Abstract
Setting:
In August 2009, a digital chest X-ray (CXR) machine was installed at a busy urban health centre in Lusaka, Zambia.
Objective:
To describe the changes in tuberculosis (TB) notifications and treatment delay ≥7 days in Zambia after introducing a digital X-ray service.
Design:
Operational retrospective research of TB notification, laboratory and CXR data for Q4 2008 (prior to digital CXR) compared to Q4 2009.
Results:
Notifications for sputum smear-negative TB increased by 8.1%, from 370/527 (70.2%) in Q4 2008 to 425/544 (78.1%) in Q4 2009, despite a 6.7% decrease in sputum smear positivity in Q4 2009. TB treatment delay decreased from 75/412 (18.2%) in Q4 2008 to 52/394 (13.2%) in Q4 2009 (P = 0.05).
Conclusion:
In Q4 2009, sputum smear-negative TB notifications increased and treatment delay decreased. However, accurate diagnosis of TB is challenging in this setting, and misdiagnosis and overtreatment may occur. Moreover, other factors in addition to the introduction of the digital X-ray service could have contributed to these findings. Nonetheless, we found that the digital X-ray service had many advantages and that it may aid in more efficient TB diagnosis.
Keywords: tuberculosis, Zambia, digital X-ray, treatment delay
Abstract
Contexte:
Un appareil de radiographie digitale du thorax (CXR) a été installé dans un centre de santé urbain très actif à Lusaka, Zambie, en août 2009.
Objectif:
Décrire des modifications des déclarations de tuberculose (TB) et de délais de traitement de ≥7 jours en Zambie, au moment de l’introduction d’un service de CXR digitale.
Schéma:
Recherche opérationnelle rétrospective sur les déclarations de TB et les données de laboratoire et de CXR pour le 4ème trimestre 2008 (avant l’introduction de la CXR digitale) par comparaison avec le 4ème trimestre 2009.
Résultats:
On a noté une augmentation de 8,1% des déclarations de TB à frottis négatifs des crachats en passant de 370/527 (70,2%) au 4ème trimestre 2008 à 425/544 (78,1%) au 4ème trimestre 2009, en dépit d’une décroissance de 6,7% des cas à frottis positifs des crachats au cours du 4ème trimestre 2009. Les délais de traitement de la TB ont diminué de 75/412 (18,2%) au 4ème trimestre 2008 à 52/394 (13,2%) au 4ème trimestre 2009 (P = 0,05).
Conclusion:
Au 4ème trimestre 2009, les déclarations de TB à frottis négatifs ont augmenté et le délai du traitement a diminué. Toutefois, un diagnostic précis de la TB constitue un défi dans ce contexte ; pour cette raison, des erreurs de diagnostic et des abus de traitement pourraient survenir. De plus, d’autres facteurs à côté de l’introduction d’un service de CXR digitale pourraient avoir contribué à nos résultats. Néanmoins, nous avons considéré que le service de CXR digitale a beaucoup d’avantages et pourrait aider à un diagnostic plus efficient de la TB.
Abstract
Marco de referencia:
En agosto del 2009 se puso en marcha un sistema de radiografía de tórax (CXR) digital en un centro de salud urbano muy activo en Lusaka, Zambia.
Objetivo:
Describir las modificaciones en la notificación de la tuberculosis (TB) y el retraso del tratamiento ≥ 7 días, que ocurrieron al introducir el servicio de CXR digital en Zambia.
Método:
Estudio retrospectivo de investigación operativa sobre la notificación de la TB y los datos de laboratorio y de CXR del cuarto trimestre (Q4) del 2008 (antes de la introducción de la CXR digital), comparados con los datos del Q4 del 2009.
