Abstract
Setting:
One reference and three zonal laboratories and 500 health facilities managing retreatment tuberculosis (TB) patients in Tanzania.
Objectives:
The National Tuberculosis and Leprosy Programme (NTLP) requires that all notified cases of retreatment TB in Tanzania have sputum samples sent for culture and drug susceptibility testing (DST). This study determined 1) if the number of annually notified retreatment patients corresponded to the number of sputum samples received by the reference laboratories, and 2) the number of culture-positive samples and the number of cases undergoing DST.
Design:
Nine-year audit of country-wide programme data from 2002 to 2010.
Results:
Of the 40 940 retreatment TB patients notified by the NTLP, 3871 (10%) had their sputum samples received at the reference and zonal laboratories for culture and DST. A total of 3761 (97%) sputum samples were processed for culture, of which 1589 (42%) were found to be culture-positive and 1415 (89%) had DST performed.
Conclusions:
There is a >90% shortfall between notified retreatment cases and numbers of sputum samples received, cultured and assessed for DST at reference and zonal laboratories. Steps needed to address this problem are discussed.
Keywords: Tanzania, retreatment TB, operational research, sputum, DST
Abstract
Contexte:
Un laboratoire de référence, trois laboratoires de zone et 500 services de santé prenant en charge le retraitement de patients tuberculeux en Tanzanie.
Objectifs:
Le Programme National de Tuberculose et de Lèpre (NTLP) exige que dans tous les cas déclarés de retraitement en Tanzanie des échantillons de crachats soient envoyés pour culture et tests de sensibilité aux médicaments (DST ). Cette étude a déterminé 1) si le nombre de patients en retraitement déclarés chaque année correspond au nombre d’échantillons de crachats reçus par les laboratoires de référence, et 2) le nombre d’échantillons à culture positive et le nombre où le DST a été exécuté.
Schéma:
Audit de 9 années des données du NTLP pour l’ensemble du pays pendant la période 2002–2010.
Résultats:
Sur les 40 940 patients en retraitement déclarés par le NTLP, chez 3871 (10%) les échantillons de crachats ont été reçus pour culture et DST dans les laboratoires de référence et de zone. Au total, 3761 échantillons de crachats (97%) ont été traités pour culture, chez 1589 (42%) la culture a été positive et chez 1415 (89%) la DST a été réalisée.
Conclusions:
Il y a un déficit de >90% entre le nombre de cas de retraitement déclarés et le nombre d’échantillons de crachats reçus, cultivés et évalués par DST dans les laboratoires de référence et de zone. Les étapes à franchir pour répondre à ce problème font l’objet d’une discussion.
Abstract
Marco de referencia:
Un laboratorio de referencia, tres laboratorios zonales y 500 centros de salud que atienden a los pacientes en retratamiento antituberculoso en Tanzanía.
Objetivos:
El Programa Nacional contra la Tuberculosis y la Lepra (NTLP) exige que en todos los casos notificados en retratamiento de tuberculosis (TB) en Tanzanía se recojan muestras de esputo, a fin de remitirlas para cultivo y pruebas de sensibilidad a los medicamentos (DST). En el presente estudio se buscó 1) verificar si el número de pacientes notificados anualmente en retratamiento corresponde al número de muestras de esputo recibidas en los laboratorios de referencia y 2) comparar el número de muestras con cultivo positivo y el número de cultivos con DST.
Métodos:
Se practicó una auditoria a escala nacional de los datos del NTLP del periodo 2002–2010.
Resultados:
De 40 940 casos notificados en retratamiento antituberculoso por el NTLP, los laboratorios de referencia y los laboratorios zonales recibieron muestras de 3871 pacientes (10%) para cultivo y DST; de estas muestras 1589 (42%) presentaron un cultivo positivo y se practicó el antibiograma en 1415 muestras (89%).
Conclusión:
Existe un déficit de >90% entre los casos notificados en retratamiento y el número de muestras de esputo recibidas y de cultivos y DST realizadas en los laboratorios de referencia y los laboratorios zonales. En el artículo se analizan las medidas necesarias a fin de superar estas deficiencias.
Tanzania is one of the sub-Saharan African countries with the highest burden of tuberculosis (TB) globally.1 In 2010, there were 63 453 notified cases of TB, the majority of which (94%) were newly registered TB cases (receiving anti-tuberculosis treatment for the first time) while 6% had been treated previously (retreatment cases).2 Retreatment patients are of public health importance as they form a sub-group in whom first-line TB treatment has not worked either because of poor treatment adherence or because of primary or secondary drug resistance. Such patients might have multidrug-resistant TB (MDR-TB, i.e., resistance to at least isoniazid [INH] and rifampicin [RMP]),3 and the National TB and Leprosy Programme (NTLP) will need to provide treatment for all those with MDR-TB.
