Abstract
Setting:
Five districts in Sri Lanka.
Objectives:
To determine: 1) the proportion of sputum smear-positive pulmonary tuberculosis (PTB) cases who failed to smear convert at 2 months, 2) their management, and 3) whether baseline characteristics and final treatment outcomes were different from those who did smear convert.
Design:
Cross-sectional retrospective review of medical files, tuberculosis (TB) registers and TB treatment records of new smear-positive PTB patients registered from January to December 2010.
Results:
Of 925 patients, 840 were available to submit sputum at 2 months, of whom 137 (16%) were smear-positive. Baseline sputum smears showing 3+ acid-fast bacilli and missing doses of anti-tuberculosis drugs during the initial phase of treatment were significantly associated with being smear-positive at 2 months. Management was poor: of 137 patients, 46 (34%) submitted sputum for culture and drug susceptibility testing and Mycobacterium tuberculosis was cultured in six cases; 120 (88%) received a 1-month extension of the initial phase, and of the 30 patients still smear-positive at 3 months there were no culture results available. Final treatment outcomes were similar, regardless of smear conversion at 2 or 3 months.
Conclusion:
Certain characteristics were risk factors for failure to smear convert at 2 months. However, treatment outcomes for all patients were good. These findings have implications for the modification of national programme recommendations.
Keywords: pulmonary tuberculosis, 2-month smear conversion, operational research, Sri Lanka
Abstract
Contexte:
Cinq districts du Sri Lanka.
Objectifs:
Déterminer : 1) la proportion de cas de tuberculose (TB) pulmonaire à frottis des crachats positifs dont les frottis n’ont pas été négativés à 2 mois, 2) leur prise en charge, et 3) dans quelle mesure les caractéristiques initiales et les résultats finaux du traitement sont différents de ceux dont les frottis ont été négativés.
Schéma:
Revue rétrospective transversale des dossiers médicaux, des registres de TB et des rapports de traitement des patients atteints d’une nouvelle TB pulmonaire à frottis positif enregistrés entre janvier et décembre 2010.
Résultats:
Sur les 925 patients, 840 ont pu fournir des crachats à 2 mois, parmi lesquels le frottis a été positif chez 137 (16%). Sont en association significative avec une positivité des frottis à 2 mois le degré de positivité 3+ des crachats initiaux pour les bacilles acido-résistants et le fait de n’avoir pas pris toutes les doses de médicament anti-tuberculose au cours de la phase initiale du traitement. La prise en charge a été médiocre : sur les 137 patients, 46 (34%) ont fourni des expectorations pour la culture et la détermination de la sensibilité aux médicaments ; on a cultivé Mycobacterium tuberculosis chez six d’entre eux ; 120 (88%) ont bénéficié d’une prolongation d’un mois de traitement. Sur 30 patients dont les frottis étaient toujours positifs à 3 mois, aucun résultat de culture n’a été disponible. Les résultats finaux du traitement sont similaires indépendamment de la négativation des frottis à 2 ou 3 mois.
Conclusion:
Certaines caractéristiques constituent des facteurs de risque d’échec de la négativation des frottis à 2 mois. Toutefois, les résultats du traitement sont bons pour l’ensemble des patients. Ces observations ont des implications pour la modification des recommandations des programmes nationaux.
Abstract
Marco de referencia:
Cinco distritos en Sri Lanka.
Objetivos:
1) Definir la proporción de casos de tuberculosis (TB) pulmonar con baciloscopia positiva que no convierten el esputo a los 2 meses de tratamiento; 2) describir su tratamiento; y 3) analizar si las características iniciales y los desenlaces terapéuticos son diferentes en los pacientes que sí convierten el esputo.
Método:
Fue este un estudio transversal retrospectivo con análisis de los expedientes médicos, los registros de TB y los registros de tratamiento de los casos nuevos de TB pulmonar con baciloscopia positiva notificados entre enero y diciembre del 2010.
Resultados:
De los 925 casos analizados, 840 pacientes aportaron una muestra de esputo a los 2 meses y 137 (16%) obtuvieron una baciloscopia positiva al segundo mes. Las baciloscopias de esputo 3+ y los casos que habían fallado a la administración de dosis de medicamentos anti-tuberculosos durante la fase inicial del tratamiento se asociaron de manera significativa con la persistencia de la baciloscopia positiva al segundo mes. El tratamiento fue deficiente en 137 casos y 46 de los pacientes con baciloscopia positiva persistente (34%) aportaron una muestra de esputo para cultivo y pruebas de sensibilidad; en 6 de estas muestras se cultivó Mycobacterium tuberculosis; en 120 pacientes (88%) se prolongó un mes el tratamiento con cuatro medicamentos y 30 pacientes seguían presentando baciloscopia positiva al tercer mes, pero no se contó con resultados del cultivo. Los desenlaces terapéuticos finales fueron equivalentes, independientemente de la persistencia de una baciloscopia positiva al segundo o tercer mes de tratamiento.
