Abstract
Setting:
Antiretroviral treatment (ART) clinic at Zomba Central Hospital, Malawi.
Design:
Retrospective analysis of records (2004–2011) of human immunodeficiency virus (HIV) infected patients with Kaposi’s sarcoma (KS).
Objectives:
To determine the number and characteristics of HIV-infected adult patients with KS on ART and vincristine (VCR) therapy and their treatment outcomes.
Results:
A total of 545 HIV-infected patients with KS (58% male, median age 33 years) were included in the study. The baseline median CD4 count was 180 cells/µl (interquartile range 111–287). Cumulative outcomes were as follows: 168 (31%) were still alive, 133 (24%) had died, 172 (32%) were lost to follow-up and 71 (13%) had transferred out; 229 had received at least one course of VCR, 171 had received less than one full course and 145 had not received VCR. The survival probability for 229 patients who received at least one course of VCR was 65% at 1 year, 42% at 2 years and 13% by 6 years. Patients who started VCR therapy before or concurrently with ART had a higher risk of death and generally a higher risk of death and loss to follow-up than those who started VCR after ART.
Conclusion:
Poor outcomes were noted in HIV-infected patients with KS in a programme setting in Malawi. Other treatment interventions, including combination and/or second-line chemotherapy and earlier ART initiation, are needed to reduce morbidity and mortality.
Keywords: Malawi, HIV/AIDS, Kaposi’s sarcoma, vincristine, treatment outcomes, operational research
Abstract
Contexte:
La clinique de traitement antiretroviral (ART) à l’Hôpital Central Zomba au Malawi.
Schéma:
Analyse rétrospective des dossiers (2004–2011) de patients atteints d’un sarcome de Kaposi (KS) et infectés par le virus de l’immunodéficience humaine (VIH).
Objectifs:
Déterminer le nombre et les caractéristiques des patients adultes infectés par le VIH et atteints d’un sarcome de Kaposi sous traitement par l’ART et la vincristine (VCR) et les résultats du traitement.
Résultats:
On a noté 545 patients infectés par le VIH et atteints de KS (58% de sexe masculin, âge médian 33 ans). Les décomptes médians de CD4 au départ ont été de 180 cellules/µl (intervalle interquartile 111–287). Les résultats cumulatifs ont été les suivants : 168 (31%) patients en vie, 133 (24%) décédés, 172 (32%) perdus de vue et 71 (13%) transférés vers l’extérieur. Au moins une cure de VCR a été administrée à 229 patients, 171 ont reçu moins d’une cure complète et 145 n’en ont pas reçu du tout. La probabilité de survie pour les 229 patients ayant reçu au moins une cure de VCR a été de 65% à 1 an, de 42% à 2 ans et de 13% jusqu’à 6 ans. Les patients qui ont commencé le traitement par la VCR avant ou conjointement avec l’ART connaissent un risque plus élevé de décès et, de manière générale, un risque plus élevé de décès et de perte de suivi que ceux qui ont commencé la VCR après l’ART.
Conclusion:
Chez les patients atteints de KS et infectés par le VIH, les résultats sont médiocres dans un contexte de programme au Malawi. D’autres interventions thérapeutiques comportant une chimiothérapie combinée et/ou de deuxième ligne ainsi qu’une mise en route plus précoce de l’ART sont nécessaires pour réduire la morbidité et la mortalité.
Abstract
Marco de referencia:
El consultorio de tratamiento antirretrovírico (ART) en el Hospital Central de Zomba en Malaui.
Métodos:
Fue este un análisis retrospectivo de las historias clínicas de los pacientes con diagnóstico de infección por el virus de la inmunodeficiencia humana (VIH) y sarcoma de Kaposi (KS).
Objetivos:
Determinar el número de pacientes adultos infectados por el VIH que presentan KS y reciben ART y vincristina (VCR) y evaluar sus características y los desenlaces terapéuticos.
Resultados:
Se encontraron 545 casos de pacientes con diagnóstico VIH y KS (58% eran hombres), con una edad mediana de 33 años. La mediana del recuento basal de células CD4 fue 180 celulas/µl (intervalo intercuartil 111–287). Los desenlaces acumulados se distribuyeron como sigue: 168 pacientes vivos (31%), 133 defunciones (24%), 172 perdidos durante el seguimiento (32%) y 71 transferidos a otros centros (13%). Doscientos veintinueve pacientes recibieron como mínimo un ciclo de VCR, 171 recibieron menos de un ciclo completo y 145 pacientes no recibieron VCR. La probabilidad de supervivencia de los 229 pacientes que recibieron como mínimo un ciclo de tratamiento con VCR fue 65% al año, 42% a los 2 años y 13% a los 6 años. En general, los pacientes que comenzaron un tratamiento con VCR antes del ART o de manera simultánea presentaron un mayor riesgo de mortalidad y de pérdida durante el seguimiento que los pacientes que iniciaron la VCR después de haber comenzado los medicamentos ART.
