Abstract
Arabinomannan (AM) is a polysaccharide of the mycobacterial capsule. The capsular polysaccharides of various microorganisms are diverse, and this diversity is important for classification of organisms into serotypes and vaccine development. In the present study we examined the prevalence and diversity of AM among Mycobacterium tuberculosis strains using four AM-binding monoclonal antibodies (MAbs). One of these MAbs, MAb 9d8, is known to bind to AM specifically. By whole-cell enzyme-linked immunosorbent assay (ELISA), the AM recognized by MAb 9d8 was detected on the surfaces of 9 of 11 strains, while 2 strains showed no reactivity with MAb 9d8. However, the AM recognized by MAb 9d8 was found in the culture supernatants of all 11 M. tuberculosis strains tested, as demonstrated by capture ELISA. Other AM-binding MAbs reacted both with the surfaces and with the culture supernatants of all 11 strains. Mice immunized with an experimental AM-recombinant Pseudomonas aeruginosa exoprotein A (rEPA) conjugate vaccine had an increased antibody response to AM and a moderate reduction in the numbers of CFU in their organs 7 days after challenge. Our results indicate that AM was detected in all M. tuberculosis strains tested, with differences in epitope distributions of certain strains. In addition, our results suggest that an experimental AM-rEPA vaccine has a moderate effect on the numbers of CFU in organs early after infection.
Tuberculosis (TB) continues to be a leading cause of morbidity and mortality worldwide. Lack of optimal means of control is considered a major contributor to this overwhelming problem. The Mycobacterium bovis Bacille-Calmette-Guérin (BCG) vaccine, which was introduced in the 1920s, reduces the rate of disseminated TB in young children but does not prevent the pulmonary form of TB (2). In recent years major efforts have been directed to the development of new candidate vaccines against TB (4) that will, it is hoped, be more effective than the currently available (BCG) vaccine. The BCG vaccine and most experimental vaccines against TB are designed to enhance cell-mediated immunity against Mycobacterium tuberculosis. However, this approach is different from that for most vaccines licensed to date for use in humans, which are thought to work by inducing effective antibody responses against the target pathogen (for a review, see references 8 and 21a). Several of the licensed vaccines successfully used at present are polysaccharide conjugate vaccines. These vaccines include the Haemophilus influenzae type b, Streptococcus pneumoniae, Salmonella enterica serovar Typhi (16), and most recently, meningococcal group C polysaccharide conjugate vaccines (25). The general consensus is that humoral immunity plays little or no role in protection against M. tuberculosis. However, in recent years several studies reported a beneficial effect of monoclonal antibodies (MAbs) directed to mycobacterial antigens on the course of infection (for a review, see reference 8). Two of these studies involved MAbs directed to mycobacterial polysaccharides (10, 24). These observations suggest that polysaccharides can potentially be useful for eliciting protective immune responses against M. tuberculosis.
The surface of M. tuberculosis contains several polysaccharide and polysaccharide-containing fractions (1). The outermost surface layer, thought by some investigators to represent a capsule, contains the polysacchrides arabinomannan (AM), glucan, and mannan, as well as a small amount of protein (5). Recent observations demonstrating that administration of a MAb to AM (24) and immunization with an AM-conjugate vaccine (11) resulted in prolonged survival suggest that AM may be potentially useful in the development of a vaccine against M. tuberculosis. However, it is unknown how prevalent and uniform AM is among M. tuberculosis isolates.
Microbial capsular polysaccharides can be antigenically variable, and this quality provides the basis for classifying pathogens into strains (or serotypes). Classification of microbial pathogens into serotypes has been useful for diagnosis, understanding of disease epidemiology, and vaccine development (17, 18). AM was previously shown to be expressed during the in vitro and in vivo growth of M. tuberculosis (23), but the prevalence and antigenic expression of AM among different M. tuberculosis isolates have not been examined. In this study we examined the antigenic expression and prevalence of AM among various M. tuberculosis isolates and discuss the potential implications of our results for vaccine design.
(This work was presented in part in the 4th World Congress on Tuberculosis, Washington, D.C., June 2002. Some of the data presented in this paper are from a thesis submitted by J. R. Schwebach in partial fulfillment of the requirements for the degree of doctor of philosophy from the Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Bronx, N.Y.)
MATERIALS AND METHODS
Mycobacterial strains.
Clinical isolates of M. tuberculosis were obtained from the Diagnostic Microbiology Laboratory at Montefiore Medical Center, Bronx, N.Y. These isolates were recovered from the sputa of patients with pulmonary TB, and all isolates were susceptible to first-line anti-TB antibiotics. M. tuberculosis Erdman (TMC 107) originated from the Trudeau Mycobacterial Culture Collection (TMC), Trudeau Institute, Saranac Lake, N.Y.; M. tuberculosis CDC 1551 was provided by the laboratory of one of the authors (W.R.J., Jr.).
Mycobacterial culture for detection of AM.
