Abstract
Setting:
South Africa reports more cases of tuberculosis (TB) than any other country, but an up-to-date, precise estimate of the costs associated with diagnosing, treating and preventing TB at the in-patient level is not available.
Objective:
To determine the costs associated with TB management among in-patients and to study the use of personal protective equipment (PPE) at a central academic hospital in Cape Town.
Design:
Retrospective and partly prospective cost analysis of TB cases diagnosed between May 2008 and October 2009.
Results:
The average daily in-patient costs were US$238; the average length of stay was 9.7 days. Mean laboratory and medication costs per stay were respectively US$26.82 and US$8.68. PPE use per day cost US$0.99. The average total TB management costs were US$2373 per patient. PPE was not always properly used.
Discussion:
The costs of in-patient TB management are high compared to community-based treatment; the main reason for the high costs is the high number of in-patient days. An efficiency assessment is needed to reduce costs. Cost reduction per TB case prevented was approximately US$2373 per case. PPE use accounted for the lowest costs. Training is needed to improve PPE use.
Keywords: cost-analysis, policy, prevention, in-patient costs
Abstract
Contexte:
On signale plus de cas de tuberculose (TB) en Afrique du Sud que dans n’importe quel autre pays, mais on ne dispose pas d’une estimation actualisée et précise des coûts liés au diagnostic, au traitement et à la prévention de la TB chez le patient hospitalisé.
Objectif:
Déterminer les coûts liés à la prise en charge des patients hospitalisés dans un hôpital académique central à Cape Town. En outre, on a étudié l’utilisation de l’équipement de protection du personnel (PPE).
Schéma:
Etude de coût rétrospective et partiellement prospective à partir de patients diagnostiqués entre mai 2008 et octobre 2009.
Résultats:
Le coût moyen d’une journée d’hospitalisation a été de US$238 par jour, la durée moyenne de séjour a été de 9,7 jours. Les coûts moyens pour le laboratoire ont été de US$6,82 par séjour, les coûts moyens de médicaments de US$8,68 par séjour et les coûts d’utilisation du PPE de US$0,99 par jour. Le coût moyen d’une prise en charge totale de la TB a été de US$2.373. Le PPE n’a pas toujours été utilisé correctement.
Discussion:
Les coûts de la prise en charge d’un patient hospitalisé sont élevés par comparaison de ceux du traitement basé sur la collectivité. La raison principale de ces coûts élevés est le nombre important de journées d’hospitalisation. Une évaluation de l’efficience est nécessaire pour produire des recommandations permettant des réductions du coût. Les réductions de coût par cas de TB évité s’élèvent approximativement à US$2.373 par cas. L’utilisation du PPE est responsable des coûts les moins élevés. Une formation est nécessaire pour améliorer l’utilisation du PPE.
Abstract
Marco de referencia:
En Sudáfrica se notifican más casos de tuberculosis (TB) que en ningún otro país, pero no se cuenta con un cálculo actualizado y preciso de los costos generados por el diagnóstico, el tratamiento y la prevención de la TB a escala intrahospitalaria.
Objetivo:
Determinar los costos asociados con el manejo de los pacientes hospitalizados por TB en un hospital universitario central en la Ciudad del Cabo. Se evaluó además la utilización de los dispositivos personales de protección (PPE).
Método:
Se llevó a cabo un estudio de costos, retrospectivo y parcialmente prospectivo, del tratamiento de los pacientes hospitalizados por TB entre mayo del 2008 y octubre del 2009.
Resultados:
El costo promedio por día de hospitalización fue US$238 y la duración promedio de la estancia fue 9,7 días. El promedio de los costos de laboratorio por hospitalización fue US$26,82 y el promedio de los costos por medicamentos fue US$8,68. El costo del uso de los dispositivos personales de protección fue US$0,99 por día. El promedio del costo total del tratamiento de la TB fue US$2373. El PPE no siempre se utilizó de manera adecuada.
Conclusión:
Los costos del tratamiento intrahospitalario de la TB son altos en comparación con los costos del manejo comunitario. La principal razón del alto costo fue la prolongación de la estancia. Es preciso realizar una evaluación de la eficiencia, a fin de obtener recomendaciones que contribuyan a disminuir los costos. La disminución de costos por cada caso de TB que se logra prevenir es de cerca de US$2373. El uso de los dispositivos personales de protección representó la categoría con más bajo costo en el presupuesto. Es necesario impartir capacitación sobre la utilización de estos PPE.