Resultados:
Se observó un aumento de 8,1% en la notificación de casos de TB con baciloscopia negativa: de 370 en 527 casos notificados en el Q4 el 2008 (70,2%), se pasó a 425 de los 544 casos registrados en el Q4 del 2009 (78,1%), pese a una disminución de 6,7% de la positividad de la baciloscopia del esputo en el trimestre estudiado del 2009. Se encontró una disminución del retraso del tratamiento antituberculoso de 75/412 casos notificados (18,2 %) en el Q4 en el 2008 hasta 52/394 casos (13,2%) en el Q4 del 2009 (P = 0,05).
Conclusión:
En el Q4 del 2009, aumentaron las notificaciones de casos de TB con baciloscopia negativa del esputo y disminuyó el retraso en el comienzo del tratamiento. Sin embargo, el diagnóstico preciso de TB es difícil en este entorno, por lo cual pueden ocurrir errores diagnósticos y tratamientos en exceso. Además, es posible que otros factores diferentes de la introducción del servicio de CXR digital hayan contribuido a estos resultados. No obstante, se observó que el servicio de CXR digital ofrecía muchas ventajas y podría favorecer un diagnóstico más eficaz de la TB.
Zambia is a country with a high burden of tuberculosis (TB) and human immunodeficiency virus (HIV), with HIV prevalence among adults estimated at 13.5% and TB notifications estimated at 309 per 100 000 population.1 There is therefore an urgent need for interventions aimed at ensuring early TB detection and effective diagnosis and treatment. In efforts to provide more efficient diagnosis of TB in Zambia, the Zambia AIDS-related Tuberculosis Project (ZAMBART) partnered with Delft Diagnostic Systems, the Lung Institute University of Cape Town and the Image Science Institute University of Utrecht in a project seeking to develop a computer-aided diagnostic (CAD) tool for reading chest X-rays (CXRs). In August 2009, a digital CXR service was placed at a busy health centre in Lusaka, Zambia, as a diagnostic aid.
TB diagnosis in resource-constrained settings can be difficult, and diagnostic delay, whether patient- or health provider-related, leads to increased spread of TB within the community.2–5 Although the gold standard for TB diagnosis is culture, in Zambia TB diagnosis is largely dependent on sputum smear microscopy, as it is rapid, simple and cheap; however, it has modest sensitivity for TB diagnosis, particularly in HIV-infected individuals.6–10 A systematic review by the World Health Organization (WHO) found that sensitivity for smear microscopy ranged between 50% and 72%, depending on the approach and the population.11 As smear microscopy is the mainstay of diagnosis in resource-constrained settings such as Zambia, various management algorithms have been proposed to optimise the number of patients correctly treated for smear-negative TB while minimising overtreatment of patients who do not have the disease.
The WHO has developed an algorithm to expedite the diagnosis of smear-negative pulmonary TB in resource-constrained settings with high HIV prevalence.12 The algorithm includes performing a clinical history and physical examination, HIV testing, sputum smear microscopy and, if negative, CXR and culture. Patients suspected of having TB after these investigations (e.g., compatible radiograph plus symptoms) should be treated for TB.
The objective of our study was to determine if provision of on-the-spot digital CXR improved TB diagnosis and reduced treatment delay. We also wanted to determine whether the increased accessibility of the digital CXR service led to over-reliance on CXR and abandonment of sputum smear microscopy. The questions we sought to answer in this study were as follows: 1) Does the presence of a digital CXR service increase the percentage of sputum smear-negatives and/or smears not done? 2) Does the presence of a digital CXR service within the health centre increase the number of patients being referred for CXR? and 3) Does the digital CXR service reduce the time from first evaluation for TB to initiation of treatment?
METHODS
This was an operational retrospective review conducted using routine TB notification data from a Lusaka urban health centre for Q4 2008 before the introduction of a digital CXR service, and for Q4 2009, after the introduction of the digital service. The digital CXR machine is an Odelca-DR machine supplied by Delft; it is housed in a 20-foot shipping container that weighs 5 tons and holds a computer and its own energy supply.