Mycobacteria from frozen stocks (1 ml) were added to 24 ml of 7H9 medium (Difco, Detroit, Mich.) with 1% glycerol enriched with oleic acid-albumin-dextrose-catalase (Becton Dickinson, Sparks, Md.) in the absence of Tween. Frozen stocks of clinical strains of M. tuberculosis were recovered from Jensen-Lewis solid medium prior to growth in 25 ml of 7H9 liquid medium. The cultures were incubated at 37°C with shaking. Culture samples were mixed with an equal volume of 10% buffered formalin, and the absorbance at 600 nm was measured. When the absorbance reached 1.0, a 5-ml aliquot of each culture was transferred to a 490-cm2 roller bottle (Corning, Inc., Corning, N.Y.) containing 95 ml of 7H9 medium without Tween 80, and the bottle was incubated at 37°C with rotation at 1.25 rpm. After 25 days cultures were collected and centrifuged at 2,000 × g for 30 min. Culture supernatants were removed and filtered twice through a 0.22-μm-pore-size filter (Millipore Corporation, Bedford, Mass.). Mycobacteria were washed twice in phosphate-buffered saline (PBS), heat killed for 2 h at 80°C, lyophilized (FTS Systems, Inc., Stone Ridge, N.Y.), and weighed.
MAbs.
MAb 9d8, an immunoglobulin G3 (IgG3) isotype immunoglobulin that recognizes AM, and MAb 5c11, an IgM isotype immunoglobulin that recognizes AM, lipoarabinomannan (LAM), and arabinogalactan (AG), were described elsewhere (9, 19). MAb CS-40, an IgG1 isotype immunoglobulin that recognizes AM and LAM (3, 19), and MAb CS-35, an IgG3 isotype immunoglobulin that recognizes AM, LAM, and AG (12, 19), were kindly provided by John T. Belisle, Colorado State University, Fort Collins.
Concentration of culture supernatant.
The supernatant from each mycobacterial culture was placed in an individual dialysis tube (Snakeskin; Pierce, Rockland, Ill.). The tubes were placed in polyethylene glycol 8000 (Fisher Scientific, Pittsburgh, Pa.) overnight at 4°C, after which the concentrated culture supernatants were collected and maintained at 4°C until testing.
Whole-cell ELISA.
Whole-cell enzyme-linked immunosorbent assay (ELISA) was performed by a method similar to that described previously (9, 23). Briefly, 50 μl of mycobacterial suspension at a concentration of 1 mg/ml in Tris-buffered saline (TBS) was added to microtiter ELISA plate wells. The plated were incubated at room temperature for 2 h and blocked by adding 200 μl of 1% bovine serum albumin (BSA) in TBS for 1.5 h at 37°C.
The wells were washed three times with TBS containing 0.05% Tween 20, MAb 9d8, 5c11, CS-40, or CS-35 at a concentration of 10 μg/ml in 1% BSA in TBS was added to the plates, and the wells were serially diluted; the plates were incubated at 37°C for 1 h or 4°C overnight. After the plates were washed, alkaline phosphatase (AP)-conjugated goat-anti mouse (GAM) (GAM-AP) IgM, IgG3, or IgG1 (Southern Biotechnology Associates, Birmingham, Ala.) at a concentration of 1 μg/ml in 1% BSA in TBS was added, and the plates were incubated for 1 h at 37°C. The plates were washed five times, and 50 μl of 1 μg of p-nitrophenylphosphate per ml in substrate buffer (0.001 M MgCl2, 0.05 M Na2CO3 [pH 9.8]) was added. The absorbance at 405 nm was measured in a Multiscan MS reader (Labsystems, Vantaa, Finland).
Capture ELISA for detection of AM.
Capture ELISA was performed by a method similar to that described previously (19, 23). Briefly, microtiter plates were incubated with 50 μl of unlabeled GAM IgG3 or IgG1 per well at a concentration of 1 μg/ml in TBS and incubated for 1 h at 37°C. The plates were blocked with 1% BSA in TBS. After the plates were washed, 1 μg of MAb 9d8, CS-40, or CS-35 per ml in 1% BSA in TBS was added. Following incubation and washing of the plate, purified AM, LAM, or AG in 1% BSA in TBS or a concentrated culture supernatant resulting from the growth of mycobacteria was added to the wells and the wells were serially diluted; the plates were then incubated for 1 h at 37°C. After the plates were washed, 1 μg of MAb 5c11 per ml in 1% BSA in TBS was added to the wells, and the plates were further incubated as described above. GAM-AP IgM was added to the plates at a concentration of 1 mg/ml. The plates were developed and read as described above.
Calculation of amount of AM generated by mycobacteia.
The concentration of AM in the concentrated culture supernatants was calculated by extrapolation from known concentrations of purified LAM (kindly provided by John T. Belisle, Colorado State University) and AM and is expressed in micrograms per milliliter. The total amount of AM in each culture supernatant was then calculated by multiplying the concentration by the volume (in milliliters) of the concentrated supernatant. The total amount of AM was then divided by the dry weight of the mycobacteria. The results were expressed in milligrams per gram (dry weight) of M. tuberculosis. Each value calculated represents the average results from two separate experiments.