Conclusión:
Algunas características de los pacientes representaron factores de riesgo de persistencia de la baciloscopia positiva del esputo al segundo mes de tratamiento. Sin embargo, los desenlaces terapéuticos de todos los pacientes fueron favorables. Estos resultados pueden inducir modificaciones en las recomendaciones del programa nacional.
Sri Lanka, an island nation with a population of 21 million, is classified as a low tuberculosis (TB) burden country, with 9755 new and relapse TB cases reported in 2011.1,2 The National Programme for Tuberculosis Control and Chest Diseases (NPTCCD) has been implementing the World Health Organization (WHO) DOTS standardised model of TB control since 1995, and provides standardised, fixed-dose combination treatment to all registered TB patients, in line with WHO guidelines.3,4 Patients are classified as having pulmonary (smear-positive and -negative) TB (PTB) or extra-pulmonary TB. In 2011, there were 245 relapse TB cases, 1% of new smear-positive TB cases failed treatment, with an estimated 21 patients with multidrug-resistant TB (MDR-TB, defined as resistance to at least isoniazid [INH] and rifampicin [RMP]), and 21 patients with human immunodeficiency virus (HIV) associated TB.2
All new sputum smear-positive PTB patients are started on a 6-month anti-tuberculosis treatment regimen, with a 2-month initial phase consisting of four drugs (INH, RMP, pyrazinamide and ethambutol), followed by a 4-month continuation phase with RMP and INH. Patients submit sputum for smear examination at 2 and 5 months and at the end of the treatment period. Those who convert to negative at 2 months start the continuation phase of treatment, while those who are still smear-positive are supposed to have a sputum specimen sent for culture and drug susceptibility testing (DST), with a 1-month extension of the initial phase and an additional sputum specimen sent for smear examination and possible culture and DST at 3 months.4 Drug-resistant TB may be identified either through the DST results at 2 months or due to failure to convert at 5 or 6 months. Such patients are considered treatment failures, and are moved to a retreatment or second-line MDR-TB treatment regimen.
In Sri Lanka, we were interested to know about sputum smear conversion rates at 2 months, whether recommendations from the NPTCCD are being followed, and whether following these recommendations has any effect on final treatment outcomes. We therefore aimed to determine, in patients registered with new smear-positive PTB in five selected districts in Sri Lanka: 1) the proportion alive, known to be on treatment and failing to smear convert at 2 months; 2) whether these patients submitted sputum specimens for culture and DST, had the initial phase of treatment extended and submitted an additional sputum sample at 3 months; and 3) whether the characteristics and treatment outcomes of such patients were different from those who did achieve conversion at 2 months.
METHODS
Study design
This was a cross-sectional descriptive study using routine programme records.
Study setting
The Sri Lanka NPTCCD comprises a central unit and 24 district chest clinics that are run by medical doctors working as district TB control officers. All district chest clinics have laboratories that perform smear microscopy, and another 150 microscopy centres provide smear microscopy services throughout the country. All smear microscopy services undergo internal and external quality control. Patients with cough of more than 2 weeks are regarded as PTB suspects and are investigated using sputum smear microscopy and chest X-ray, according to national guidelines. Patients are diagnosed as smear-positive if at least two of three sputum specimens are positive for acid-fast bacilli (AFB) and ≥1 AFB are detected per 100 high power fields under light microscopy.4 Smear-positive sputum is graded as 3+, 2+, 1+ and scanty, according to national guidelines.4 Patients are generally treated on an ambulatory basis from the first day, and are monitored during treatment using a TB treatment card and a card documenting directly observed treatment (DOT). End of treatment outcomes are recorded in the TB patient registers.