South Africa ranks fifth among the 22 high tuberculosis (TB) burden countries in terms of incidence, and seventh in terms of overall TB burden. The 2011 World Health Organization report on TB indicates a prevalence of 795 per 100 000 population and an incidence of 981/100 000 in South Africa.1 At 1000/ 100 000 population, the Western Cape Province has the highest recorded TB incidence in South Africa.2 TB is the most common opportunistic infection and the leading cause of death in persons with HIV/AIDS (human immunodeficiency virus/acquired immune-deficiency syndrome).3–5
TB infection prevention and control (IPC) is a combination of processes and measures aimed at minimising the risk of TB transmission, both in health care facilities and at the community level. Effective management of TB in hospitals is essential to prevent transmission among patients, health care workers and visitors.5 Health care workers are at higher risk due to frequent exposure to patients with TB. The number of persons with HIV/AIDS attending health care facilities has increased in South Africa; such persons are at an increased risk of developing TB. Moreover, if health care workers themselves are immune-suppressed they have an even higher risk of developing TB and transmitting nosocomial infection.3,6
The Academic Unit for Infection Prevention and Control (UIPC), based in the University of Stellenbosch, Western Cape, South Africa, has set up an IPC programme with specific policies and in-service training to reduce TB transmission among patients, visitors and health care workers in a central academic hospital (CAH) in Cape Town. IPC at the CAH includes context-specific administrative processes (protocols, segregation and isolation of infectious patients), ventilation (natural and mechanical) and the use of personal protective equipment (PPE), such as masks, respirators and gloves.3 It has been shown that the implementation of administrative controls, environmental controls and PPE effectively reduces transmission of TB in health care facilities.6
Although TB care in South Africa is mainly community-based, prevalence surveys and field observations show that a significant number of patients continue to present to hospitals.7 At the CAH, TB patients are often admitted due to comorbidities, such as HIV/AIDS and treatment for drug-resistant TB.
The emergence of multidrug-resistant (MDR-) and extensively drug-resistant TB (XDR-TB) are of particular concern to IPC and health care cost management. These TB strains are resistant to the main anti-tuberculosis drugs, and require a longer treatment regimen of 20 months or more, resulting in higher treatment costs.8 Reduction in TB, including MDR- and XDR-TB, is dependent on effective diagnosis, treatment and improved communications between the hospital, the laboratory and its down-referral clinics.9
In South Africa, no studies assessing the costs of in-patient-based TB care, including diagnosis, management and IPC, have been published. In a resource-limited environment, an adequate costing study is necessary for data on the cost implications of IPC per patient diagnosed and treated.10 It was therefore important for the UIPC to investigate the cost of in-patient TB management, including the use of TB-IPC, in the CAH. This study will fill an important gap in the literature by providing cost estimates of in-patient-based TB care and by identifying areas of weakness in IPC measures. Furthermore, cost reduction per TB case prevented can be determined.
The objectives of the study were to estimate the costs associated with the management (diagnosis, treatment, IPC) of pulmonary TB in-patients at a CAH, and to evaluate adherence to TB-IPC protocol recommendations, such as PPE use, during pulmonary TB in-patient care at a CAH.
METHODOLOGY
Design
A retrospective and partly prospective cost analysis was performed. The analysis was conducted from a provider’s perspective, as we examined the costs to the health service, including in-patient processes and hospital care. This means that all direct and non-direct health care costs were included; however, direct non-health care costs, such as patient travel and time costs, were excluded.11–13 The unit cost of health services was defined as the full economic costs per patient treated.11,12 To be able to determine the costs in as detailed a manner as possible, the ingredient-based costing method was used, i.e., each component of TB management was calculated and a unit cost was derived for each.10 For this, cost-analysis market prices were used, as they represented the most precise estimation of the costs in daily practice.11 Costs were expressed in terms of monetary value (2009 US$).