Double staining with acid-fast stain and immunohistochemistry of organ tissues from with M. tuberculosis-infected mice.
Murine infection was performed as described previously (23). Briefly, female BALB/c mice (age, 6 to 8 weeks; Charles River Laboratories, Wilmington, Mass.) were infected intravenously with 106 M. tuberculosis Erdman bacilli. The organs were harvested 42 days after infection, fixed in 10% formalin, and embedded in paraffin. Sections of 4 to 5 mm were placed on glass microscope slides. After removal of the paraffin, the slides were coated with carbolfuschin KF (Becton Dickinson, Sparks, Md.) for 45 min. The slides were washed with distilled H2O and decolorized with acid-alcohol. Immunohistochemistry was performed as described previously (10). Briefly, the slides were incubated with 10 μg of MAb 5c11 per ml in 2% BSA for 2 h, followed by incubation with GAM-AP IgM (Southern Biotechnology Associates, Inc.) diluted 1:200 in 2% BSA. Color was developed with a 5-bromo-4-chloro-3-indolylphosphate-Nitro Blue Tetrazolium substrate tablet in 10 ml of distilled H2O (Sigma, St. Louis, Mo.). Between each step the slides were washed with PBS. Tissue samples from the lungs of uninfected BALB/c mice were used as negative controls. Additional negative controls consisted of samples of lung tissue from infected mice that were incubated as described above, with the exception that MAb 5c11 was not included. Some sections were counterstained with hematoxylin (Biomeda Corp., Foster City, Calif.).
Immunization of mice with an AM-conjugate vaccine.
AM isolated from a clinical strain of M. tuberculosis (23) and purified as described previously (23) was conjugated to recombinant Pseudomonas aeruginosa exoprotein A (rEPA) by using 1-cyano-4 dimethylaminopyridinium tetrafluoroborate (CDAP) (14). Female BALB/c mice (age, 6 to 8 weeks; Charles River Laboratories) were immunized with 5 μg of AM-rEPA vaccine in 100 μl of 50% Freund's incomplete adjuvant (Sigma) in PBS. Booster doses of 5 μg of vaccine in 100 μl of PBS were given at 2 and 4 weeks. Immunizations and booster doses were administered subcutaneously at the side of the base of the tail. Control mice received unconjugated rEPA or PBS. Four mice were used to study the immunogenicity of the AM-rEPA vaccine, and they were bled through the retroorbital plexus prior to and at 2, 3, 4, 5, and 6 weeks after the initial immunization. The titers of IgG to AM in serum were measured by ELISA, as described previously (23).
Infection of immunized mice with mycobacteria.
Mice immunized with AM-rEPA conjugate vaccine were challenged intravenously with M. tuberculosis Erdman or M. bovis BCG Pasteur (which originated from frozen stocks of known titer) by infection through the tail vein. Challenge doses consisted of 1 × 106 M. tuberculosis and 2.5 × 105 BCG organisms in 200 μl of PBS containing 0.05% Tween 80 (PBS-T). The mycobacteria originated from a frozen vial containing a known quantity (in CFU per milliliter). The lungs, livers, and spleens of three mice from each of the experimental and control groups were harvested at 1, 2, 3, and 5 weeks after infection. A subset of 5 mice from each group infected with M. tuberculosis was designated for use in the survival study. The organs harvested were homogenized in PBS-T with a Seward Stomacher 80 Lab System (Seward, London, United Kingdom). The homogenates were serially diluted in PBS-T, and aliquots were plated on 7H10 (Difco, Detroit, Mich.). Colony counting was performed 3 weeks after plating, and the total number of CFU in each organ was calculated.
RESULTS
Detection of AM-containing polysaccharides in tissue.
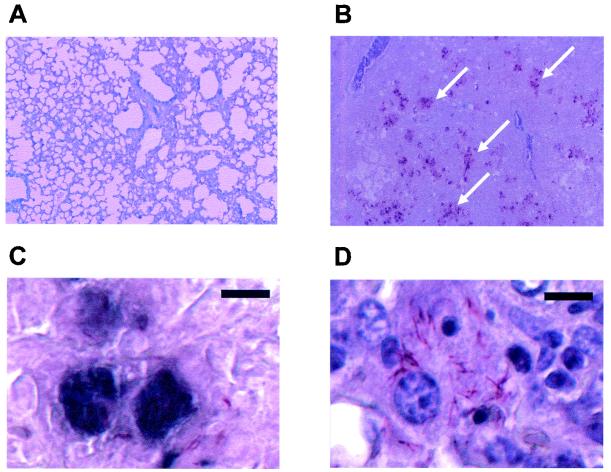
To visually assess the presence and location of AM-containing polysaccharide during infection, tissues of mice infected with M. tuberculosis were stained with an acid-fast stain followed by immunohistochemistry analysis with MAb 5c11 (Fig. 1). Staining revealed multiple acid-fast bacilli dispersed throughout lung tissue samples. Only occasional acid-fast bacilli were observed in spleen and liver tissue samples. Immunostaining of lung tissue localized around the acid-fast bacilli and extended beyond their contours (Fig. 1B to D). The use of sequential tissue sections (Fig. 1C and D) and hematoxylin counterstain localized the acid-fast bacilli and most of the immunostaining inside the cells. Occasional immunostaining of rod-shaped bacilli was noticed as well. No immunostaining was noticed in liver or spleen tissue samples or negative control samples. Immunostaining with MAb 5c11 provides a qualitative evaluation of the abundance of polysaccharide containing AM in the lungs of infected mice.