Study sites
A total of five districts were included in the study using the following sampling method: the sample size was calculated at 825 patients based on the total number of new smear-positive patients diagnosed in 2010, a 25% failure to smear convert at 2 months, a design effect of 2, and 50% incomplete treatment cards. The 24 district chest clinics were then categorised into two groups based on the national average sputum conversion rate among new sputum smear-positive patients at 2 months (75% in 2010): n = 12 for those below and n = 12 for those above the average. All districts were assigned a random number using a computer-generated random number list, and were randomly chosen from the two categories until the final sample size for new sputum smear-positive patients was met. The five districts included in the study were Anuradapura, Matale and Karunegala (below the average for sputum smear conversion) and Kalutara and Galle (above the average for sptum smear conversion; Figure 1).
FIGURE 1.
Districts in Sri Lanka included in the study.
Study participants
All consecutive new sputum smear-positive PTB cases registered from 1 January to 31 December 2010 in the selected five district chest clinics were included in the study.
Data collection and data variables
The data were collected between March and May 2012 using a structured proforma especially designed for the study. Data variables included baseline characteristics such as TB registration number, date of registration, age, sex, ethnicity, initial sputum smear examination results, presence of radiographic cavities with information recorded on treatment cards, presence of co-morbidities such as HIV serostatus, treatment characteristics such as type of DOT provider, number of anti-tuberculosis doses missed during the initial phase of treatment, treatment outcomes at 2 months (i.e., alive on treatment, died, loss to follow-up or transferred out), sputum results at 2 months, the management of those still smear-positive at 2 months including results of culture and DST, and final treatment outcomes. Information about study variables was retrieved for each patient using patient case files, TB patient registers and TB treatment records kept in the selected district chest clinics. The data were extracted by study investigators.
Data entry and analysis
Data were double-entered, validated and analysed using EpiData (EpiData Association, Odense, Denmark; freely available at www.epidata.dk). The descriptive analysis was performed in relation to the study’s objectives. Baseline characteristics, treatment characteristics and treatment outcomes were compared in relation to whether patients had smear conversion at 2 months. The χ2 test was used to compare categorical variables, and levels of significance were set at 5%.
Ethical approval was obtained from the ethical committee of Sri Jayawardanapura University and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France.
RESULTS
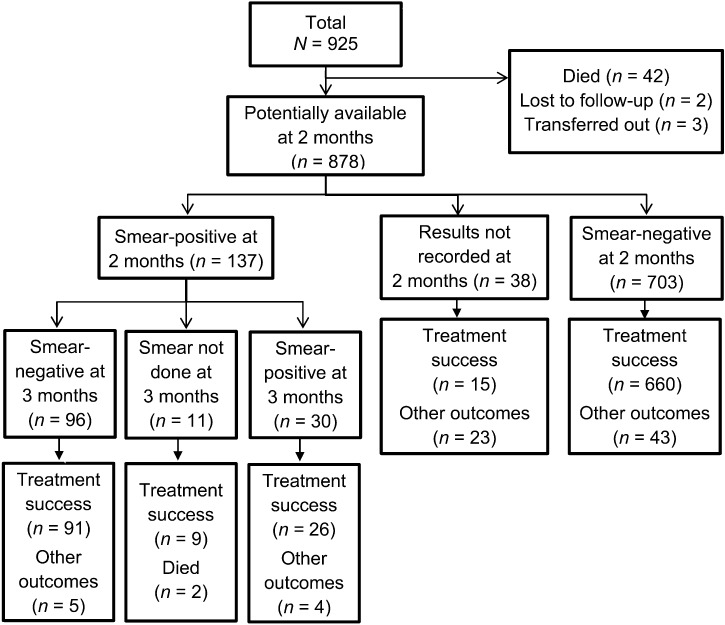
In 2010, 925 new smear-positive PTB patients were registered, of whom 840 were recorded as submitting sputum specimens, and 137 (16%) were smear-positive at 2 months. Figure 2 shows the flow of patients who submitted sputum specimens at 2 months, and those who were identified as smear-positive and -negative. The chart also shows the number of smear-positive cases at 2 months who submitted further sputum specimens at 3 months and their smear examination results. For all these groups, the final treatment outcomes (treatment success and other outcomes) are also shown.
FIGURE 2.
Flow chart of new smear-positive patients registered and treated in the five districts of Sri Lanka in 2010.
Baseline and initial phase treatment characteristics of patients who were smear-positive at 2 months and those who had converted at 2 months are shown in Table 1. Having at least one sputum smear showing 3+ AFB at baseline and missing doses of anti-tuberculosis drugs during the initial phase of treatment were significantly more common in patients who remained smear-positive at 2 months compared with those who had converted. The only other characteristic associated with positive 2-month sputum smears was being HIV-negative in comparison to HIV status unknown; no patient was found to be HIV-positive.
TABLE 1.