Data collection
TB management costs at the CAH consisted of in-patient days, laboratory tests, medications and PPE, including masks, respirators, gloves, aprons and disinfectant. To calculate the costs of an in-patient day, we used the basic accounting system at the hospital, including all patients admitted and treated for pulmonary TB during the period from May 2008 to October 2009. Accounting heads were used to determine in-patient day costs; the costs were then added up and divided by the patient-day equivalent (PDE). PDE was calculated by adding the number of in-patients plus one third of out-patient visits. This ratio is based on primary data collected from Western Cape hospitals, which assumes that an in-patient day requires three times more overhead resources than an out-patient day.14 In other words, this ratio provides information on the costs of one day of hospital admission or three out-patient visits. The components of the costs of an in-patient stay are listed in Table 1. Costs for laboratory tests and medications were not included in calculating the cost of an in-patient day, as we chose to calculate these costs specifically for TB.
TABLE 1.
Types of expenditure and categories of costs of in-patient day*
| Category | Expenditure type |
| Personnel | Personnel: salaries, pension, medical aid allowance, bonus, scarce skills, housing allowance, commuting, normal overtime, incentives, sessional, periodical, discretionary leave, shift allowance, other remuneration, uniform allowances |
| Agency staff: administration, medical, nursing, pharmacy | |
| Joint staff: Cape Peninsula University, University of Stellenbosch, basic accounting system journals | |
| Goods and services | Surgical supplies, blood, X-ray consumables, private medical services (magnetic resonance imaging, positron emission tomography/computed tomography), assistive devices, clinical engineering, hospital engineering, cleaning services, security services, linen/laundry management, waste removal, photo stats (photocopier rental, copies), burial services |
| Overheads | Overhead: catering, communication, stationery, training, computer services and systems, courier and postal, power, water, uniforms purchased, pest control, consultants and contractors, grounds and gardens, steam, municipal services, gases and rentals, fuel oil and lubricants |
| Capital equipment | Capital equipment, current equipment |
Although not a monthly cost, the added cost of financial assets, software and intangible assets were included in the study analysis.
Other costs included diagnostic laboratory tests and drug prescriptions. Clinicom, an electronic patient database, was accessed to identify patients diagnosed and admitted to the hospital with pulmonary TB between May 2008 and October 2009. A total of 200 patients were included in the study.
To determine laboratory costs, we used the National Health Laboratory Service (NHLS) database. This is updated daily, capturing laboratory test requests for each patient. For patients found in the Clinicom database, we selected only TB-specific laboratory tests, as shown in Table 2. TB-specific medication data were extracted from the electronic database of the hospital pharmacy (Table 3). The laboratory test prices from the NHLS and medication prices from the pharmacy were used to determine the prices of the items identified and measured.
TABLE 2.
TB laboratory tests performed at the NHLS
| Fluid preparation for TB microscopy |
| PCR tuberculosis |
| Mycobacterium tuberculosis complex |
| Identification of mycobacteria |
| Special TB culture materials |
| TB fluorescence microscopy |
| Standard TB microscopy |
| DST against PZA |
| TB differentiation |
| DST per drug |
| Culture TB |
| No growth on culture |
| Radiometric mycobacterium antibiotic sensitivity |
| Identification of M. tuberculosis |
TB = tuberculosis; NHLS = National Health Laboratory Service; PCR = polymerase chain reaction; DST = drug susceptibility testing; PZA = pyrazinamide.
TABLE 3.
Anti-tuberculosis drugs from pharmacy
| EMB HCl |
| Ethionamide |
| INH |
| Kanamycin sulphate |
| Moxifloxacin |
| Ofloxacin |
| PZA |
| RMP + EMB + INH + PZA combination |
| RMP |
| RMP + INH combination |
| RMP + INH + PZA combination tablet |
| Streptomycin |
| Terizidone |
EMB = ethambutol; HCl = hydrogen chloride; INH = isoniazid; PZA = pyrazinamide; RMP = rifampicin.
Use of personal protective equipment
In line with the TB-IPC protocol of the hospital, all in-patients with pulmonary TB were reported to the IPC nursing team. PPE use per day was measured using several randomly selected observations on the wards by the principal investigator. The type and quantity of PPE used by personnel and visitors when in contact with TB patients were documented. These observations were used to investigate the volume of PPE for the costing analysis and to evaluate the TB-IPC protocol. Nine patients were observed for 24 h each, spread over a time period of 4 weeks.