Conclusión:
Dentro de un marco programático, se observaron desenlaces clínicos desfavorables en los pacientes con diagnóstico de infección por el VIH y KS en Malaui. Con el propósito de disminuir la morbilidad y la mortalidad de estos pacientes es necesario introducir nuevas intervenciones terapéuticas como los tratamientos combinados o el tratamiento de segunda línea e iniciar de manera más temprana el ART.
In most African countries in the sub-Saharan region, Kaposi’s sarcoma (KS) is the most common human immunodeficiency virus (HIV) related malignancy. Despite increasing access to antiretroviral treatment (ART) and the use of chemotherapy, the burden of KS is still high in resource-limited settings, and outcomes of HIV-infected patients with KS remain poor.1–5
Malawi, one of the poorest countries in Southern Africa, is severely affected by the HIV epidemic. The current HIV prevalence in the country is 12%, with over 500 000 patients cumulatively started on ART as of 31 December 2011.6,7 In December 2011, the national ART data reported that 14 431 (3%) patients on ART had KS,7 and that KS accounted for 34% of all malignancies (HIV-related and non-HIV-related) in the country.1 The national ART guidelines recommend vincristine (VCR) as standard chemotherapy for patients with moderate to severe forms of KS and bleomycin as second-line treatment in addition to ART.8 However, bleomycin is not available in most ART clinics in the country and patients therefore receive multiple courses of VCR in case of non-response or relapse after the initial course of treatment. There is no lifetime dose for VCR, but toxicity often limits prolonged treatment.4,9,10 The cost of one course of VCR per person is about US$105. The cost of one course of bleomycin per patient is 10 times greater and that of 1 year of ART (stavudine [d4T] + lamivudine [3TC] + nevirapine [NVP]) two thirds greater than that of VCR.
Routine ART data at the Zomba Central Hospital, a referral hospital in South East Malawi that has implemented the national ART guidelines since 2004, show growing numbers of KS patients failing or relapsing after a course of VCR; 30% of these receive multiple courses of VCR while in care. There is a need to better understand this phenomenon and study long-term outcomes of patients on ART. Several studies reporting on short-term outcomes of KS patients on ART or chemotherapy have been published,2,10–12 but data on long-term KS patient outcomes on ART and single-agent chemotherapy in Africa are still scarce.
Our aim was therefore to document long-term KS treatment outcomes of adult patients aged ≥15 years diagnosed between 2004 and 2011 in Zomba, Malawi, and to evaluate how these relate to management strategies. We specifically set out 1) to describe the number and proportion of HIV-infected adult patients diagnosed and registered with KS and who were on ART, and the number and proportion receiving VCR; 2) to determine the survival and standardised cumulative ART outcomes for all KS patients; and 3) to report on successful completion, failure and relapse rates of VCR monotherapy.
METHODS
Study design
This was a descriptive, retrospective cohort analysis using records of HIV-infected KS patients registered at the Zomba Central Hospital ART clinic between 2004 and 2011.
Setting
The study took place at the Zomba Central Hospital ART Clinic, a referral hospital for eight hospitals in the South-East Zone of Malawi. Patients presenting with lesions resembling KS are assessed and a diagnosis is made on clinical grounds at the ART clinic. For visceral KS, additional investigations may include X-ray, ultrasound scanning and effusion analysis. The only histopathology diagnostic service is 60 km away from the Zomba Central Hospital, and as this is a paying service it was unaffordable for most patients.
All patients with KS undergo HIV testing according to national ART guidelines.8 All HIV-infected KS patients are categorised as World Health Organization (WHO) clinical Stage 4 and meet the eligibility criteria for ART, and all are started on standard first-line ART consisting of nucleoside reverse transcriptase inhibitors and non-nucleoside reverse transcriptase inhibitors (d4T/3TC/NVP). HIV-infected patients with KS may be enrolled for VCR chemotherapy before ART, at the same time as ART or after ART has been started. Clinicians categorise the stage of KS based on the Simplified Classification System,13 which was adopted for its applicability in a laboratory-constrained hospital (Table 1).