Study setting and population
The urban health centre serves a population of 146 000 and notifies 2000 TB cases per year, with HIV co-infection rates of approximately 70%. In 2008 and 2009, patients who presented to the clinic with a cough of >3 weeks were considered TB suspects and were sent by the clinician to an onsite laboratory to submit a spot sputum sample. At the laboratory, they were given two more sputum containers and asked to return with additional sputum samples the next day. CXR was requested for sputum smear-negative patients or if sputum smear results were unavailable on the second day of evaluation. All patients were offered HIV counselling and testing.
Laboratory quality assessment was performed on a quarterly basis on approximately 25 randomly selected sputum smears, which were read by an independent reader off site. The number of classification errors was calculated and reported as percentage of agreement. The microscopic preparation was evaluated for evenness, size, thickness, staining, cleanliness and specimen quality.
Study enrolment
Patients were included in the study if they were newly notified to the urban health centre during Q4 2008 or 2009, and were recorded in both a TB and a laboratory register. Patients were assigned a TB case identification number, which was recorded in the registry along with their date of presentation, demographic data, address, patient type (new, relapse, transferred in, treatment resumed, other), smear microscopy results, CXR results, TB type (pulmonary and/or extra-pulmonary), HIV status, if anti-tuberculosis treatment was started and if so, then the date of initiation, TB treatment regimen and outcome of treatment (cure, failure, treatment complete, died, transferred out, lost to follow-up). The date of specimen submission, demographic data, address, visual appearance of sputum and smear microscopy results were entered in the laboratory register. The sputum smear microscopy results were available within 24 h. In 2008, if the first sputum smear was negative or not performed, the patient was referred to a tertiary referral centre for a CXR, and the film may or may not have been available for review by the clinician at the second or third clinic visit. In 2009, if the first sputum smear was negative or not performed, the patient signed a consent form to allow digital CXR to be used for research purposes and then underwent digital CXR on the first or second day of evaluation. The digital CXR was available immediately for review by the clinician; however, treatment was initiated only when sputum smear results also became available. In both years, there were occasions when sputum smear-positive patients also underwent CXR. Data from the TB and laboratory registries were captured and double-entered into a Sequel Server database (Microsoft, Redwoods, WA, USA).
Data analysis
Specimen dates, treatment dates and outcome data were obtained from the TB and laboratory registers. The TB and laboratory registers were merged and analysed using Stata, version 11 (StataCorp, College Station, TX, USA). Variables analysed included baseline demographics, HIV status, smear status, TB notifications and treatment delay. Measurement of treatment delay was reported as days from date of first sputum specimen submission in the laboratory to treatment initiation; ≥7 days was arbitrarily chosen to represent a delay in treatment commencement, based on the accepted practice of expedited commencement of anti-tuberculosis treatment in high HIV prevalence settings. Categorical variables were created within Stata for treatment delay (time ≥7 days). The differences in categorical variables were calculated using χ2 tests. Odds ratios (ORs) of differences between categorical variables were calculated. P = 0.05 was considered statistically significant. Logistic regression analysis was performed to determine factors (or characteristics) associated with treatment delay. Significantly associated factors (P = 0.05) identified through univariable analysis were then introduced into a multivariable model and retained in the final model if P < 0.1 on the likelihood-ratio test.
Ethics
This study was approved by the University of Zambia research ethics committee.
RESULTS
In Q4 2008 and in Q4 2009, respectively 634 and 663 new TB patients were registered. Baseline demographics show a median age of 31 in both years. Over 55% of the newly registered TB patients were men in both years. Of the sputum smear-negative patients who underwent HIV testing, respectively 233/283 (82.3%) and 308/380 (81.1%) were HIV-infected in 2008 and 2009. The percentage of CXRs performed among smear-negative patients was 99% in both years. The proportion of new smear-negative TB notifications increased by 8.1% in 2009 (P = 0.003); however, positive sputum smears decreased by 6.7% in 2009. Overall treatment outcomes for TB improved in 2009 (Table 1).
TABLE 1.