To offer the optimum treatment regimen to retreatment cases, national guidelines have required for over 10 years that their sputum samples undergo culture for Mycobacterium tuberculosis and drug susceptibility testing (DST). Monitoring this process would inform the NTLP about whether or not national policy is being implemented. Although such monitoring is not part of routine supervision and reporting, anecdotal evidence suggests that a considerable proportion of sputum samples of retreatment cases do not reach the reference or zonal laboratories, and of those that do arrive, some do not have growth on culture or may be found to be infected with mycobacteria other than TB, commonly known as atypical mycobacteria. DST of culture samples might also be incomplete.
To our knowledge, no recent studies have been performed in sub-Saharan Africa to quantify the above-mentioned gaps. We thus conducted a study to determine 1) if the number of annually notified retreatment cases corresponded to the number of sputum sam-ples received by the reference laboratories, and 2) the number of samples that were culture-positive and had DST results.
METHODS
Study setting and population
The study was conducted between June and November 2012 in Tanzania, an African country with a population of about 46 million, with a high TB and human immunodeficiency virus burden. The study included 1) all TB patients who were notified as retreatment cases between 2002 and 2010, and 2) culture and DST results at the reference and three zonal laboratories.
Study design
This was a cross-sectional analysis of routine programme data.
Management of sputum samples from notified retreatment tuberculosis cases
Retreatment TB cases receive a retreatment drug regimen according to national and World Health Organization guidelines.4 More than 500 public and private health facilities conduct sputum smear microscopy for TB diagnosis in Tanzania. Each patient diagnosed as being a retreatment case is required to submit one sputum sample for culture at one of the three zonal reference laboratories located in different regions of the country or at the Central Tuberculosis Reference Laboratory (CTRL) located in Dar es Salaam. The primary responsibility for sending sputum specimens of retreatment cases lies with the health facilities that diagnose the retreatment cases. If a specimen grows on culture at the zonal laboratory, the positive isolates are then sent to the CTRL for DST. Sputum samples and isolates are transported through the normal postal service.
Routine culture and drug susceptibility testing methods
While anti-tuberculosis DST is only performed at the CTRL, sputum culture is conducted at four sites: the zonal TB reference laboratories at Bugando Medical Centre, Kilimanjaro Christian Medical Centre and Mbeya Referral Hospital and the CTRL. Culture is performed on Löwenstein Jensen solid media, and positive culture isolates are sent to the CTRL for DST. DST is performed for four of the first-line anti-tuberculosis drugs: RMP, INH, ethambutol and streptomycin, using the resistance ratio method.5
Data collection and analysis
Data were obtained from nine annual NTLP reports and 21 laboratory registers from the zonal and reference laboratories covering this 9-year period. Data were double-entered by two independent encoders, and validated using EpiData entry software, version 3.1 (EpiData Association, Odense, Denmark). Discordances were resolved by cross-checking with the paper registers. Data were exported to SPSS version 17.1 (SPSS Statistics, Chicago, IL) for analysis.
Definition of terms
Retreatment TB patients comprised the following categories: 1) ‘treatment failures’ were patients with a positive sputum smear at ≥5 months of treatment; 2) ‘relapses’ were patients who successfully completed treatment and who returned with smear-positive pulmonary TB (PTB); 3) ‘return after loss to follow-up’ were patients who returned to the programme sputum smear-positive after being lost to follow-up (>2 months); and 4) ‘retreatment others’ were patients who didn’t fit the above three definitions, such as those previously treated but with unknown outcome of that previous treatment, and/or who returned for treatment with smear-negative PTB or bacteriologically negative extra-pulmonary TB.6,7
Ethics approval
This study met the approved criteria for analysis of routinely collected programme data of the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, and received clearance from the National Health Research Ethics Committee of the Medical Research Coordinating Committee in Tanzania.
RESULTS
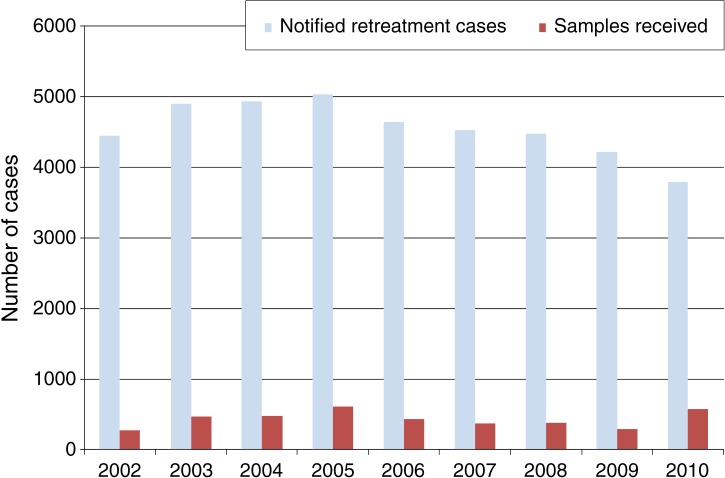
Of 40 940 notified retreatment cases included in the study, 900 (2%) had failed treatment, 1890 (5%) had returned after being lost to follow-up, 15 283 (38%) had relapsed and 22 867 (60%) cases were ‘retreatment others’. Of these retreatment patients, 3871 (10%) sputum samples were received at the central reference and zonal laboratories. The Figure shows the shortfall in sputum samples received in comparison with notified retreatment cases for the period 2002–2010.