FIG. 1.
Double staining of lung tissue of a mouse using acid-fast staining followed by immunohistochemistry analysis with MAb 5c11. (A) Lung tissue of an uninfected mouse (original magnification, ×100); (B) lung tissue of a mouse infected with M. tuberculosis (original magnification, ×100) demonstrating areas of immunostaining (arrows); (C) lung tissue of a mouse infected with M. tuberculosis (bar, 10 μm) demonstrating immunostaining in dark blue; (D) acid-fast staining of lung tissue of a mouse infected with M. tuberculosis without the addition of MAb 5c11 (bar, 10 μm); panel D demonstrates an area equivalent to that shown in panel C following counterstaining with hematoxylin. The figure was generated with an Epson Perfection 1650 scanner and Adobe Photoshop (version 7.0) for the personal computer.
Presence of AM on surfaces of M. tuberculosis isolates.
We previously demonstrated that the AM recognized by MAb 9d8 was found in culture supernatants of M. tuberculosis CDC 1551 and Erdman (23). However, while it was detected on the surface of the Erdman strain, it was not found on the surface of CDC 1551. This finding prompted us to examine whether AM is present on the surfaces of different M. tuberculosis strains. The presence of AM on the surfaces of M. tuberculosis isolates was examined by whole-cell ELISA with several AM-binding MAbs with different specificities (19). MAb 9d8 was previously shown to react specifically with AM (19) without cross-reacting with other arabinose-containing surface polysaccharides. On the other hand, other AM-binding MAbs also bind to the AM found in LAM, and some cross-react with AG (19). Previous studies have shown that the amount of AM generated by M. tuberculosis increases in a time-dependent manner (23). For this reason, the mycobacteria were grown for 25 days prior to testing. Comparable amounts of mycobacteria were used to coat the ELISA wells before they were reacted with the MAbs. MAbs CS-40, 5c11, and CS-35 were found to react with the cell surfaces of all M. tuberculosis strains (Table 1). MAb 9d8 reacted with the cell surfaces of 9 of the 11 M. tuberculosis strains tested. No reactivity of this MAb was detected with the cell surface of M. tuberculosis CDC 1551 or CI-9 (Table 1). These results suggest that the target epitope of MAb 9d8 is present on the surfaces of most strains tested but is absent from the surfaces of two isolates.
TABLE 1.
Reactivities of AM-binding MAbs with cell surfaces and culture supernatants of M. tuberculosis strains
| M. tuberculosis strain | Reactivity of AM-binding MAbs:
|
|||
|---|---|---|---|---|
| 9d8
|
CS-40, CS-35, and 5c11
|
|||
| Cell surface | Culture supernatant | Cell surface | Culture supernatant | |
| Erdman | + | + | + | + |
| CDC 1551 | − | + | + | + |
| CI 1a | + | + | + | + |
| CI 2 | + | + | + | + |
| CI 3 | + | + | + | + |
| CI 4 | + | + | + | + |
| CI 5 | + | + | + | + |
| CI 6 | + | + | + | + |
| CI 7 | + | + | + | + |
| CI 8 | + | + | + | + |
| CI 9 | − | + | + | + |
CI, clinical isolate.
Capture ELISA for detection of AM epitopes.