TABLE 1.
Simplified classification of KS13
| Stage 1 | Localised nodular KS with <15 cutaneous lesions |
| Stage 2 | Localised, invasive and aggressive KS |
| Stage 3 | Disseminated mucocutaneous lymphadenopathy KS, with or without skin lesions and no visceral involvement |
| Stage 4 | Disseminated visceral KS |
KS = Kaposi’s sarcoma.
In terms of management, as national guidelines recommend, asymptomatic patients with Stage 1 KS are started on ART alone. Patients with Stage 1 KS with severe pain or swelling and those with Stages 2–4 KS are routinely scheduled for VCR chemotherapy. A full course of VCR consists of weekly intravenous injections of 2 mg VCR for the first 6 weeks, followed by one injection every 2 weeks for the next 12 weeks, followed by one injection every 4 weeks for the next 6 months.8 Patients who fail treatment or who relapse with severe symptoms may be offered a second, and sometimes a third course of VCR as they continue ART and KS follow-up.
Patients
All HIV-infected adult patients aged ≥15 years attending the Zomba Central Hospital enrolled for ART from 2004 to 2011 and who had a diagnosis of KS were included in the study.
Data collection and analysis
Data were collected from routine patient records into a specially designed questionnaire; these included ART and VCR start dates, age, sex, KS stage, baseline CD4 count (measured as cells/μl), VCR treatment relapse, VCR treatment failure and standardised ART outcomes, which were censored at the end of December 2011. We defined as VCR treatment failure the failure of KS lesion(s) to regress or the progression of KS lesions at the end of a course of VCR, and relapse as the recurrence of KS lesion(s) ≥3 months after initial resolution or regression on completing a course of VCR.
Data were double-entered into EpiData Version 3.1 (EpiData Association, Odense, Denmark) and were analysed using both EpiData and SPSS (Statistical Product and Service Solutions, Chicago, IL, USA). Descriptive analyses included computing proportions for categorical data, and medians with interquartile ranges (IQRs) for continuous data. χ2 tests were used to compare categorical variables and non-parametric tests to compare medians. Survival curves were constructed using Kaplan-Meier estimates. Multivariable logistic regression analyses were performed to estimate risk of death and loss to follow-up related to timing of ART and VCR. Levels of significance were set at 0.05 for all statistical testing.
Ethics approval
The study was approved by the National Health Sciences Research Committee of Malawi and the Ethics Advisory Group (EAG) of the International Union Against Tuberculosis and Lung Disease, Paris, France.
RESULTS
Of 18 800 HIV-infected patients registered for ART between 2004 and 2011, 545 were diagnosed with KS, representing 3% of all patients on ART. Of the 545 HIV-infected patients with KS, 318 (58%) were males (Table 2). The median age was 33 (IQR 30–39) years. No baseline CD4 counts were recorded for 57% of the patients, and for those with baseline CD4 counts the median CD4 was 180 cells/μl (IQR 111–278). Over 80% of patients had CD4 counts of <350 cells/μl. Nearly 20% of the patients had KS Stage 4 disease with visceral involvement.
TABLE 2.
Characteristics of 545 HIV-infected patients with KS on ART and VCR, 2004–2011, Zomba, Malawi
| Patient characteristic | Completed at least one course of VCR (n = 229) n (%) | Received less than one full course of VCR (n = 171) n (%) | Did not receive any VCR (n = 145) n (%) | P value | |
| Male sex | 143 (62) | 96 (56) | 79 (54) | 0.27 | |
| Age, years, median [IQR] | 34 [30–40] | 33 [27–40] | 32 [28–38] | 0.13 | |
| CD4 count, cells/µl | |||||
| Not done | 74 (33) | 123 (72) | 110 (76) | 0.001 | |
| <50 | 19 (12) | 6 (12) | 7 (20) | 0.71 | |
| 50–199 | 68 (44) | 21 (43) | 10 (29) | 0.47 | |
| 200–350 | 42 (27) | 14 (29) | 10 (29) | 0.98 | |
| >350 | 25 (16) | 8 (16) | 8 (23) | 0.63 | |
| KS stage | 0.02 | ||||
| Stage 1 | 29 (13) | 25 (15) | 33 (23) | 0.03 | |
| Stage 2 | 102 (45) | 59 (35) | 53 (37) | 0.08 | |
| Stage 3 | 58 (25) | 50 (29) | 43 (30) | 0.49 | |
| Stage 4 | 40 (18) | 37 (22) | 16 (11) | 0.05 | |
| Outcomes | |||||
| Alive on ART | 106 (47) | 36 (21) | 26 (18) | 0.001 | |
| Died | 74 (32) | 35 (20) | 24 (17) | 0.001 | |
| Lost to follow-up | 32 (14) | 82 (48) | 58 (40) | 0.001 | |
| Transferred out | 16 (7) | 18 (11) | 37 (26) | 0.001 | |
HIV = human immunodeficiency virus; KS = Kaposi’s sarcoma; ART = antiretroviral therapy; VCR = vincristine; IQR = interquartile range.