Patient characteristics, including HIV status, sputum smear results and treatment outcomes
| Q4 2008 (n = 634) n (%) | Q4 2009 (n = 663) n (%) | |
| Median age, years | 31 | 31 |
| Sex | ||
| Male | 361 (56.9) | 369 (55.7) |
| Female | 273 (43.1) | 291 (43.9) |
| Unknown | 0 | 3 (0.4) |
| HIV status | ||
| HIV-infected | 351 (55.4) | 437 (65.9) |
| Non-HIV-infected | 122 (19.2) | 163 (24.6) |
| Unknown | 161 (25.4) | 63 (9.5) |
| Smear status | ||
| Positive | 157 (24.7) | 119 (18.0) |
| Negative | 370 (58.4) | 425 (64.0) |
| Unknown | 107 (16.9) | 119 (18.0) |
| Chest X-ray | ||
| Performed | 529 (83.4) | 591 (89.1) |
| Not performed | 105 (16.6) | 72 (10.9) |
| Disease type | ||
| Pulmonary | 560 (88.3) | 571 (86.1) |
| Extra-pulmonary | 71 (11.2) | 90 (13.6) |
| Unknown | 3 (0.5) | 2 (0.3) |
| Treatment outcome | ||
| Treatment success* | 498 (78.5) | 595 (89.7) |
| Failure | 2 (0.3) | 0 |
| Died | 30 (4.7) | 15 (2.3) |
| Default | 3 (0.5) | 1 (0.2) |
| Transfer out | 46 (7.3) | 38 (5.7) |
| Unknown | 55 (8.7) | 13 (2.1) |
Includes those who achieved cure, as evidenced by sputum smear status changing from positive to negative, as well as those who completed treatment.
HIV = human immunodeficiency virus; Q = quarter.
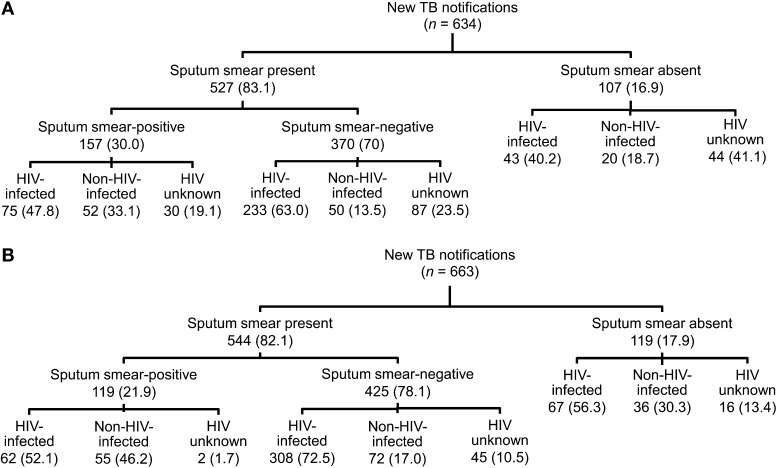
Of the 634 newly diagnosed patients in 2008, 527 (83.1%) had at least one smear recorded in the laboratory registry system (Figure 1); 370/527 (70.2%) TB suspects had a negative sputum smear result and 157 (29.8%) were sputum smear-positive. The exact number of sputum sample submissions was available for 289/370 (78.1%) smear-negative patients; 262/289 (90.7%) submitted two sputum samples and 113/289 (39.1%) submitted three. Of the 529 patients who underwent CXR, 428 (80.9%) still had a sputum smear registered in the laboratory.
FIGURE 1.
Patient enrolment: A) Q4 2008; B) Q4 2009. TB = tuberculosis; HIV = human immunodeficiency virus.
Of the 663 newly registered patients in 2009, 544 (82.1%) had at least one smear recorded in the laboratory registry system (Figure 1); 425/544 (78.1%) TB suspects were sputum smear-negative and 119 (21.9%) were positive. The exact number of sputum sample submissions was available for 321/425 (75.5%) smear-negative patients; 301/321 (93.8%) submitted two samples and 214/321 (66.7%) submitted three. Of the 591 patients who underwent CXR, 478 (80.9%) still had a sputum smear registered in the laboratory.