FIGURE.
Number of annually notified retreatment cases and annual number of sputum samples received by reference laboratories for culture and drug susceptibility testing in Tanzania, 2002–2010.
The Table shows the comparison of the notified retreatment cases and the progressive losses in numbers at the various stages from sputum reception at the laboratory to mycobacterial culture and eventual DST.
TABLE.
Annually notified retreatment cases and sputum samples received and processed by zonal and reference laboratories for culture and DST in Tanzania, 2002–2010
| Year | Pre-laboratory phase |
Laboratory phase |
|||
| Notified retreatment cases | Samples received | Samples with culture done | Samples culture-positive | Samples with DST done | |
| N | n (%) | n (%) | n (%) | n (%) | |
| 2002 | 4 445 | 277 (6) | 271/277 (98) | 69/271 (26) | 21/69 (30) |
| 2003 | 4 896 | 471 (10) | 423/471 (90) | 90/423 (21) | 31/90 (34) |
| 2004 | 4 931 | 476 (10) | 463/476 (97) | 100/463 (22) | 96/100 (2) |
| 2005 | 5 032 | 608 (12) | 586/608 (96) | 331/586 (57) | 322/331 (96) |
| 2006 | 4 635 | 431 (9) | 417/431 (97) | 191/417 (46) | 190/191 (99) |
| 2007 | 4 525 | 369 (8) | 366/369 (99) | 221/366 (60) | 216/221 (98) |
| 2008 | 4 474 | 376 (8) | 372/379 (98) | 197/372 (53) | 169/197 (86) |
| 2009 | 4 217 | 291 (7) | 291/291 (100) | 162/291 (56) | 148/162 (91) |
| 2010 | 3 785 | 572 (15) | 572/572 (100) | 228/572 (40) | 222/228 (97) |
| Total | 40 940 | 3871 (10) | 3761 (97) | 1589 (42) | 1415 (89) |
DST = drug susceptibility testing.
DISCUSSION
This study shows that less than one in every 20 notified retreatment TB cases underwent DST, primarily due to failure to receive sputum samples at the reference and zonal laboratories. There is thus a major gap between existing policy guidelines and the performance of the NTLP.
The strengths of this study are that we conducted our audit over a 9-year period, data were sourced from programme registers and nationally endorsed reports, and as the analysis included countrywide data it is likely to be representative on a national level. We also followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies8 and ethical considerations for operational research.9 A limitation is that we were unable to conduct a stratified analysis by category of retreatment TB because this information was inconsistently filled out in the laboratory request forms and the laboratory registers. Other limitations include the inability to validate the previously collected routine data and the possibility that reference laboratories discarded sputum samples that consisted only of saliva or with long transfer times without being recorded.
The findings from this study raise a number of issues that merit discussion. First, the number of sputum samples from retreatment cases that did not reach the laboratory level was unacceptably high, at 91%. We do not know the reasons for this, but possibilities include poor guideline implementation by clinicians, weaknesses in the sputum transportation system,10 shortcomings in recording and limited laboratory capacity.11,12 Further detailed assessment is needed through prospective studies. Second, immediate measures should be put in place to correct this situation, including the introduction of quarterly monitoring and reporting of gaps in sputum samples received from retreatment cases, and regular supervisory visits to check the accuracy of the data. An audit of the sputum transport chain is also needed to identify areas that would benefit from direct support. Third, the evidence from this audit shows that the performance of the laboratories is good, especially in recent years; the main problem thus appears to lie in getting sputum specimens from the peripheral health facilities to the laboratories.
The primary purpose of sending sputum samples systematically from retreatment cases to the laboratory is to detect drug-resistant TB and administer appropriate treatment regimens. Failure of the laboratory to receive sputum specimens implies that those with drug-resistant TB will not be diagnosed and they therefore risk receiving sub-optimal treatment. This in turn could lead to the development and transmission of MDR-TB, which is a major public health concern. Although MDR-TB estimates in Tanzania in 2006 were low, at 3.2% in retreatment cases, the first-line treatment regimen in Tanzania has since been shortened (to include RMP) and the DOTS model has changed.13 Both these changes could potentially increase the risk of MDR-TB, and this makes it more important than ever to conduct DST in retreatment patients.
