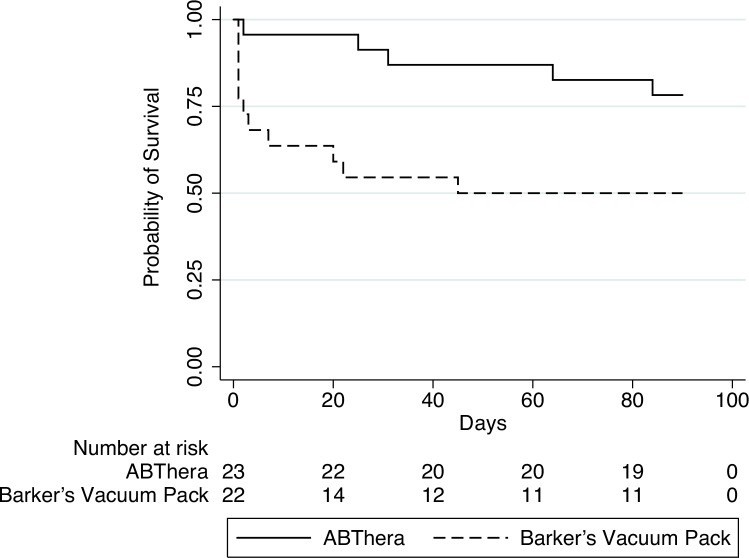
Intention-to-treat analysis revealed a 90-day mortality of 21.7% in the ABThera group versus 50.0% in the Barker's vacuum pack group [HR, 0.32; 95% confidence interval (CI), 0.11–0.93; P = 0.04]. Compared with the more gradual decline in survival probability in the ABThera group, the probability of survival decreased rapidly in the first 5 days among those treated with the Barker's vacuum pack (Fig. 3). Of the 8 deaths that occurred during this early time period (7 of which were in the Barker's vacuum pack group), half were secondary to persistent hemorrhagic shock whereas the other half were due to severe sepsis/septic shock with or without multiple organ dysfunction syndrome. The HR for 90-day mortality among the subgroup of patients with abdominal injury was 0.14 (95% CI, 0.02–1.14; P = 0.07) whereas it was 0.53 (95% CI, 0.14–1.98; P = 0.34) among those with intra-abdominal sepsis. These subgroup estimates were not significantly different (P = 0.24).
FIGURE 3.
Survival probability according to treatment group.
Prespecified efficacy and safety outcomes (compared using intention-to-treat principles) are summarized in Table 3. The median number of days alive and without mechanical ventilation in the first 30 days of randomization was 27 (IQR, 0–28) in the ABThera group versus 18 (IQR, 0–28) in the Barker's vacuum pack group (P = 0.08). There was insufficient evidence to support a difference in the median number of ICU- or hospital free days between groups. Finally, although there was no difference in the risk of RRT [risk ratio (RR), 0.99; 95% CI, 0.49–2.00; P > 0.99)] in the first 30 days after randomization between the groups, the median number of days alive and without RRT during this period was 30 (IQR, 19–30) in the ABThera group versus 24 (IQR, 1–30) in the Barker's vacuum pack group (P = 0.05).
TABLE 3. Prespecified Secondary Efficacy and Safety Outcomes by Treatment Group.