Data analysis
The cost of an in-patient day and that of a day of PPE use were multiplied by the average length of stay of a TB patient at the hospital; this was then added to laboratory and medication costs. We used a ZAR-USD exchange rate of 1 ZAR = USD 0.10729, as at the beginning of 2009. Excel (Microsoft, Redwoods, WA, USA) and SPSS (Statistical Package for the Social Sciences Inc, Chicago, IL, USA) were used to process data.
Ethical approval
Ethical approval was granted by the Health Research Ethics Committee at the Faculty of Health Sciences, University of Stellenbosch, Cape Town, and operational approval by the Department of Health, Western Cape Province. Participant information and informed consent was provided. Patients were informed that the results of the study would be made public and published.
RESULTS
In-patient day costs were calculated using all patients with pulmonary TB at the hospital. The average cost of an in-patient day was US$238 (95% confidence interval [CI] 193–238; range 205–299; Table 4).
TABLE 4.
Cost of an in-patient TB day
| Month | Cost category, US$ |
||||||
| Personnel | Goods and services | Overheads | Capital equipment | Other costs* | PDE | Costs of in-patient day | |
| 2008 | |||||||
| May | 6 490 749 | 2 316 798 | 23 589 | 40 758 | 0 | 43 180 | 205 |
| June | 7 323 770 | 3 065 345 | 11 146 | 13 913 | 0 | 42 586 | 245 |
| July | 6 454 427 | 1 952 390 | 11 168 | 167 414 | 0 | 43 757 | 196 |
| August | 6 574 636 | 3 002 514 | 10 083 | 7 328 | 0 | 41 609 | 231 |
| September | 6 852 024 | 2 771 488 | 16 318 | 6 011 | 0 | 42 184 | 229 |
| October | 6 837 718 | 2 604 261 | 6 752 | 142 553 | 0 | 44 129 | 217 |
| November | 6 791 684 | 2 308 745 | 5 002 | 22 646 | 0 | 41 626 | 219 |
| December | 6 799 549 | 2 039 322 | 28 829 | 69 738 | 2 999 | 37 682 | 237 |
| 2009 | |||||||
| January | 6 915 963 | 2 706 912 | 37 614 | 31 659 | 0 | 38 295 | 253 |
| February | 7 047 069 | 1 540 510 | 15 467 | 63 722 | 1 296 | 40 065 | 216 |
| March | 7 156 361 | 3 116 003 | 10 101 | 782 157 | 0 | 44 021 | 251 |
| April | 6 692 803 | 2 496 321 | 1 125 | 166 866 | 0 | 40 837 | 229 |
| May | 7 278 237 | 3 365 177 | 1 802 | 113 453 | 0 | 44 890 | 240 |
| June | 7 053 694 | 3 137 800 | 12 257 | 32 815 | 0 | 41 855 | 245 |
| July | 7 884 633 | 3 196 888 | 3 994 | 65 574 | 41 781 | 43 457 | 258 |
| August | 7 044 381 | 2 993 785 | 13 655 | 887 396 | 1 017 | 43 430 | 252 |
| September | 7 149 828 | 2 926 934 | 427 | 28 303 | 124 | 42 375 | 238 |
| October | 9 935 384 | 2 836 720 | 3 458 | 297 291 | 730 | 43 788 | 299 |
Financial assets, software and intangible assets.
PDE = patient-day equivalent.
Laboratory and medication costs
Of the 200 TB patients included in the analysis, 24.5% (n = 49) had no records in either the NHLS or the pharmacy database, and a value of zero was therefore used for their laboratory and medication costs. These patients were included in the differential analysis as they received treatment and were perceived to represent in-patient costs for pulmonary TB at the hospital most accurately.
Mean laboratory costs were US$26.82 (95%CI 36.59–90.22, range 0.00–174.88). Patients with high costs underwent several costly laboratory tests, possibly when MDR-TB was suspected. No data on the definitive diagnosis (i.e., susceptible or resistant) of these patients were available. Mean medication costs were US$8.68 (95%CI 34.88–52.23, range 0.00–133.36). Patients with high laboratory costs often also had higher medication costs.