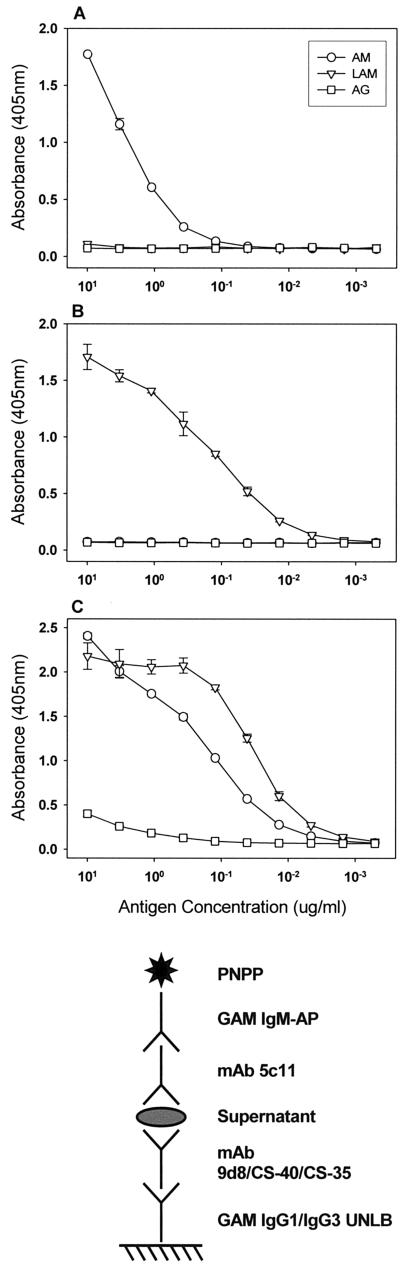
The method of capture ELISA was intended for the detection of AM in culture supernatants of M. tuberculosis strains. It has been demonstrated that the mycobacterial capsular material can be recovered from culture medium supernatant (20). We first tested the capture properties of the various AM-binding MAbs using the purified mycobacterial carbohydrate fractions AM, LAM, and AG. In these assays, MAb 5c11 was used as the detection antibody and MAbs 9d8, CS-40, and CS-35 were used as the capture antibodies. MAb 9d8 captured an epitope present in AM but not LAM (Fig. 2A), MAb CS-40 captured an epitope present in LAM but not AM (Fig. 2B), and MAb CS-35 captured an epitope common to AM and LAM (Fig. 2C). The level of capture of AG by MAb CS-35 was minimal but exceeded the level of background binding and may have been due to the cross-reactivity of MAbs CS-35 and 5c11 with AG. The ability of MAb CS-35 to capture both AM and LAM as well as to bind to AG (19) is probably due to the predominance of arabinose in the epitope recognized by this MAb (13).
FIG. 2.
Capture of AM, LAM, and AG by different MAbs. The graphs indicate the ELISA configuration. The capture antibodies were MAbs 9d8 (A), CS-40 (B), and CS-35 (C). Symbols represent the averages of two measurements; error bars denote 1 standard deviation. The experiment was performed twice with similar results. PNPP, p-nitrophenylphosphate; UNLB, unlabeled.
Detection of AMs in culture supernatants of mycobacteria.
Our previous studies demonstrated that the AM recognized by MAb 9d8 is found in the culture supernatant of M. tuberculosis strain CDC1551 but not on the cell surface (23). This finding suggests that this epitope is either easily removed or completely shed from the surface. To evaluate whether this finding applies to other M. tuberculosis strains, capture ELISA was used to detect AM in the culture supernatants of various clinical isolates. AM was detected in the culture supernatants of all 11 M. tuberculosis strains (Table 1), as manifested by the presence of the epitope of each MAb in the culture supernatants of all strains. The amount of the AM captured by MAb CS-40 in the various culture supernatants was between 1.8 and 25.1 mg per g (dry weight) of M. tuberculosis, with a mean of 9.82 mg/g and a standard deviation of 7.31 mg/g. The amount of the AM epitope captured by MAb CS-35 was between 0.6 and 4.6 mg per g (dry weight), with a mean of 3.18 mg/g and a standard deviation of 1.63 mg/g. The amount of AM captured by MAb 9d8 could not be calculated with accuracy due to the large internal variability in the assay results. It is worth noting that the AM epitope recognized by MAb 9d8 was found in the culture supernatants of CI-9 and CDC 1551, despite its absence from the surfaces of these strains. The variability in epitope concentration among the different strains, as well as the difference in the average concentration of each epitope between the groups (P = 0.008 by t test between the two quantifiable epitopes), is of interest. In this regard, biochemically analyzed AM fractions from several laboratory strains of M. tuberculosis were previously reported to have strain-to-strain variations in quantities and the ratio of the quantity of arabinose to the quantity of mannose (20).
Immunogenicity of AM-rEPA vaccine in mice.
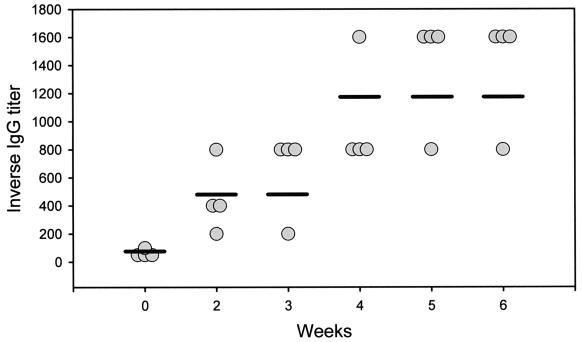
Mice immunized with AM-rEPA vaccine were tested for antibody titers by ELISA. Titers of IgG antibodies to AM were measured prior to vaccination and then weekly from weeks 2 to 6. The baseline titers of IgG antibodies to AM were 1:50 or 1:100. All mice immunized demonstrated gradual increases in antibody titers after vaccination. The titers reached 1:1,600 at 5 to 6 weeks after primary immunization (Fig. 3).
FIG. 3.
Titers of IgG antibodies to AM in mice immunized with the AM-rEPA vaccine, as measured by ELISA. Circles, titers for individual mice at a given time; horizontal bars, median titers.
Effect of AM-rEPA vaccine on in vivo infection with M. tuberculosis.