| Median (IQR) | |||
|---|---|---|---|
| Variable | ABThera (n = 23) | Barker's Vacuum Pack (n = 22) | P |
| Efficacy outcomes | |||
| Number of days alive and without the following condition or treatment within the first 30 d | |||
| Open abdomen | 27 (0–28) | 18 (0–28) | 0.08 |
| Mechanical ventilation | 18 (12–25) | 12 (0–26) | 0.12 |
| Renal replacement therapy | 30 (19–30) | 24 (1–30) | 0.05 |
| ICU stay | 17 (11–23) | 9 (0–23) | 0.10 |
| Hospital stay | 0 (0–6) | 0 (0–11) | 0.43 |
| Safety outcomes | |||
| Enterocutaneous or enteroatmospheric fistula, n (%) | 2 (8.7) | 1 (4.6) | 0.52 |
| Intra-abdominal pressure, mm Hg, median (IQR) | |||
| 0–24 hr (n = 33) | 16 (11–19) | 14 (11–17) | |
| 24–48 hr (n = 35) | 16 (12–19) | 16 (12–18) | |
| 48–72 hr (n = 8) | 15 (11–21) | 19 (18–20) | |
| Grade III/IV intra-abdominal hypertension, n (%)* | |||
| 0–24 hr | 1/20 (5.0) | 0/13 (0) | >0.99 |
| 24–48 hr | 2/19 (10.5) | 1/16 (6.7) | >0.99 |
| 48–72 hr | 1/3 (33.3) | 1/5 (20.0) | >0.99 |
| Recurrent abdominal compartment syndrome, n (%)† | 0 (0) | 0 (0) | NA |
*Defined as a mean daily value of intra-abdominal pressure of 21 mm Hg or more.
†Defined as an intra-abdominal pressure of more than 20 mm Hg with new onset organ failure in a patient with an open abdominal wound.
NA indicates not applicable.
The median number of days alive and without an open abdomen was 27 (IQR, 0–28) in the ABThera group versus 18 (IQR, 0–28) in the Barker's vacuum pack group (P = 0.08). The cumulative incidence of primary fascial closure at 90 days was similar between groups (HR, 1.6; 95% CI, 0.82–3.0; P = 0.17) (Fig. 4) and did not differ when stratified by whether patients underwent abbreviated laparotomy for abdominal injury or intra-abdominal sepsis (P = 0.68). The risk of development of enterocutaneous/atmospheric fistulae was similar between the groups. There was also no difference in intra-abdominal pressures or the development of severe (grade III or IV) intra-abdominal hypertension between groups. No patient developed abdominal compartment syndrome.
FIGURE 4.
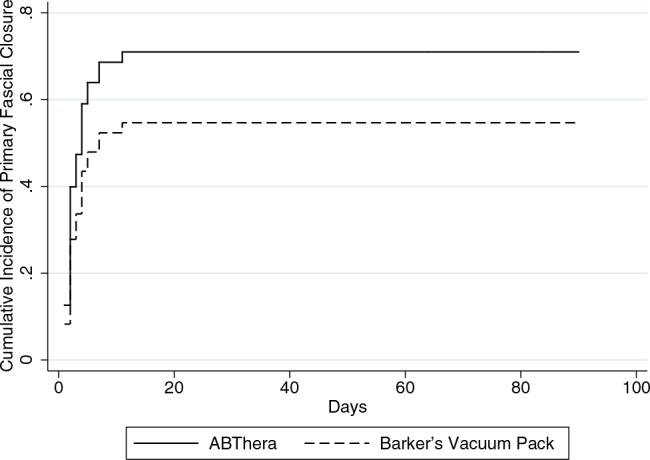
Cumulative incidence of primary fascial closure according to treatment group.
Exploratory Analyses
Median plasma concentrations of IL-6 at baseline were significantly higher among nonsurvivors (2263.3 pg/mL; IQR, 1615.5–3776.6 pg/mL) than among survivors (679.0 pg/mL; IQR, 210.1–3123.8 pg/mL) (<0.001). Using a time-dependent Cox proportional hazards model, each measure of IL-6 (baseline, 24, and 48 hours) was used to assess the relationship between IL-6 and mortality in the subsequent time periods up to 72 hours. In this time-dependent model with each 1 unit increase in plasma IL-6, there was an associated 1.61 (95% CI, 1.15–2.24) times increase in the hazard of mortality (P = 0.005) in the following time period. Although this association between baseline IL-6 and mortality was maintained after adding treatment group into the model (HR, 1.45; 95% CI, 1.03–2.05, P = 0.033), the effect of the ABThera on survival was nonsignificant after accounting for the time-dependent effects of IL-6 (HR, 0.65; 95% CI, 0.05–1.29; P = 0.10). In a secondary exploratory analysis, the estimated HR for mortality was similar after adjusting for baseline differences in Charlson Comorbidity Index and arterial lactate concentrations (HR, 0.23; 95% CI, 0.07–0.72; P = 0.01).