Costs of personal protective equipment
The average cost of a day of PPE use was US$0.99 (95%CI 0.26–1.72, range 0.42–1.40). PPE costs for suspected MDR-TB cases were slightly higher than for drug-susceptible TB, as more masks were used. PPE included surgical masks, gloves, aprons and hand disinfectant. N95 respirators were not used, although these were recommended for MDR-TB cases (Table 5).
TABLE 5.
PPE use
| Patient | Ward/single | TB type | Staff |
Visitors |
||||||
| No. of entries | Surgical masks n (%) | Gloves* n (%) | Hand disinfectant n (%) | Apron n (%) | No. of entries | Surgical masks n (%) | Hand disinfectant n (%) | |||
| 1 | Ward | TB | 13 | 3 (23) | 8 (31) | 3 (23) | 1 (8) | 13 | 1 (8) | 0 |
| 2 | Ward | TB | 25 | 4 (16) | 4 (8) | 1 (4) | 0 | 3 | 0 | 0 |
| 3 | Single | MDR-TB | 14 | 12 (86) | 4 (14) | 4 (29) | 4 (29) | 0 | 0 | 0 |
| 4 | Single | MDR-TB | 10 | 5 (50) | 12 (60) | 4 (40) | 0 | 0 | 0 | 0 |
| 5 | Single | TB | 12 | 1 (8) | 2 (8) | 2 (17) | 1 (8) | 1 | 1 (100) | 0 |
| 6 | Ward | TB | 22 | 3 (14) | 10 (23) | 4 (18) | 0 | 11 | 1 (9) | 0 |
| 7 | Single | TB | 7 | 2 (29) | 2 (14) | 1 (14) | 4 (57) | 4 | 1 (25) | 2 (50) |
| 8 | Ward | TB | 25 | 2 (8) | 12 (24) | 4 (16) | 1 (4) | 8 | 0 | 1 (13) |
| 9 | Ward | TB | 38 | 1 (3) | 8 (11) | 3 (8) | 0 | 9 | 0 | 0 |
Per pair.
PPE = personal protective equipment; TB = tuberculosis; MDR-TB = multidrug-resistant TB.
Total tuberculosis management costs
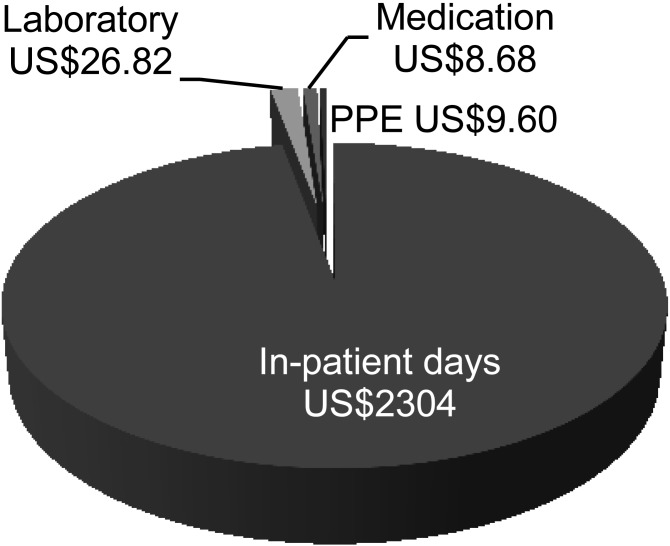
Using a random sample of 70 TB patients from our population, we calculated the average length of stay to be 9.7 days (range 0–47). The length of the study lent itself to a point prevalence analysis, which gave an idea of the average length of stay during the time period. The in-patient day costs and cost of one day of PPE use were multiplied by the average length of stay and added to laboratory and medication costs. The total TB management costs for an in-patient with TB, using a length of stay of 0 days and one of 47 days, were respectively US$36 and US$11 246. The average cost of an in-patient TB stay at the hospital was US$2373 (Figure).
FIGURE.
Total tuberculosis management costs at the central academic hospital. In-patient day costs per day and PPE costs per day were multiplied by average length of stay (9.7 days). PPE = personal protective equipment.