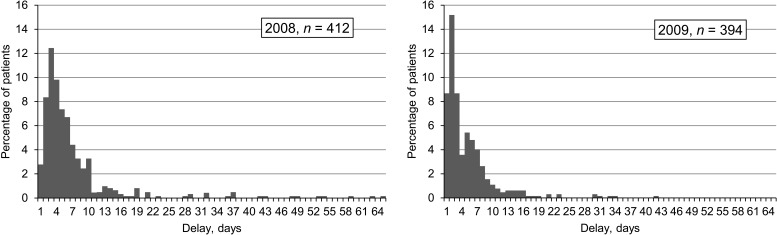
Laboratory and treatment data and dates of sputum submission and treatment commencement were available for 412/634 (65.0%) patients in 2008 and 394/663 (59.4%) in 2009. Anti-tuberculosis treatment was delayed in 75 (18.2%) TB patients in Q4 2008 and in 52 (13.2%) in Q4 2009 (P = 0.05). A histogram illustrating the distribution of time to starting treatment in Q4 2008 and 2009 demonstrates that more patients were started on anti-tuberculosis treatment earlier in 2009 (Figure 2). Sputum smear-negative status was associated with increased treatment delay (OR 6.0, 95% confidence interval [CI] 2.83–12.67), while year 2009 was associated with reduced treatment delay (OR 0.68, 95%CI 0.46–1.0). A test for interaction between HIV infection and sputum smear status showed that sputum smear negativity was independently associated with treatment delay. TB treatment delay, defined as ≥7 days, was not associated with poorer outcomes, i.e., treatment failure, death or default. On multivariate analysis, year 2008 and negative sputum smear were statistically significant in the final model for predicting treatment delay (Table 2).
FIGURE 2.
Distribution of treatment delay.
TABLE 2.
Associations with treatment delay
| Variable | Delay* ≥7 days n (%) | No delay <7 days n (%) | P value for χ2 test | Unadjusted univariate OR (95%CI) | Adjusted multivariate OR (95%CI) | P value |
| Year | 0.05 | 0.01 | ||||
| 2008 | 75 (18.2) | 337 (81.8) | 1.0 | 1.0 | ||
| 2009 | 52 (13.2) | 342 (86.8) | 0.7 (0.5–1.0) | 0.6 (0.4–0.9) | ||
| Sex | 0.69 | |||||
| Male | 70 (15.0) | 398 (85.0) | 1.0 | |||
| Female | 57 (19.9) | 280 (80.1) | 1.2 (0.8–1.7) | |||
| Unknown | 0 | 1 (100) | — | |||
| HIV status | 0.28 | |||||
| Non-HIV-infected | 24 (13.2) | 158 (86.8) | 1.0 | |||
| HIV-infected | 87 (17.4) | 414 (82.6) | 1.4 (0.9–2.2) | |||
| Unknown | 16 (13.0) | 107 (87.0) | 1.0 (0.5–1.9) | |||
| Smear status | <0.001 | <0.001 | ||||
| Positive | 8 (3.9) | 195 (96.1) | 1.0 | 1.0 | ||
| Negative | 119 (19.7) | 484 (80.3) | 6.0 (2.8–12.7) | 6.3 (3.0–13.3) | ||
| Age, years | 0.123 | |||||
| 0–14 | 6 (19.3) | 25 (80.7) | 1.0 | |||
| 15–24 | 11 (10.6) | 93 (89.4) | 0.5 (0.2–1.5) | |||
| 25–34 | 56 (17.5) | 264 (82.5) | 0.9 (0.4–2.2) | |||
| 35–44 | 30 (12.7) | 207 (87.3) | 0.6 (0.2–1.6) | |||
| ≥45 | 24 (21.1) | 90 (78.9) | 1.1 (0.4–3.0) | |||
| Disease site | 0.65 | |||||
| Pulmonary | 116 (15.6) | 630 (84.4) | 1 | |||
| Extra-pulmonary | 10 (17.5) | 47 (82.5) | 1.2 (0.6–2.4) | |||
| Missing | 1 (33.3) | 2 (66.7) | — | |||
| Outcome | 0.842 | |||||
| Treatment success | 110 (16.2) | 568 (83.8) | 1 | |||
| Treatment failure/died/default | 4 (15.4) | 22 (84.6) | 0.9 (0.3–2.8) | |||
| Transfer out | 7 (12.3) | 50 (87.7) | 0.7 (0.3–1.6) | |||
| Missing | 6 (13.3) | 39 (86.7) | 8.8 (0.3–1.9) |
≥7 days was arbitrarily chosen to represent a delay in treatment commencement based on the accepted practice of expedited anti-tuberculosis treatment commencement in high HIV prevalent settings.