DISCUSSION
In this RCT, we observed a large survival difference and improved RRT free days among patients randomized to the ABThera versus Barker's vacuum pack. However, in contrast to present theory (but similar to the findings of a recent multicenter prospective cohort study), when compared with the Barker's vacuum pack dressing, we observed reduced peritoneal fluid drainage with the ABThera and a similar primary fascial closure rate between the groups. We also found no significant difference in the systemic concentration of IL-6 or other proinflammatory cytokines at baseline versus 24 or 48 hours between the groups.
After severe abdominal injury or intra-abdominal sepsis, the damaged gut has been reported to be a source for proinflammatory cytokines that drive the systemic inflammatory response and the production of multiple organ dysfunction syndrome.14–16 Visceral ischemia-reperfusion injury after hemorrhage or sepsis upregulates expression of both proinflammatory (eg, IL-6 and TNF-α) and anti-inflammatory (eg, IL-10) cytokines in the bowel.17–22 These mediators are released into peritoneal fluid before being taken up into mesenteric lymph and plasma.21,23 Cytokine-rich mesenteric lymph primes neutrophils and may induce distant organ injury.24,25 As such, several animal and human studies have reported that peritoneal and systemic proinflammatory cytokine concentrations after abdominal injury or intra-abdominal sepsis differentiate between survivors and nonsurvivors of these conditions.26 Moreover, it has been consistently demonstrated that IL-6 concentrations after injury or sepsis correlate with clinical outcomes.27,28
Animal data suggest that TAC devices that employ negative pressure to the peritoneal cavity may decrease systemic inflammation and prevent multiorgan dysfunction.8,29 Although survival was improved with the ABThera in this trial, in our prespecified analysis this did not seem to be mediated by an improvement in peritoneal fluid drainage or reduced markers of systemic inflammation. However, these analyses relate only to those who survived greater than 24 hours, and patients who died exhibited significantly higher concentrations of IL-6 than those who survived. Furthermore, levels of IL-6 at multiple time points after randomization were independently associated with reduced survival, and the effect of the ABThera on improved survival became nonsignificant when the time-dependent effects of IL-6 on outcome were adjusted for. Thus, it may be possible that the ABThera mediated an accelerated clearance of cytokines from the systemic circulation during the first 24 hours (and not at later time points), but that we did not observe this as we did not collect blood during this period. However, even if this did occur, we are unsure whether accelerated removal of peritoneal cytokines with the ABThera during only the early postoperative period could have led to improved survival among injured patients with persistent, severe intra-abdominal hemorrhage. Finally, although IL-6 was chosen as the primary endpoint given its persistent correlation with adverse outcomes in surgical patients,27 it may be possible that the ABThera has little influence on single mediators after abbreviated laparotomy, but that it instead influences the proteome of proinflammatory and anti-inflammatory mediators in the postoperative period. Future work will therefore examine mediator behavior using multidimensional analyses (eg, latent class, discriminant, and/or Dynamic Bayesian Network inference analysis).10,29
Very few studies yet exist to support our finding of improved survival with the ABThera. A systematic review and meta-analysis of largely uncontrolled cohort studies suggested that vacuum-assisted closure devices may be associated with improved outcomes among patients requiring abbreviated laparotomy.30 However, another systematic review reported in 2012 found only 11 comparative studies (including 2 RCTs and 9 cohort studies) examining the effectiveness and safety of negative pressure peritoneal therapy dressings versus alternate TAC methods in predominantly mixed populations of trauma and non–trauma patients.9,31 Only 1 RCT included in this systematic review compared negative pressure peritoneal therapy with an alternate TAC technique,32 and this RCT observed no difference in outcomes. Since then, a prospective multicenter cohort study reported the ABThera to be associated with an improved survival and abdominal fascial closure rates when compared with the Barker's vacuum pack technique.11