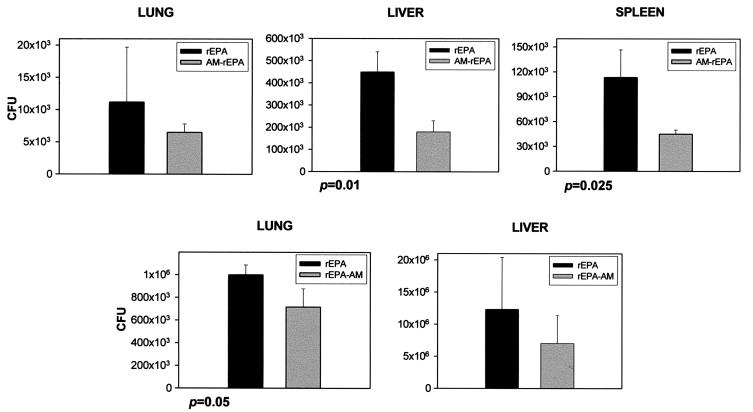
The mycobacterial burdens in the organs of mice immunized with AM-rEPA vaccine were measured 1, 2, 3, and 5 weeks after infection with M. bovis BCG or M. tuberculosis Erdman. A moderate reduction in the number of CFU was observed 1 week after challenge (Fig. 4). The differences were statistically significant for the livers and spleens of mice that had received M. bovis BCG and the lungs of mice that had received M. tuberculosis. No statistically significant differences in the numbers of CFU were noted at later times. The median survival time was 94 days for mice immunized with the rEPA-AM vaccine and 102 days for control mice; all mice died by 110 days, regardless of the group, and no statistically significant differences were found between the two groups (P = 0.9725 by the log rank test).
FIG. 4.
Mycobacterial burdens in organs of mice immunized with the AM-rEPA vaccine and controls receiving rEPA alone after infection with M. bovis BCG (three panels at the top) and M. tuberculosis (two panels at the bottom). Bars represent the average CFU from 3 mice 1 week after infection. Error bars denote 1 standard deviation.
DISCUSSION
The surface of M. tuberculosis contains polysaccharides and polysaccharide-containing fractions such as LAM, AG, and peptidoglycan (1). In addition, an outermost layer of M. tuberculosis was described by some investigators and identified as a capsule (21). This layer consists mainly of polysaccharides, of which AM is an important component (5). Polysaccharide capsules surround many pathogens, and they can be diverse. For example, a total of 6 capsular serotypes have been described for H. influenzae (17), 90 capsular serotypes have been described for S. pneumoniae (18), and 4 capsular serotypes have been described for Cryptococcus neoformans (6). Classification of microbial pathogens into serotypes has been useful for diagnosis, understanding of disease epidemiology, and vaccine development. In general, polysaccharide-based vaccines are type specific and provide protection only against bacteria with capsular polysaccharides that are antigenically similar to those used in the vaccine (16). The purpose of this study was to characterize AM in terms of its prevalence and antigenic variability.
Immunohistochemistry staining of tissues of mice that had been infected with M. tuberculosis with an AM-binding MAb demonstrated circumscribed areas of staining in lung tissue that extended beyond the surface of the acid-fast bacilli (Fig. 1). These results suggest that AM-containing polysaccharide is generated and potentially shed from the bacilli during infection in vivo, resulting in the presence of large areas containing mycobacterial polysaccharide. The paucity of staining of liver and spleen tissues may be a result of the lower numbers of CFU in these organs or the ability of these organs to efficiently clear the polysaccharides. In this regard, a previous study (10) demonstrated that the liver and the spleen are very active in the pharmacokinetics of LAM, with the hepatobiliary system having an important role in clearance.
The use of several AM-binding MAbs with different specificities allowed the immunological identification of various AM epitopes, as demonstrated by the capture of purified fractions (Fig. 2). The use of several MAbs was particularly important, given the data from a recent study demonstrating that the AM recognized by MAb 9d8 did not react with the cell surface of M. tuberculosis CDC 1551 (23), suggesting that the epitope recognized by MAb 9d8 was not present on the cell surface. This epitope was, however, present in the culture supernatant of this strain, suggesting that it is easily removable or shed. This phenomenon was not observed with M. tuberculosis Erdman, for which MAb 9d8 reacted with both the surface and the culture supernatant. In the present study, the four AM-binding MAbs reacted with all strains tested (Table 1). MAbs CS-40, CS-35, and 5c11 reacted with the cell surfaces and culture supernatants of all strains tested. MAb 9d8 reacted with the culture supernatants of all strains tested and the cell surfaces of nine strains, but it did not react with the cell surface of CI-9 or CDC 1551 (Table 1). These results suggest differences in the MAb 9d8 epitope distribution among different strains. They may also suggest strain-to-strain variations in the stability of attachment of this polysaccharide epitope to the cell surface. Different degrees of bonding between the capsular material and the cell surface have previously been described for other bacteria (22). Our results support the concept that MAb 9d8 binds to an epitope found exclusively on the outermost surface of M. tuberculosis, while the epitopes recognized by the other MAbs may also be present underneath the outermost layer. In this regard, it is worth noting that arabinose and mannose are found in the capsular AM as well as in LAM. A previous study suggested that LAM and AM share antigenic determinants yet maintain antigenic differences, as demonstrated by the ability of MAb 9d8 to bind to AM and not LAM (19).