OR = odds ratio; CI = confidence interval; HIV = human immunodeficiency virus.
Laboratory quality assessment data from Q4 2008 and 2009 showed 100% agreement on sputum smear classification. Furthermore, in each quarter, slide evenness, size, thickness, staining, cleanliness and specimen quality was 88–100% acceptable.
DISCUSSION
After the introduction of the digital CXR machine in Q4 2009, there was a modest increase in TB notifications (n = 39), a 8.1% increase in new sputum smear-negative TB notifications, as well as a statistically significant decrease in treatment delay, despite a 6.7% decrease in sputum smear positivity. Moreover, the decrease in sputum smear positivity in 2009 was not explained by a change in laboratory quality: quality assessments performed in both years showed 100% agreement on sputum smear classification.
The same proportion of CXRs was performed among smear-negative TB suspects in 2008 and 2009. The proportion of CXRs performed among TB suspects without sputum smear results did not increase after the introduction of digital CXR. There was a 27.6% increase in submissions of three sputum samples in 2009. We found that CXR was not being performed in lieu of smear microscopy, but that it was being used by clinicians as a diagnostic aid in conjunction with microscopy. Therefore, any concerns that clinicians would use CXR to diagnose TB instead of requesting sputum smear was not substantiated by this study. However, in less controlled settings, we do not know whether the introduction of a digital CXR service would be associated with a reduction in laboratory use and/or laboratory quality.
Digital CXR had many advantages in this resource-constrained setting. First, it did not require much training; second, it did not require the use of developers/reagents or film; third, the results were available immediately for review by the clinician, and last, it was highly accessible, as it was placed at the health centre close to where patients sought care, and was provided free of charge. When no machine was available, patients were referred to a tertiary referral centre approximately 8 km away; they would have incurred transport costs plus the cost of the film (US$2).
There are several limitations to this study. First, TB diagnosis was not confirmed by culture or molecular techniques; thus, despite the 6.7% decrease in sputum smear positivity in 2009, it is unclear if there was an increase in overtreatment of incorrectly diagnosed smear-negative TB suspects after digital CXR was introduced or if a higher percentage of TB suspects with disease were correctly diagnosed due to the digital service. Second, data were only available for 82–83% of the patients who submitted sputum, and the findings are therefore limited to these patients. Furthermore, we cannot draw any conclusions about the clinical relevance of a 7-day decrease in time to treatment initiation, given its lack of association with improved outcomes. Third, as this was a retrospective analysis of information gathered from the TB register, we are not able to determine how many suspects were lost to follow-up. Last, the study showed that there was a decreased treatment delay in 2009; however, the introduction of the digital CXR service may not be entirely responsible for this finding. The decrease could have been confounded by other interventions aimed at reducing community TB rates: TB programme strengthening has been a priority of Zambia’s Ministry of Health as well as of several independently funded research projects, and concerted efforts to reduce TB and HIV may explain the increase in data completeness, the higher percentage of patients with documented HIV status, the increase in sputum sample submissions and more TB notifications in 2009.