Two hundred and twenty-nine (42%) patients had received at least one complete course of VCR, 171 (31%) had started but not completed a single course of VCR and 145 (27%) had not received any VCR. A higher proportion of patients with Stage 1 disease (P = 0.03) and a lower proportion with Stage 4 disease (P = 0.05) had not received VCR (Table 2).
Among the 545 patients, 168 (31%) were alive and 133 (24%) had died, with higher proportions found in the group who had completed at least one full course of VCR (Table 2). A total of 172 (31%) patients were lost to follow-up, with higher proportions lost to follow-up in the groups that did not complete a single course of VCR (48%) and did not receive VCR (40%) than the group that received at least one complete course (14%). Seventy-one (13%) patients transferred out, with a higher transfer out rate in the group that did not receive VCR (26%) than both the group that received at least one complete course (7%) and the group that did not complete a single course of VCR (11%; Table 2).
Of 229 patients who completed at least one course of VCR, 106 (47%) were alive as of December 2011; 74 (70%) had completed treatment without complications, 24 (23%) had relapsed and 9 (8%) had failed treatment. No significant differences in sex, age, CD4 count strata and KS stage characteristics were found among these groups.
The baseline characteristics of the patients were compared in relation to timing of VCR and ART among patients who completed at least one course of VCR and among all patients who received VCR; no significant differences were observed between the groups. Table 3 illustrates the results of this analysis among all patients who received both VCR and ART: 400 patients received both ART and VCR; four (1%) had no VCR starting date. Of the remaining patients with known VCR start dates, 90 (23%) started VCR chemotherapy before ART, 109 (28%) started simultaneously with ART, and 197 (49%) started after ART. The baseline characteristics of the patients in these different groups were similar with regard to sex, age and CD4 count where known, but were different in terms of KS stage. There were also significant differences in cumulative treatment outcomes between the groups (Table 3).
TABLE 3.
Characteristics of patients who started VCR before ART, concurrently with ART and after ART between 2004 and 2011 at Zomba Central Hospital, Malawi
| Characteristic* | VCR before ART (n = 90) n (%) | VCR concurrently with ART (n = 109) n (%) | VCR after ART (n = 197) n (%) | P value |
| Male sex | 56 (62) | 64 (59) | 116 (59) | 0.74 |
| Age, years, median [IQR] | 38 [34–43] | 34.5 [32–39] | 35 [32–39] | 0.73 |
| CD4 count, cells/µl | 0.001 | |||
| Not done | 40 (44) | 79 (74) | 75 (38) | 0.001 |
| <50 | 7 (14) | 2 (7) | 16 (13) | 0.48 |
| 50–199 | 26 (52) | 12 (40) | 51 (42) | |
| 200–350 | 9 (18) | 12 (40) | 34 (28) | |
| >350 | 8 (16) | 4 (13) | 21 (17) | |
| KS stage | 0.004 | |||
| Stage 1 | 9 (10) | 7 (6) | 38 (19) | 0.004 |
| Stage 2 | 30 (33) | 46 (42) | 82 (42) | 0.35 |
| Stage 3 | 25 (28) | 33 (30) | 50 (25) | 0.65 |
| Stage 4 | 26 (29) | 23 (21) | 27 (14) | 0.008 |
| Outcome | 0.001 | |||
| Alive | 33 (37) | 13 (12) | 93 (49) | 0.001 |
| Dead | 36 (40) | 48 (44) | 25 (13) | 0.001 |
| Lost to follow-up | 14 (16) | 38 (35) | 62 (33) | 0.006 |
| Transferred out | 7 (8) | 10 (9) | 10 (5) | 0.94 |
| Death + loss to follow-up | 50 (56) | 86 (79) | 87 (44) | 0.001 |
4 of 400 who started VCR and ART were missing a VCR start date.