It was recently demonstrated that although antibodies to AM were prevalent in human sera, antibodies with MAb 9d8 specificity were detected only in a small number of patients with TB (19). The data raise questions regarding the prevalence of the MAb 9d8-specific AM epitope among clinical M. tuberculosis strains. The ubiquity of this epitope, as demonstrated in the present study, suggests that the absence of antibodies to AM with MAb 9d8 specificity in many TB patients (19) is related to the nature of the antibody response to this epitope rather than to differences in the prevalence of the MAb 9d8-specific AM epitope among different strains.
The biological significance of the differences in the MAb 9d8 epitope distribution among various M. tuberculosis strains is unclear. Whether differences in the degree of attachment of capsular material could potentially affect the interaction of various M. tuberculosis strains with the host immune system remains to be explored. It is of interest that human exposure to M. tuberculosis CDC 1551 was found to result in high rates of purified protein derivative skin test conversions with large reactions (26). In mice, this strain induced a more rapid and vigorous host immune response, with earlier granuloma formation and increased cytokine production, compared to the immune responses of other clinical isolates (15).
Previous studies demonstrated that MAb 9d8 prolongs the survival of mice infected with a lethal dose of M. tuberculosis (24) and that MAb 5c11 enhances the clearance of LAM in a murine model (10). These findings highlight the potential immunological importance of AM. In fact, one study reported that AM has an inhibitory effect on lymphocyte proliferation (7). In an effort to evaluate the potential of a polysaccharide-based vaccine against M. tuberculosis, we challenged mice that had been immunized with the AM-rEPA vaccine. The vaccine was found to be immunogenic, eliciting high titers of antibodies to AM, and led to reductions in the numbers of CFU early in the course of infection. The effect observed was not sustained and did not affect the overall course of infection. These results suggest that an AM-based vaccine has the potential to affect the course of infection in its early stages, prior to the development of specific cell-mediated immunity. A study from another group recently reported prolonged survival of C57BL/6 mice and guinea pigs immunized with an AM-conjugate vaccine (11). Taken together, the results of these studies are encouraging and suggest that mycobacterial polysaccharides may be considered in the development of a vaccine against TB.
In summary, our work demonstrated that AM is ubiquitous among M. tuberculosis strains, which expressed all four AM epitopes tested, with the MAb 9d8-specific AM epitope present on the surfaces of most strains. In addition, an experimental AM-conjugate vaccine was found to lower the numbers of CFU early in the course of infection. Additional studies are required to analyze the elements that are important for the development of an optimal polysaccharide vaccine, such as antigen specificity, type of conjugate and adjuvant used, the resulting antibody responses, as well as the effect of polysaccharide vaccine on various strains of M. tuberculosis. The latter is especially important, given the differences in the MAb 9d8 epitope distribution noted among M. tuberculosis strains. In addition, it is important to determine the exact role of mycobacterial polysaccharides in the immunopathogenesis of TB.
Acknowledgments
We are indebted to Michael H. Levi and Hitesh Patel for providing clinical M. tuberculosis strains. We thank Jorge Bermudez, Bing Chen, and Xiaojuan Wang for valuable assistance. Many thanks go to John T. Belisle (Colorado State University) for providing LAM, AG, and MAbs CS-40 and CS-35, through NIH contract N01AI75320 (Tuberculosis Research Materials and Vaccine Testing), and to Bettina Fries and Diane McFadden for critical reading of the manuscript and many valuable suggestions.
This work was supported in part by National Institutes of Health (NIH) grants AI001691 and AI053192 and a grant from the Sequella Global Tuberculosis Foundation to A.G-F. A.C. is supported by NIH grants AI033142, AI033774, AI052733, and HL059842, and W.R.J., Jr., is a Howard Hughes Medical Institute investigator and is supported by NIH grants AI026170 and AI043268. A.G-F., A.C., and W.R.J., Jr., are Center for AIDS Research investigators at the Albert Einstein College of Medicine.
REFERENCES
- 1.Besra, G. S., and D. Chatterjee. 1994. Lipids and carbohydrates of Mycobacterium tuberculosis, p. 285-306. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, D.C.
- 2.Centers for Disease Control and Prevention. 1996. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Counsil for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. 45:1-18. [PubMed] [Google Scholar]
- 3.Chatterjee, D., K. Lowell, B. Rivoire, M. R. McNeil, and P. J. Brennan. 1992. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J. Biol. Chem. 267:6234-6239. [PubMed] [Google Scholar]
- 4.Collins, H. L., and S. H. E. Kaufmann. 2001. Prospects for better tuberculosis vaccines. Lancet Infect. Dis. 1:21-28. [DOI] [PubMed] [Google Scholar]
- 5.Daffe, M., and P. Draper. 1998. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 39:131-203. [DOI] [PubMed] [Google Scholar]
- 6.Diamond, R. D. 2000. Cryptococcus neoformans, p. 2707-2718. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, vol. 2. Churchill Livingstone, New York, N.Y.