Smear-negative pulmonary TB is an increasing clinical problem in developing countries affected by the dual TB-HIV epidemic. Management algorithms that have been validated by local studies should improve case detection. The low sensitivity of smear microscopy for pulmonary TB presents a diagnostic dilemma and a major obstacle to TB control in sub-Saharan Africa. Improved diagnostics are urgently needed in these areas to ensure accurate diagnoses and to prevent the use of potentially toxic medications in non-infected individuals. Overall, this study shows the limitations of accurate TB diagnosis in the absence of culture or molecular techniques and the potential for misdiagnosis and overtreatment of TB suspects; however, digital CXR has many advantages, and may be used in conjunction with smear microscopy to aid TB diagnosis in resource-constrained settings. The digital CXR service would need to be tested on a larger scale to confirm our findings. We hope that our data will contribute to the growing body of literature that informs improved education, diagnosis and treatment of TB patients.
Acknowledgments
The authors thank the Zambian Ministry of Health for technical support and the health district staff for accommodating the data collection. The authors gratefully acknowledge funding from N L Agentschap, Delft Diagnostic Systems, the Dutch Ministry of Economic Affairs and Oldelft Benelux. DZ received funding from the US National Institute on Drug Abuse (grant number T32DA13911).
Disclosure: Although funding was received through a grant from Delft Diagnostic Systems, they had no input in the writing of this manuscript.
References
- 1.World Health Organization. Geneva, Switzerland: WHO; 2011. Zambia: health profile. [Google Scholar]
- 2.Whitehorn J, Ayles H, Godfrey-Faussett P. Extra-pulmonary and smear-negative forms of tuberculosis are associated with treatment delay and hospitalisation. Int J Tuberc Lung Dis. 2010;14:741–744. [PubMed] [Google Scholar]
- 3.Sonnenberg P, Glynn J R, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. HIV and pulmonary tuberculosis: the impact goes beyond those infected with HIV. AIDS. 2004;18:657–662. doi: 10.1097/00002030-200403050-00010. [DOI] [PubMed] [Google Scholar]
- 4.Harries A D, Nyirenda T E, Godfrey-Faussett P, Salaniponi F M. Defining and assessing the maximum number of visits patients should make to a health facility to obtain a diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7:953–958. [PubMed] [Google Scholar]
- 5.Harries A D, Hargreaves N J, Gausi F, Kwanjana J H, Salaniponi F M. High early death rate in tuberculosis patients in Malawi. Int J Tuberc Lung Dis. 2001;5:1000–1005. [PubMed] [Google Scholar]
- 6.Apers L, Mutsvangwa J, Magwenzi J, et al. A comparison of direct microscopy, the concentration method and the Mycobacteria Growth Indicator Tube for the examination of sputum for acid-fast bacilli. Int J Tuberc Lung Dis. 2003;7:376–381. [PubMed] [Google Scholar]
- 7.Barnes P F, Bloch A B, Davidson P T, Snider D E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 8.Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:97–107. [PubMed] [Google Scholar]
- 9.Parry C M, Kamoto O, Harries A D, et al. The use of sputum induction for establishing a diagnosis in patients with suspected pulmonary tuberculosis in Malawi. Tubercle Lung Dis. 1995;76:72–76. doi: 10.1016/0962-8479(95)90583-9. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi K, Lambert M L, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis. 2003;3:288–296. doi: 10.1016/s1473-3099(03)00609-1. [DOI] [PubMed] [Google Scholar]
- 11.Expert Group Meeting. Geneva, Switzerland: WHO; 2009. Approaches to improve sputum smear microscopy for tuberculosis diagnosis, Geneva, 31 October 2009. [Google Scholar]
- 12.World Health Organization. Geneva, Switzerland: WHO; 2007. Improving the diagnosis and treatment of smear-negative pulmonary and extra-pulmonary tuberculosis among adults and adolescents. Recommendations for HIV-prevalent and resource-constrained settings. [Google Scholar]