Characteristics of patients in relation to sputum smear conversion at 2 months of treatment
| Patient characteristic | Smear-negative at 2 months n (%) | Smear-positive at 2 months n (%) | Total patients n | P value |
| Total | 703 (84) | 137 (16) | 840 | |
| Sex | ||||
| Male | 513 (82) | 109 (18) | 622 | 0.1075 |
| Female | 190 (87) | 28 (13) | 218 | |
| Ethnicity | ||||
| Sinhalese | 599 (85) | 108 (15) | 707 | |
| Tamil | 53 (77) | 16 (23) | 69 | |
| Muslim | 41 (77) | 12 (23) | 53 | 0.2209 |
| Other ethnic group | 4 (80) | 1 (20) | 5 | |
| Not recorded | 6 (100) | 0 | 6 | |
| Age group, years | ||||
| 0–14 | 3 (75) | 1 (25) | 4 | |
| 15–24 | 104 (90) | 12 (10) | 116 | |
| 25–34 | 123 (89) | 15 (11) | 138 | |
| 35–44 | 114 (81) | 27 (19) | 141 | 0.1668 |
| 45–54 | 148 (81) | 34 (19) | 182 | |
| 55–64 | 121 (83) | 24 (17) | 145 | |
| ≥65 | 86 (80) | 22 (20) | 108 | |
| Age not recorded | 4 (67) | 2 (33) | 6 | |
| Presence of cavities on chest X-ray | ||||
| Present | 189 (82) | 41 (18) | 230 | |
| Not present | 383 (85) | 69 (15) | 452 | 0.6641 |
| Not recorded | 131 (83) | 27 (17) | 158 | |
| HIV status | ||||
| Negative | 44 (68) | 21 (32) | 65 | 0.0003 |
| Unknown | 659 (85) | 116 (15) | 775 | |
| Highest sputum grade at baseline | ||||
| Scanty | 29 (91) | 3 (9) | 32 | |
| 1+ | 302 (91) | 29 (9) | 331 | |
| 2+ | 183 (87) | 28 (13) | 211 | <0.0001 |
| 3+ | 163 (69) | 73 (31) | 236 | |
| Not recorded | 26 (87) | 4 (13) | 30 | |
| DOT provider in first 2 months | ||||
| Chest clinic | 99 (78) | 28 (22) | 127 | |
| Other DOT centres | 563 (84) | 104 (16) | 667 | 0.1158 |
| Not recorded | 41 (89) | 5 (11) | 46 | |
| Drug doses missed in first 2 months | ||||
| No missed doses | 523 (86) | 85 (14) | 608 | |
| 1–7 | 26 (76) | 8 (24) | 34 | |
| 8–30 | 25 (83) | 5 (17) | 30 | 0.0443 |
| 31–60 | 5 (83) | 1 (17) | 6 | |
| Not recorded | 124 (77) | 38 (23) | 162 |
HIV = human immunodeficiency virus; DOT = directly observed treatment.
Of the 137 patients who were smear-positive at 2 months, 46 (34%) submitted sputum specimens for culture and DST, 60 (44%) did not provide a specimen, and for the remaining 31 no records were available. Of the 46 patients who submitted sputum, 6 (13%) were culture-positive, and all had fully susceptible mycobacteria. Of the six culture-positive patients, three were cured, one was lost to follow-up, one was a treatment failure and one had no outcome recorded. Of the 137 smear-positive patients, 120 (88%) underwent a one-month extension of the initial phase of treatment, 13 (9%) started the continuation phase without an extension, and four patients had no records. At 3 months, 126 patients submitted another sputum specimen for smear examination, of whom 30 (23%) were still smear-positive (Figure 2). There was no record of whether these smear-positive patients submitted sputum specimens for culture and DST as per guidelines.
Final treatment outcomes for patients smear-positive and -negative at 2 months, regardless of whether they received an extended phase of treatment, were similar, with treatment success rates of 92% and 94%, respectively (Table 2). In both groups, failure rates were low, at 1%. All 13 (100%) patients who started the continuation phase without an extension and 3 of 4 (75%) with no records were successfully treated. The treatment success rate was 95% for those with negative sputum smears at 3 months, which was not significantly different from those still smear-positive at 3 months (87%).
TABLE 2.