Use of personal protective equipment
The use of surgical masks can serve as an indicator of adherence to TB-IPC protocol, as they have to be worn when entering the patient’s room. The use of other types of PPE, such as gloves and aprons, is compulsory during clinical procedures and does not indicate adherence to the TB-IPC protocol. Staff wore a mask during 26.3% of the room entries, compared to only 15.8% for visitors (Table 5). It was observed during data collection that a patient with suspected MDR-TB left the isolation room without permission. On another occasion, another MDR-TB suspect was not admitted to an isolation room. These observations suggest that IPC measures and PPE use were not always applied in clinical practice despite TB-IPC protocol recommendations.
DISCUSSION
A number of policy recommendations can be formulated from our study results. Our findings show that the total in-patient TB management costs at the hospital were on average US$2373 per TB patient, and that the main cost driver was the large number of in-patient days (97%). Laboratory tests, medication and PPE costs represented a much lower proportion. In general, the cost of TB control as a proportion of public health expenditure is relatively low.1 To reduce costs, the duration of in-patient stay should be shortened. It is, however, impossible to say where cost savings may be made on the basis of this study alone. An efficiency assessment of the TB management programme has to be made before any recommendations can be proposed on cost reduction concerning in-patient days.
The literature shows that community-based TB management is more cost-effective than clinic-based care.15 The cost of community-based TB care in South Africa is estimated to be approximately US$392–US$766 per patient.15 It is difficult to compare these costs with the costs found in our study, as hospitalisation is often required for complex cases, such as MDR-TB patients and those with comorbidities (HIV/AIDS). This could be a reason for the high costs found in our study. It is not our intention to suggest that such patients should be treated in the community, unless they are well stabilised. Community-based treatment should not be seen as an alternative to in-patient care. More research is needed for information about the cost-effectiveness of both treatment options.
We identified problems with adherence to the TB-IPC protocol in terms of the appropriate use of PPE, which is critical for the prevention of transmission and nosocomial TB infection.6 We may conclude that the overall use of PPE (especially masks) was poor among staff (26.3%), and even more so among visitors (15.8%). Patients with suspected MDR-TB were poorly monitored by staff. It should also be noted that PPE costs represented only a small proportion of the total costs (0.4%). This small investment could lead to major changes. If PPE is used optimally, new cases can be prevented, resulting in considerable cost reductions. For each TB case prevented using IPC measures, the hospital could save on average US$2373. Emphasis on PPE implementation is therefore important. Other options for improving adherence to the TB-IPC protocol should be investigated.
This study has some limitations that should be addressed in future research. First, it did not address the efficiency and effectiveness of anti-tuberculosis treatment at the hospital. Second, a substantial part of our study population did not have a record of costs at the NHLS or the pharmacy database. The laboratory and medication costs were slightly higher when these patients were excluded. Third, the cost of HIV/AIDS management was not included. In South Africa, 53% of patients with TB are tested for HIV and of these, 60% are co-infected. The literature available on additional costs due to HIV co-infection is limited. The costs of HIV/AIDS management are known; however, due to the chronic nature of the disease, it is difficult to associate the additional costs incurred by TB patients. Further studies should include the extra costs for HIV/AIDS diagnosis and treatment. We assumed that TB-HIV co-infection led to longer duration of hospital stay. In this study, HIV prevalence was difficult to measure due to the stigma attached to HIV/AIDS in South Africa and unclear notations in patients’ medical records.
Fourth, we assumed that patients who received different prescriptions and large quantities of medication were suspected MDR-TB cases, as treatment costs for MDR-TB are substantially higher than for drug-susceptible TB.8 Recent data show that 1.8–6.7% of pulmonary TB patients in South Africa have MDR-TB.1 These patients account for higher laboratory costs due to drug susceptibility testing. Unfortunately, in our population the definitive diagnosis (TB or MDR-TB) was unknown. We do know that MDR-TB is associated with higher PPE costs. The literature shows that MDR-TB requires a prolonged treatment regimen, which means that these patients would have an increased length of stay.8 As in-patient day costs account for most of our TB management costs, the costs for MDR-TB treatment should be much higher.