VCR = vincristine; ART = antiretroviral therapy; IQR = interquartile range; KS = Kaposi’s sarcoma.
Multivariable logistic regression analyses of death and loss to follow-up in relation to timing of VCR and ART initiation, controlled for sex, age, CD4 strata and KS stage, was undertaken to further analyse possible differences in outcomes between the groups (Table 4). Those given VCR before or concurrently with ART had a higher risk of death and generally a higher risk of death and loss to follow-up compared with those who started VCR after ART.
TABLE 4.
Multivariable logistic regression analysis of death and LTFU with timing of VCR and ART initiation controlled for sex, age, CD4 strata and KS stage in KS patients who received both VCR and ART
| VCR before vs. after ART median [IQR] | VCR concurrently with vs. after ART median [IQR] | VCR before or concurrently with vs. after ART median [IQR] | ||
| Death | 3.8 [2.0–7.1] | 3.9 [2.1–7.2] | 3.9 [2.3–6.6] | |
| Death + LTFU | 1.3 [0.7–2.2] | 3.4 [1.9–6.1] | 2.0 [1.3–3.2] | |
| LTFU | 0.4 [0.2–0.7] | 1.0 [0.6–1.7] | 0.7 [0.4–1.1) | |
LTFU = loss to follow-up; VCR = vincristine; ART = antiretroviral therapy; KS = Kaposi’s sarcoma.
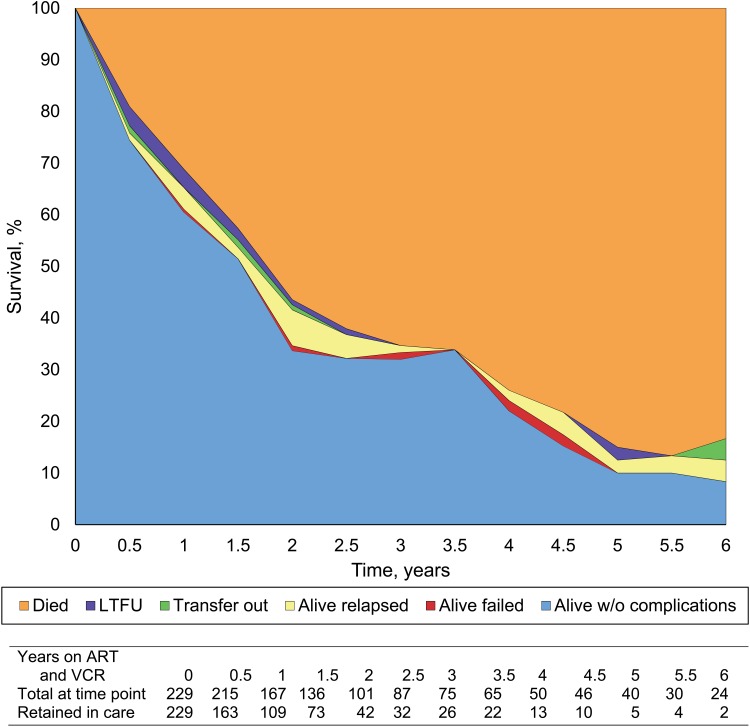
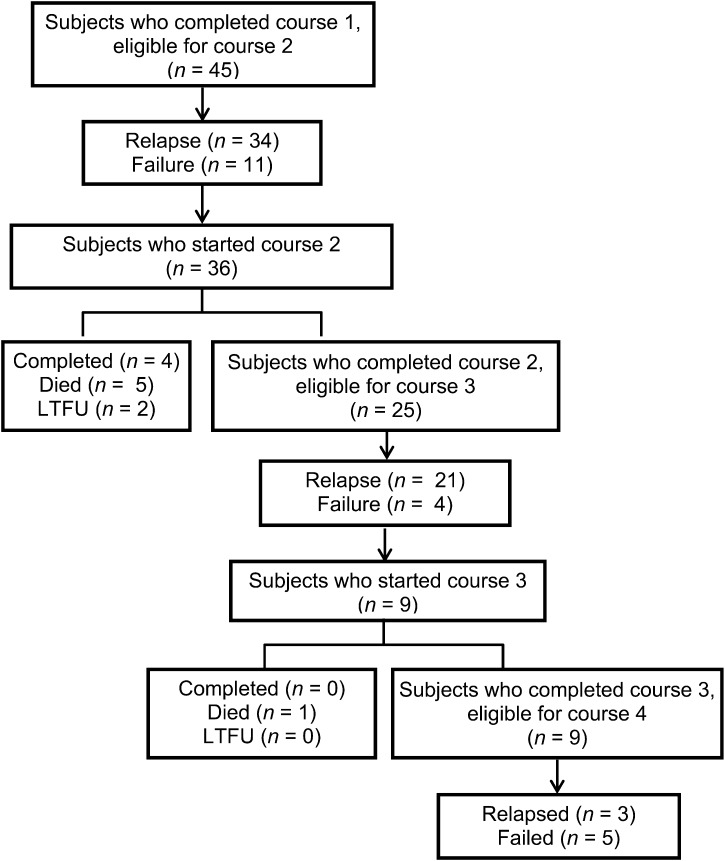
A sub-analysis of the group who completed at least one course of VCR was carried out (Figure 1). The survival probability for the 229 patients who completed at least one course of VCR was 65% at 1 year, 42% at 2 years, and further decreased to <13% by 6 years. Figure 2 shows the outcomes of patients who received multiple courses of VCR. Altogether 45 patients among those who received at least one complete course of VCR were alive and needing to go for a second VCR course due to failure (n = 11) and relapse (n = 34); 36 patients received a second course and 25 were eligible to start a third course due to failure or relapse. Only 9 of the 25 patients actually received a third course, and 8 of these 9 patients failed or relapsed.
FIGURE 1.
Survival curves for HIV-infected patients with KS on ART and VCR, Zomba, Malawi, 2004–2011. Outcomes of KS patients on ART and VCR treatment. KS = Kaposi’s sarcoma; ART = antiretroviral treatment; VCR = vincristine.
FIGURE 2.
Outcomes of patients on ART who received multiple courses of VCR, Zomba, Malawi, 2004–2011. LTFU = loss to follow-up; ART = antiretroviral treatment; VCR = vincristine.
DISCUSSION