Treatment outcomes in relation to smear conversion at 2 months
| Treatment outcome | Smear-negative at 2 months n (%) | Smear-positive at 2 months n (%) | Total n (%) |
| Treatment success | 660 (94) | 126 (92) | 786 |
| Failure | 5 (1) | 2 (1) | 7 (1) |
| Died | 5 (1) | 3 (2) | 8 (1) |
| Loss to follow-up | 9 (1) | 2 (1) | 11 (1) |
| Transferred out | 0 | 1 (1) | 1 (0) |
| Outcome not recorded | 21 (3) | 3 (2) | 24 (3) |
| Treatment changed | 3 (0) | 0 | 3 (0) |
| Total | 703 | 137 | 840 |
DISCUSSION
This is the first study in Sri Lanka to assess the characteristics, management and treatment outcomes of new smear-positive PTB patients who failed to smear convert at 2 months. Of the sputum smear-positive patients who were alive and able to submit sputum specimens at 2 months, 16% remained smear-positive. There are various explanations for this high rate of smear positivity at the end of the initial phase of treatment: 1) extensive disease with lung cavitations taking a longer time to convert, 2) co-morbid conditions, 3) poor adherence to medication, and 4) drug-resistant TB.
Having a high baseline grade of smear positivity was associated with failure to smear convert, a finding consistent with other reports,5–8 and this probably signifies high initial bacterial loads which take longer to clear during the initial phase of treatment. The fact that the majority of these sputum specimens submitted for culture at 2 months failed to grow Mycobacterium tuberculosis suggests that these are dead bacilli being excreted.
In contrast to findings in other settings,5–8 older age and the presence of cavities on chest X-rays were not associated with remaining smear-positive at 2 months. However, as the data on radiographic cavities were extracted from the treatment cards, these data may not be robust, thus diminishing the reliability of our negative observation.
About 10% of all patients missed one or more doses during the initial phase of treatment, and, in keeping with other reports,9 this was associated with remaining smear-positive at 2 months. Poor adherence might be associated with sub-therapeutic levels of anti-tuberculosis drugs in the initial phase of treatment that in turn might explain delayed clearance of AFB from sputum. We do not know the reasons why HIV-negative persons were more likely to be smear-positive at 2 months compared with those whose HIV status was not known.
Finally, remaining smear-positive at 2 months may indicate good quality smear microscopy services. All services underwent internal and external quality control, although in this retrospective study we have no data on how well the control system was conducted. Nevertheless, it is well recognised that good laboratory technicians can detect low grades of smear positivity, and that smear positivity rates at 2 months in such circumstances can be as high as 25%, even if the initial phase of treatment is well supervised and drugs are of good quality.10 As stated above, these bacilli might already have been dead, as reflected by negative M. tuberculosis cultures, and this might be the reason why the final treatment outcomes were similar between those smear converting at 2 months and those who were still smear-positive at 2 and 3 months of treatment.
Despite national guidelines recommending that patients who remain smear-positive at 2 months should have sputum specimens examined for culture and DST, an extended initial phase and re-examination of sputum specimens by culture if the smears are still AFB-positive, adherence to these recommendations was poor. The record keeping for the process was also poor. Of the small number of sputum specimens that grew M. tuberculosis, it was reassuring that all were fully susceptible to the drugs, and there was no record of resistance to RMP and INH. Despite these management failures at 2 months, final TB treatment outcomes in all groups of patients were good, and few failures were reported at the end of treatment. There is some discussion about the value of sputum smear examination at 2 months in predicting final treatment outcomes,11,12 but recent evidence shows that extending the initial phase of treatment does not reduce failure.13 Our findings are in line with these observations.
The strengths of this study were the large number of patients studied and the adherence to the conduct and reporting of the study according to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.14 Limitations include the usual operational problems of working with routine programmatic records that may have inaccuracies, inconsistencies and missing data.
This study has implications that can improve programmatic performance. First, as we found that patients with higher baseline bacillary loads and those who missed drug doses in the initial phase had a higher frequency of smear-positive sputum at 2 months, these patients should be accorded extra attention by programme providers, with priority given to ensuring that sputum specimens are collected and examined at this interim stage. Although we found no association with radiographic cavities, this is a risk factor identified by others,5–8 and such patients also require extra attention. Second, although no patient was found to be HIV-positive, there should be a more organised approach to test and record HIV status according to programme guidelines. Third, the recommendations for the management of patients who fail to smear convert at 2 months are poorly followed, and while these recommendations still apply, more attention needs to be paid to sending sputum specimens for culture and DST. This latter problem is not confined to Sri Lanka: wherever the process of sputum transport from peripheral centres to laboratories that perform culture and DST is assessed, there are shortcomings.15,16 Finally, however, the recommendations need to be re-assessed, because in Sri Lanka the process of performing culture and DST at 2 months and extending the initial phase of treatment makes no difference to final treatment outcomes. The current WHO treatment guidelines recommend no extension of the initial phase for patients on treatment with RMP throughout who fail to smear convert,3 and Sri Lanka now has local evidence to support a change of policy.