Although our findings may agree with those of the aforementioned cohort study, they must be interpreted with caution given the potential limitations of this RCT. Because of ethical concerns, the allocated TAC was required to be utilized for only 24 hours in per-protocol analyses. Although longer application may have potentially revealed greater mediator clearance, therapies were applied for median times of 42 and 44 hours in the ABThera and Barkers vacuum pack groups, respectively. Thus, we expected to observe a treatment signal as these times were both longer than the therapeutic application period in the previous animal study of these treatments.8 It might also be considered that our finding of improved survival may be due to covariate imbalance at baseline given the relatively small sample size of the study. However, this finding was robust to sensitivity analyses adjusting for differences in Charlson Comorbidity Index and arterial lactate between the groups. Furthermore, although the recruited patients represented a heterogeneous group of those with abdominal injury and intra-abdominal sepsis, the magnitude and characteristics of their peritoneal and systemic inflammatory responses were similar. This patient sample also aligns with those recruited into previously conducted TAC studies9 and managed by general/trauma surgeons. Most importantly, however, as we were unable to confirm the suggested mechanism by which the ABThera may lead to improved outcomes, and because our findings do not seem to be the result of confounding, they may potentially represent a type I error and therefore must be confirmed by future, multicenter trials. As the majority of eligible patients with abdominal injury and intra-abdominal sepsis were randomized in this study, recruitment of patients into these trials is likely to be feasible.
CONCLUSIONS
This trial observed a survival difference between patients randomized to the ABThera versus Barker's vacuum pack that did not seem to be mediated by improved peritoneal fluid drainage, fascial closure rates, or increased clearance of well-known mediators of systemic inflammation. As our findings could potentially be the result of a type I error, further work is required to explain the potential mechanisms of improved outcomes and confirm our findings before they are used to inform surgical practice. These trials should likely be separately done among patients with abdominal injury or intra-abdominal sepsis given that their recruitment and randomization seem to be safe and feasible.
Supplementary Material
ACKNOWLEDGMENTS
Dr Kirkpatrick has received a NannoMaxx ultrasound machine from the Sonosite Corporation for unrestricted point of care ultrasound research and has received travel assistance to attend cadaver labs from the Syntheses, Lifecell, and Innovative Trauma Care Inc.
Drs Kirkpatrick and Roberts had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. The authors acknowledge Caroline Leger and Mandy Tse of the Snyder Research Laboratory for assistance with study development and laboratory analysis. The investigators thank the attending surgeons, and house, operating room, and critical care staff for assisting with the conduct of the study, including Drs Ian Anderson, Donald Buie, Elijah Dixon, Michael Dunham, John Kortbeek, Rohan Lall, Lloyd Mack, Anthony MacLean, Daphne Mew, Janice Pasieka, May Lynn Quan, Bruce Rothwell, Francis Sutherland, Walley Temple, and Jeff Way. The investigators also acknowledge the assistance of Anita Verdonk for collecting background information; Stacy Ruddel and Christine Skinner, who screened patients and recorded data; and Jean-Francois Ouellet, MD, for study conceptualization.