A final point is whether the laboratories could cope with the increased workload associated with all retreatment patients submitting their sputum specimens for culture and DST. From an operational perspective, this might require first prioritising culture and DST for high-risk groups such as those who fail treatment. The way forward is to strengthen the overall laboratory capacity and foster decentralisation, with the use of rapid DST methods. Test assays such as Xpert® MTB/RIF (Cepheid, Inc., Sunnyvale, CA, USA) are promising, but remain expensive.14,15
CONCLUSION
This study reveals a considerable gap between notified retreatment cases and sputum samples received for culture and DST, and immediate steps should be taken to address this problem. These include setting up monitoring and evaluation of this particular aspect of TB control, supervision to ensure data accuracy and a review of sputum transport/transfer logistics to identify areas that would benefit from direct support.
Acknowledgments
This research was supported through an operational research course that was jointly developed and run by the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease, Paris, France (The Union), and the Operational Research Unit, Médecins Sans Frontières, Brussels–Luxembourg. Additional support for running the course was provided by the Institute for Tropical Medicine, Antwerp, Belgium; the Center for International Health, University of Bergen; and the University of Nairobi, Kenya.
Funding for the course was from an anonymous donor, the Department for Inter-national Development, United Kingdom, and Médecins Sans Frontières, Brussels Operational Center, MSF–Luxembourg.
Conflict of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis control. WHO report 2011. WHO/HTM/TB/2011.16. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 2.Tanzania Ministry of Health and Social Welfare. National Tuberculosis and Leprosy Programme. Annual report 2010. Dar es Salaam, Tanzania: Ministry of Health and Social Welfare; 2011. [Google Scholar]
- 3.Burgos M, DeRiemer K, Small P M, Hopewell P C, Daley C L. Effect of drug resistance on the generation of secondary cases of tuberculosis. J Infect Dis. 2003;188:1878–1884. doi: 10.1086/379895. [DOI] [PubMed] [Google Scholar]
- 4.Tanzania Ministry of Health and Social Welfare. National Tuberculosis and Leprosy Programme. Manual of the National Tuberculosis and Leprosy Programme in Tanzania. 2010 ed. Dar es Salaam, Tanzania: Ministry of Health and Social Welfare; 2010. [Google Scholar]
- 5.Rieder H L, Chonde T M, Myking H, et al. The Public Health Service National Tuberculosis Reference Laboratory and the National Laboratory Network. Paris, France: International Union Against Tuberculosis and Lung Disease; 1998. [Google Scholar]
- 6.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. WHO/HTM/TB/2009.420. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 7.Srinath S, Sharath B, Santosha K, et al. Tuberculosis ‘retreatment others’: profile and treatment outcomes in the state of Andhra Pradesh, India. Int J Tuberc Lung Dis. 2011;15:105–109. [PubMed] [Google Scholar]
- 8.von Elm E, Altman D G, Egger M, Pocock S J, Gotzsche P C, Vandenbroucke J P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 9.Edginton M, Enarson D, Zachariah R, et al. Why ethics is indispensable for good-quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harries A D, Michongwe J, Nyirenda T E, et al. Using a bus service for transporting sputum specimens to the Central Reference Laboratory: effect on the routine TB culture service in Malawi. Int J Tuberc Lung Dis. 2004;8:204–210. [PubMed] [Google Scholar]
- 11.Chonde T M, Basra D, Mfinanga S G M, et al. National anti-tuberculosis drug resistance study in Tanzania. Int J Tuberc Lung Dis. 2010;14:967–972. [PubMed] [Google Scholar]
- 12.Mfinanga G S, Ngadaya E, Mtandu R, et al. The quality of sputum smear microscopy diagnosis of pulmonary tuberculosis in Dar es Salaam,Tanzania. Tanzan Health Res Bull. 2007;9:164–168. doi: 10.4314/thrb.v9i3.14323. [DOI] [PubMed] [Google Scholar]
- 13.Egwaga S, Mkopi A, Range N, et al. Patient-centred tuberculosis treatment delivery under programmatic conditions in Tanzania: a cohort study. BMC Med. 2009;7:80. doi: 10.1186/1741-7015-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Stop TB Partnership Retooling Task Force, Stop TB Partnership New Diagnostics Working Group. New laboratory. Diagnostic tools for tuberculosis control. Geneva, Switzerland: WHO; 2009. http://www.stoptb.org/retooling Accessed May 2013. [Google Scholar]
- 15.Vassall A, van Kampen S, Sohn H, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]