Abstract
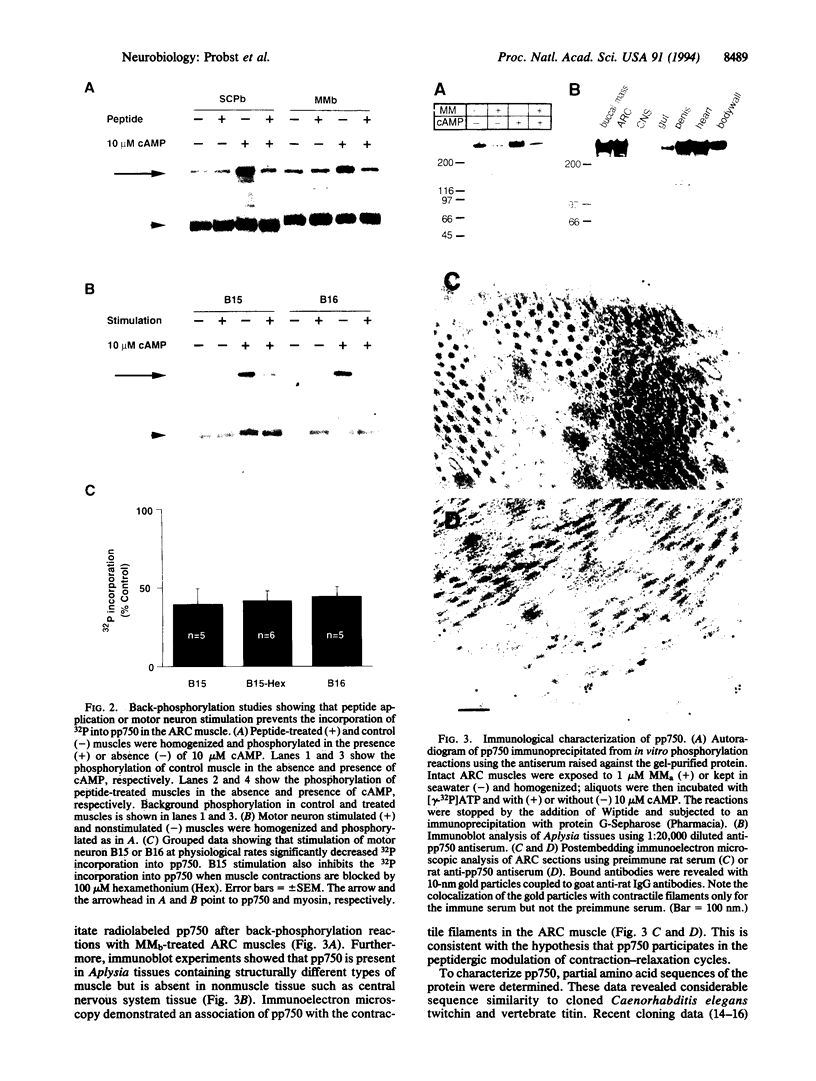
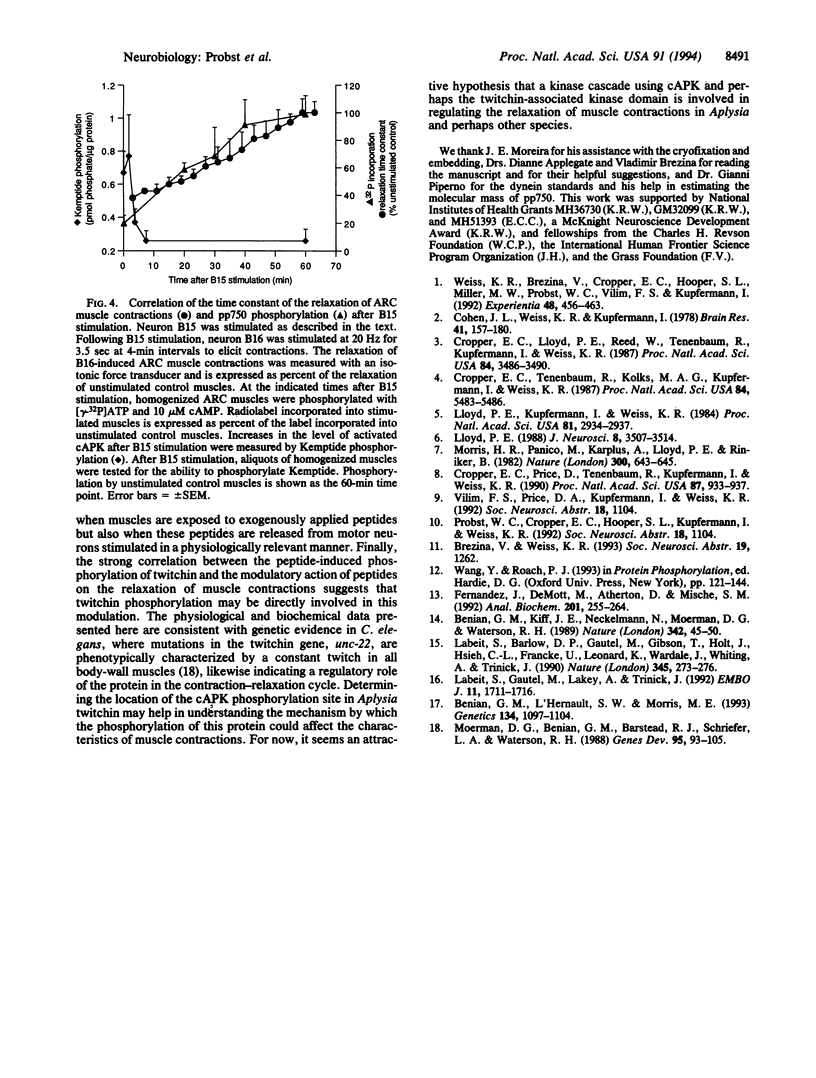
Acting through a cAMP-cAMP-dependent protein kinase (cAPK) cascade, members of two neuropeptide families, the small cardioactive peptides and myomodulins, modulate contraction amplitude and relaxation rate in the accessory radula closer (ARC) muscle of the marine mollusc Aplysia californica. An approximately 750-kDa phosphoprotein was identified in the ARC muscle as the major substrate for cAPK activated either by application of neuropeptides or by peptides released by motorneuron stimulation at physiological frequencies. Immunoblot and immunoelectron microscopy experiments revealed the widespread presence of this protein in Aplysia muscles and its colocalization with contractile filaments in the ARC muscle. Sequence analysis of proteolytic peptide fragments derived from the protein indicated that it is structurally related to the muscle protein twitchin. Finally, the level of neuropeptide-induced phosphorylation of the protein correlated well with peptidergic modulation of the relaxation rate of the muscle. We propose that twitchin in Aplysia, and perhaps in other species, may mediate the modulation of the relaxation rate of muscle contractions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benian G. M., Kiff J. E., Neckelmann N., Moerman D. G., Waterston R. H. Sequence of an unusually large protein implicated in regulation of myosin activity in C. elegans. Nature. 1989 Nov 2;342(6245):45–50. doi: 10.1038/342045a0. [DOI] [PubMed] [Google Scholar]
- Benian G. M., L'Hernault S. W., Morris M. E. Additional sequence complexity in the muscle gene, unc-22, and its encoded protein, twitchin, of Caenorhabditis elegans. Genetics. 1993 Aug;134(4):1097–1104. doi: 10.1093/genetics/134.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. L., Weiss K. R., Kupfermann I. Motor control of buccal muscles in Aplysia. J Neurophysiol. 1978 Jan;41(1):157–180. doi: 10.1152/jn.1978.41.1.157. [DOI] [PubMed] [Google Scholar]
- Cropper E. C., Lloyd P. E., Reed W., Tenenbaum R., Kupfermann I., Weiss K. R. Multiple neuropeptides in cholinergic motor neurons of Aplysia: evidence for modulation intrinsic to the motor circuit. Proc Natl Acad Sci U S A. 1987 May;84(10):3486–3490. doi: 10.1073/pnas.84.10.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper E. C., Price D., Tenenbaum R., Kupfermann I., Weiss K. R. Release of peptide cotransmitters from a cholinergic motor neuron under physiological conditions. Proc Natl Acad Sci U S A. 1990 Feb;87(3):933–937. doi: 10.1073/pnas.87.