Andrew W. Kirkpatrick conceptualized the study, conducted the literature search, created the study design, published the methodology, devised the analytic method, submitted the ethics request, obtained funding, recruited patients, collected data, analyzed the data, drafted the manuscript, revised the manuscript, and approved the final manuscript for publication. Derek J. Roberts conducted the literature search, created the study design, published the methodology, devised the analytic method, analyzed the data, drafted the manuscript, revised the manuscript, and approved the final manuscript for publication. Peter D. Faris created the study design, published the methodology, devised the analytic method, analyzed the data, revised the manuscript, and approved the final manuscript for publication. Craig N. Jenne created the study design, published the methodology, analyzed the data, revised the manuscript, and approved the final manuscript for publication. Chad G. Ball conceptualized the study, published the methodology, recruited patients, collected data, drafted the manuscript, revised the manuscript, and approved the final manuscript for publication. Corina Tiruta conducted the literature search, created the study design, published the methodology, collected data, analyzed the data, revised the manuscript, and approved the final manuscript for publication. Zhengwhen Xiao conducted the literature search, collected data, analyzed the data, revised the manuscript, and approved the final manuscript for publication. Jessalyn K Holodinsky analyzed the data, revised the manuscript, and approved the final manuscript for publication. Paul B. McBeth conceptualized the study, conducted the literature search, created the study design, revised the manuscript, and approved the final manuscript for publication. Christopher J. Doig conceptualized the study, analyzed the data, revised the manuscript, and approved the final manuscript for publication. Paul Kubes conceptualized the study, analyzed the data, revised the manuscript, and approved the final manuscript for publication.
Dr Roberts is supported by an Alberta Innovates—Health Solutions Clinician Fellowship Award, a Knowledge Translation (KT) Canada Strategic Training in Health Research Fellowship, a KT Canada Research Stipend, and funding from the Canadian Institutes of Health Research.
Footnotes
Disclosure: This study was funded through an investigator-initiated grant from Kinetic Concepts Incorporated (the manufacturer of the ABThera) and a research award from the Calgary Surgical Research Development Fund, Department of Surgery, University of Calgary. The investigators had complete control and the only access to the data at all phases of the study. The sponsor had no role at any phase in the design, conduct, analysis, or decision to publish the results, which are the sole privilege of the investigators. Dr Ball has received travel assistance to attend cadaver labs from Innovative Trauma Care Inc. The remaining authors have no conflicts of interest to declare.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Campbell A, Chang M, Fabian T, et al. Management of the open abdomen: from initial operation to definitive closure. Am Surg. 2009;75(11 suppl):S1–S22. [PubMed] [Google Scholar]
- 2.Diaz JJ, Jr, Cullinane DC, Dutton WD, et al. The management of the open abdomen in trauma and emergency general surgery: part 1-damage control. J Trauma. 2010;68:1425–1438. [DOI] [PubMed] [Google Scholar]
- 3.Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godat L, Kobayashi L, Costantini T, et al. Abdominal damage control surgery and reconstruction: world society of emergency surgery position paper. World J Emerg Surg. 2013;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotondo MF, Zonies DH. The damage control sequence and underlying logic. Surg Clin N Amer. 1997;77:761–777. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JW, Gracias VH, Schwab CW, et al. Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma. 2001;51:261–271. [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick AW, Laupland KB, Karmali S, et al. Spill your guts! Perceptions of Trauma Association of Canada member surgeons regarding the open abdomen and the abdominal compartment syndrome. J Trauma. 2006;60:279–286. [DOI] [PubMed] [Google Scholar]
- 8.Kubiak BD, Albert SP, Gatto LA, et al. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock. 2010;34:525–534. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DJ, Zygun DA, Grendar J, et al. Negative-pressure wound therapy for critically ill adults with open abdominal wounds: a systematic review. J Trauma Acute Care Surg. 2012;73:629–639. [DOI] [PubMed] [Google Scholar]