In conclusion, this study shows that the costs associated with in-patient TB management at a CAH in South Africa are high. It is important to investigate whether anti-tuberculosis treatment needs to be moved to community-based care. PPE was not used properly. This indicates that training is required to ensure better adherence to TB-IPC protocol recommendations, given that the cost reduction per TB case prevented at the hospital was US$2373 per saved case.
References
- 1.World Health Organization. Global tuberculosis control 2011. WHO/HTM/TB/2011.16. Geneva, Switzerland: WHO; 2011. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf Accessed June 2012. [Google Scholar]
- 2.Mehtar S. Lowbury Lecture 2007: infection prevention and control strategies for tuberculosis in developing countries—lessons learnt from Africa. J Hosp Infect. 2008;69:321–327. doi: 10.1016/j.jhin.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health, South Africa. The draft national infection prevention and control policy for TB, MDR-TB and XDR-TB 2007. Pretoria, South Africa: DOH; 2007. http://www.doh.gov.za/docs/policy/2007/part1.pdf Accessed June 2012. [Google Scholar]
- 4.Department of Health, South Africa. The South African tuberculosis control plan: practical guidelines 2004. Pretoria, South Africa: DOH; 2004. http://www.kznhealth.gov.za/chrp/documents/Guidelines/Guidelines%20National/ Tuberculosis/South Africa%20TB%20Guidelines%202004.pdf Accessed June 2012. [Google Scholar]
- 5.Department of Health South Africa. The national infection prevention and control policy and strategy 2007. Pretoria, South Africa: DOH; 2007. http://www.doh.gov.za/docs/policy/2007/ipc-policy.pdf Accessed June 2012. [Google Scholar]
- 6.World Health Organization. WHO policy on TB infection control in health-care facilities, congregate settings and households. WHO/HTM/TB/2009.419. Geneva, Switzerland: WHO; 2009. http://whqlibdoc.who.int/publications/2009/9789241598323_eng.pdf Accessed June 2012. [PubMed] [Google Scholar]
- 7.Upkelar M. Stopping tuberculosis: time to turn urgent attention to hospitals. Int J Tuberc Lung Dis. 2008;12:986. [PubMed] [Google Scholar]
- 8.Maher D, Floyd K, Raviglione M, on behalf of the World Health Organization . Strategic framework to decrease the burden of TB/HIV. WHO/CDS/TB/2002.296; WHO/HIV_AIDS/2002.2. Geneva, Switzerland: WHO; 2002. http://whqlibdoc.who.int/hq/2002/WHO_CDS_TB_2002.296.pdf Accessed June 2012. [Google Scholar]
- 9.Loveday M, Thomson L, Chopra M, Ndlela Z. A health systems assessment of the KwaZulu-Natal tuberculosis programme in the context of increasing drug resistance. Int J Tuberc Lung Dis. 2008;12:1042–1047. [PubMed] [Google Scholar]
- 10.Department of Health, South Africa. Tuberculosis strategic plan for South Africa 2007–2011. Pretoria, South Africa: http://www.info.gov.za/view/DownloadFileAction?id = 72544 Accessed June 2012. [Google Scholar]
- 11.Cheese A, Parker D. Cost-analysis in primary health care—a training manual for programme managers. Geneva, Switzerland: WHO; 1994. http://www.who.int/immunization_financing/data/methods/en/caphc_creese.pdf Accessed June 2012. [Google Scholar]
- 12.Drummond F, Sculpher M, Torrance G W, O’Brien B J, Stoddart G L. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- 13.Cleary S M, McIntyre D, Boulle A M. The cost-effectiveness of antiretroviral treatment in Khayelitsha, South Africa—a primary data analysis. Cost Eff Resour Alloc. 2006;4:20. doi: 10.1186/1478-7547-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnum H, Kutzin J. Resource use, costs, financing. Baltimore, MD, USA: The John Hopkins University Press; 1993. Public hospitals in developing countries; pp. 196–197. [Google Scholar]
- 15.Sinanovic E, Floyd K, Dudley L, Azevedo V, Grant R, Maher D. Costs and cost-effectiveness of community-based care for tuberculosis in Cape Town, South Africa. Int J Tuberc Lung Dis. 2003;7(Suppl 1):S56–S62. [PubMed] [Google Scholar]