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper E. C., Tenenbaum R., Kolks M. A., Kupfermann I., Weiss K. R. Myomodulin: a bioactive neuropeptide present in an identified cholinergic buccal motor neuron of Aplysia. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5483–5486. doi: 10.1073/pnas.84.15.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J., DeMott M., Atherton D., Mische S. M. Internal protein sequence analysis: enzymatic digestion for less than 10 micrograms of protein bound to polyvinylidene difluoride or nitrocellulose membranes. Anal Biochem. 1992 Mar;201(2):255–264. doi: 10.1016/0003-2697(92)90336-6. [DOI] [PubMed] [Google Scholar]
- Labeit S., Barlow D. P., Gautel M., Gibson T., Holt J., Hsieh C. L., Francke U., Leonard K., Wardale J., Whiting A. A regular pattern of two types of 100-residue motif in the sequence of titin. Nature. 1990 May 17;345(6272):273–276. doi: 10.1038/345273a0. [DOI] [PubMed] [Google Scholar]
- Labeit S., Gautel M., Lakey A., Trinick J. Towards a molecular understanding of titin. EMBO J. 1992 May;11(5):1711–1716. doi: 10.1002/j.1460-2075.1992.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd P. E. Fast axonal transport of modulatory neuropeptides from central ganglia to components of the feeding system in Aplysia. J Neurosci. 1988 Sep;8(9):3507–3514. doi: 10.1523/JNEUROSCI.08-09-03507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd P. E., Kupfermann I., Weiss K. R. Evidence for parallel actions of a molluscan neuropeptide and serotonin in mediating arousal in Aplysia. Proc Natl Acad Sci U S A. 1984 May;81(9):2934–2937. doi: 10.1073/pnas.81.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman D. G., Benian G. M., Barstead R. J., Schriefer L. A., Waterston R. H. Identification and intracellular localization of the unc-22 gene product of Caenorhabditis elegans. Genes Dev. 1988 Jan;2(1):93–105. doi: 10.1101/gad.2.1.93. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Panico M., Karplus A., Lloyd P. E., Riniker B. Elucidation by FAB-MS of the structure of a new cardioactive peptide from Aplysia. Nature. 1982 Dec 16;300(5893):643–645. doi: 10.1038/300643a0. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Brezina V., Cropper E. C., Hooper S. L., Miller M. W., Probst W. C., Vilim F. S., Kupfermann I. Peptidergic co-transmission in Aplysia: functional implications for rhythmic behaviors. Experientia. 1992 May 15;48(5):456–463. doi: 10.1007/BF01928164. [DOI] [PubMed] [Google Scholar]