- 7.Ellner, J. J., and T. M. Daniel. 1979. Immunosuppression by mycobacterial arabinomannan. Clin. Exp. Immunol. 35:250-257. [PMC free article] [PubMed] [Google Scholar]
- 8.Glatman-Freedman, A. 2003. Advances in antibody-mediated immunity against Mycobacterium tuberculosis: implications for a novel vaccine strategy. FEMS Immunol. Med. Microbiol. 39:9-16. [DOI] [PubMed] [Google Scholar]
- 9.Glatman-Freedman, A., J. M. Martin, P. F. Riska, B. R. Bloom, and A. Casadevall. 1996. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J. Clin. Microbiol. 34:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glatman-Freedman, A., A. J. Mednick, N. Lendvai, and A. Casadevall. 2000. Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect. Immun. 68:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamasur, B., M. Haile, A. Pawlowski, U. Schroder, A. Williams, G. Hatch, G. Hall, P. Marsh, G. Kallenius, and S. B. Svenson. 2003. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine 21:4081-4093. [DOI] [PubMed] [Google Scholar]
- 12.Hunter, S. W. H., H. Gaylord, and P. J. Brennan. 1986. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 261:12345-12351. [PubMed] [Google Scholar]
- 13.Kaur, D., T. L. Lowary, V. D. Vissa, D. C. Crick, and P. J. Brennan. 2002. Characterization of the epitope of anti-lipoarabinomannan antibodies as the terminal hexaarabinofuranosyl motif of mycobacterial arabinans. Microbiology 148:3049-3057. [DOI] [PubMed] [Google Scholar]
- 14.Konadu, E., J. Shiloach, D. A. Bryla, J. B. Robbins, and S. C. Szu. 1996. Synthesis, characterization, and immunological properties in mice of conjugates composed of detoxified lipopolysaccharide of Salmonella paratyphi A bound to tetanus toxoid with emphasis on the role of O acetyls. Infect. Immun. 64:2709-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manca, C., L. Tsenova, C. E. Barry III, A. Bergtold, S. Freeman, P. A. J. Haslett, J. M. Musser, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis CDC 1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 162:6740-6746. [PubMed] [Google Scholar]
- 16.Marchant, C. D., and M. L. Kumar. 2002. Immunizations, p. 232-262. In H. B. Jenson and R. S. Baltimore (ed.), Pediatric infectious diseases: principles and practices. The W. B. Saunders Co., Philadelphia, Pa.
- 17.Moxon, E. R., and T. F. Murphy. 2000. Haemophilus influenzae, p. 2369-2378. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, vol. 2. Churchill Livingstone, New York, N.Y.
- 18.Musher, D. M. 2000. Streptococcus pneumoniae, p. 2128-2147. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, vol. 2. Churchill Livingstone, New York, N.Y.
- 19.Navoa, J. D., S. Laal, L. Pirofski, G. McLean, J. B. Robbins, R. Schneerson, Z. Dai, A. Casadevall, and A. Glatman-Freedman. 2003. Specificity and diversity of antibodies to Mycobacterium tuberculosis arabinomannan. Clin. Diagn. Lab. Immunol. 10:88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortalo-Magne, A., A. B. Andersen, and M. Daffe. 1996. The outermost capsular arabinomannans and other mannoconjugates of virulent and avirulent bacilli. Microbiology 142:927-935. [DOI] [PubMed] [Google Scholar]
- 21.Ortalo-Magne, A., M.-A. Dupont, A. Lemassu, A. B. Anderson, P. Gounon, and M. Daffe. 1995. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology 141:1609-1620. [DOI] [PubMed] [Google Scholar]
- 21a.Robbins, J. B., R. Schneerson, and S. C. Szu. 1996. Hypothesis: how licensed vaccines confer protective immunity. Adv. Exp. Med. Biol. 397:169-182. [DOI] [PubMed] [Google Scholar]
- 22.Roberts, I. S. 1996. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 50:285-315. [DOI] [PubMed] [Google Scholar]
- 23.Schwebach, J. R., A. Casadevall, R. Schneerson, Z. Dai, X. Wang, J. B. Robbins, and A. Glatman-Freedman. 2001. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect. Immun. 69:5671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teitelbaum, R., A. Glatman-Freedman, B. Chen, J. B. Robbins, E. Unanue, A. Casadevall, and B. R. Bloom. 1998. A MAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA 95:15688-15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trotter, C. L., M. E. Ramsay, and E. B. Kaczmarski. 2002. Meningococal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Commun. Dis. Public Health 5:220-225. [PubMed] [Google Scholar]
- 26.Valway, S. E., M. C. Sanchez, T. F. Shinnick, I. Orme, T. Agerton, D. Hoy, J. S. Jones, H. Westmoreland, and I. M. Onorato. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338:633-639. [DOI] [PubMed] [Google Scholar]