- 10.Roberts DJ, Jenne CN, Ball CG, et al. Efficacy and safety of active negative pressure peritoneal therapy for reducing the systemic inflammatory response after damage control laparotomy (the Intra-peritoneal Vacuum Trial): study protocol for a randomized controlled trial. Trials. 2013;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheatham ML, Demetriades D, Fabian TC, et al. Prospective study examining clinical outcomes associated with a negative pressure wound therapy system and Barker's vacuum packing technique. World J Surg. 2013;37:2018–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G, Gould AL. Comparison of alternative strategies for analysis of longitudinal trials with dropouts. J Biopharm Stat. 2002;12:207–226. [DOI] [PubMed] [Google Scholar]
- 13.Fine J, Gray R. A proportional hazards model for the sub-distribution of a competing task. J Am Stat Assoc. 1999;94:496–497. [Google Scholar]
- 14.Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction, syndrome. Crit Care Med. 2001;29:S99–S106. [DOI] [PubMed] [Google Scholar]
- 15.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177–196. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J, Wei Z, Liu X, et al. The role of intestinal mucosa injury induced by intra-abdominal hypertension in the development of abdominal compartment syndrome and multiple organ dysfunction syndrome. Crit Care. 2013;17:R283. 10.1186/cc13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rongione AJ, Kusske AM, Ashley SW, Reber HA, McFadden DW. Interleukin-10 prevents early cytokine release in severe intraabdominal infection and sepsis. J Surg Res. 1997;70:107–112. [DOI] [PubMed] [Google Scholar]
- 18.Yao YM, Redl H, Bahrami S, Schlag G. The inflammatory basis of trauma/shock-associated multiple organ failure. Inflamm Res. 1998;47:201–210. [DOI] [PubMed] [Google Scholar]
- 19.Wortel CH, van Deventer SJ, Aarden LA, et al. Interleukin-6 mediates host defense responses induced by abdominal surgery. Surgery. 1993;114:564–570. [PubMed] [Google Scholar]
- 20.Scheingraber S, Bauerfeind F, Bohme J, Dralle H. Limits of peritoneal cytokine measurements during abdominal lavage treatment for intraabdominal sepsis. Am J Surg. 2001;181:301–308. [DOI] [PubMed] [Google Scholar]
- 21.Shah SK, Jimenez F, Walker PA, et al. Peritoneal fluid: a potential mechanism of systemic neutrophil priming in experimental intra-abdominal sepsis. Am J Surg. 2012;203:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartelli M, Catena F, Di Saverio S, et al. Current concept of abdominal sepsis: WSES position paper. World J Emerg Surg. 2014;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SK, Jimenez F, Letourneau PA, et al. Strategies for modulating the inflammatory response after decompression from abdominal compartment syndrome. Scand J Trauma Resusc Emerg Med. 2012;20:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavriani G, Domingos HV, Soares AL, et al. Lymphatic system as a path underlying the spread of lung and gut injury after intestinal ischemia/reperfusion in rats. Shock. 2005;23:330–336. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Wu Q, Li Q, et al. Mesenteric lymphatic ducts ligation decreases the degree of gut-induced lung injury in a portal vein occlusion and reperfusion canine model. J Surg Res. 2009;154:45–50. [DOI] [PubMed] [Google Scholar]
- 26.Hendriks T, Bleichrodt RP, Lomme RM, De Man BM, van Goor H, Buyne OR. Peritoneal cytokines predict mortality after surgical treatment of secondary peritonitis in the rat. J Am Coll Surg. 2010;211:263–270. [DOI] [PubMed] [Google Scholar]
- 27.Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Analytic review: interleukin-6 in surgery, trauma, and critical care: part I: basic science. J Intensive Care Med. 2011;26:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011;26:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emr B, Sadowsky D, Azhar N, et al. Removal of inflammatory ascites is associated with dynamic modification of local and systemic inflammation along with prevention of acute lung injury: in vivo and in silico studies. Shock. 2014;41:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quyn AJ, Johnston C, Hall D, et al. The open abdomen and temporary abdominal closure systems—historical evolution and systematic review. Colorectal Dis. 2012;14:e429–e438. [DOI] [PubMed] [Google Scholar]
- 31.Henteleff HJ, Parry NG, Burlew CC. What is the comparative efficacy of negative-pressure wound therapy vs alternate temporary abdominal closure techniques in open abdominal wounds? J Am Coll Surg. 2014;218:1251–1253. [DOI] [PubMed] [Google Scholar]
- 32.Bee TK, Croce MA, Magnotti LJ, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma. 2008;65:337–342; discussion 342–334. [DOI] [PubMed] [Google Scholar]