CONCLUSION
This study shows that 16% of patients with smear-positive PTB failed to smear convert at 2 months, but that final treatment outcomes were similar for all patients regardless of smear conversion at 2 or 3 months. These findings have implications that could modify national programme recommendations.
Acknowledgments
This research was supported through an operational research course that was jointly developed and run by the Centre for Operational Research, The International Union Against Tuberculosis and Lung Disease, South-East Asia Office, Delhi, India; the Operational Research Unit, Médecins Sans Frontières, Brussels Operational Center, Luxembourg.
Conflict of interest: none declared.
References
- 1.National Programme for Tuberculosis Control and Chest Diseases. Quarterly reports. Ministry of Healthcare and Nutrition, Sri Lanka. Colombo, Sri Lanka: NPTCCD; 2011. [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2012. WHO/HTM/TB/2012.6. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 3.World Health Organization. Treatment of tuberculosis. Guidelines. 4th ed, 2009. WHO/HTM/TB/2009.420. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 4.National Programme for Tuberculosis Control and Chest Diseases. Revised general manual for tuberculosis control, Sri Lanka, 2005. Ministry of Healthcare and Nutrition, Sri Lanka. Colombo, Sri Lanka: NPTCCD; 2005. [Google Scholar]
- 5.Telzak E E, Fazal B A, Pollard C L, Turett G S, Justman J E, Blum S. Factors influencing time to sputum conversion among patients with smear-positive pulmonary tuberculosis. Clin Infect Dis. 1997;25:666–670. doi: 10.1086/513772. [DOI] [PubMed] [Google Scholar]
- 6.Domínguez-Castellano A, Muniain M A, Rodriguez-Baño J, et al. Factors associated with time to sputum smear conversion in active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7:432–438. [PubMed] [Google Scholar]
- 7.Kuaban C, Bame R, Mouangue L, Djella S, Yomgni C. Non conversion of sputum smears in new smear positive pulmonary tuberculosis in Yaounde, Cameroon. East Afr Med J. 2009;86:219–225. doi: 10.4314/eamj.v86i5.54192. [DOI] [PubMed] [Google Scholar]
- 8.Singla R, Osman M M, Khan N, Al-Sharif N, Al-Sayegh M O, Shaikh M A. Factors predicting persistent sputum smear positivity among pulmonary tuberculosis patients 2 months after treatment. Int J Tuberc Lung Dis. 2003;7:58–64. [PubMed] [Google Scholar]
- 9.Xu W, Lu W, Zhou Y, Zhu L, Shen H, Wang J. Adherence to anti-tuberculosis treatment among pulmonary tuberculosis patients: a qualitative and quantitative study. BMC Health Serv Res. 2009;9:169. doi: 10.1186/1472-6963-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Compendium of indicators for monitoring and evaluating national tuberculosis programs. WHO/HTM/TB/2004.344. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 11.Rieder H L. Sputum smear conversion during directly observed treatment for tuberculosis. Tubercle Lung Dis. 1996;77:124–129. doi: 10.1016/s0962-8479(96)90026-x. [DOI] [PubMed] [Google Scholar]
- 12.Trébucq A, Rieder H L. Two excellent management tools for national tuberculosis programmes: history of prior treatment and sputum status at two months. Int J Tuberc Lung Dis. 1998;2:184–186. [PubMed] [Google Scholar]
- 13.Aung K J M, Declercq E, Ali Md A, et al. Extension of the intensive phase reduces relapse but not failure in a regimen with rifampicin throughout. Int J Tuberc Lung Dis. 2012;16:455–461. doi: 10.5588/ijtld.11.0216. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harries A D, Michongwe J, Nyirenda T E, et al. Using a bus service for transporting sputum specimens to the Central Reference Laboratory: effect on the routine TB culture service in Malawi. Int J Tuberc Lung Dis. 2004;8:204–210. [PubMed] [Google Scholar]
- 16.Qi W, Harries A D, Hinderaker S G. Performance of culture and drug susceptibility testing in pulmonary tuberculosis patients in northern China. Int J Tuberc Lung Dis. 2011;15:137–139. [PubMed] [Google Scholar]