This is one of the first studies in sub-Saharan Africa to evaluate long-term treatment outcomes of HIV-infected patients with KS being routinely treated with ART and single-agent VCR chemotherapy. Cumulatively, only 31% of all KS patients enrolled from 2004 to 2011 on ART were alive and still in care at the end of 2011. A previous study in Malawi by Makombe et al. reported that at 6 and 12 months significantly fewer KS patients were alive and significantly more had died or defaulted compared to non-KS patients in public sector clinics in Malawi.5 The most recent national data on ART patients in Malawi showed that of the 57 033 patients who had started ART 5 years previously, 29 087 (51%) were alive and still on ART at 5 years.7 Even for patients receiving a full course of VCR therapy, treatment outcomes were poor, with a survival probability of 13% for patients completing 5–6 years of treatment (Figure 1).
Although more males (58%) with KS diagnosis were registered for ART, no significant differences in baseline characteristics and cumulative treatment outcomes were observed between male and female patients. This is in contrast to a study of characteristics of HIV-1-associated Kaposi’s sarcoma among women and men in South Africa, which reported poorer prognosis and more severe forms of KS in female patients.14 At the time that patients presented for care in the current study, the majority had CD4 cell counts <350 cells/μl and nearly one fifth had disseminated visceral KS. It is likely that such advanced disease for both HIV and KS was responsible for the generally poor treatment outcomes.
There were some interesting differences in patients who received and either completed or did not complete a course of VCR and those who did not receive VCR. First, the data suggest that national guidelines may not be strictly adhered to, as a proportion of patients with Stage 1 KS were placed on VCR. Second, the defined outcomes were clearer for those who completed a single course of VCR, with loss to follow-up and transfer out being considerably less frequent in this group compared with the other groups. Taken together, these findings suggest that the selection of patients completing VCR treatment may have more to do with the ability to attend the clinic than clinical need. Patients unable to attend due to severity of illness, distance, poverty or other factors may be less likely to start VCR or complete a course of treatment. If they are unable to attend, they are often lost to follow-up from the point of view of the central clinic. Routine contact tracing has not been feasible in this setting, but anecdotally many of those patients who are lost to follow-up are thought to have died, with reports not being relayed back to the clinic staff. These data may strengthen the argument for better referral systems and contact tracing. Furthermore, decentralising VCR chemotherapy to peripheral ART facilities might improve the outcomes of KS patients and lead to improved documentation of KS outcomes.
Another potential option to improve patient outcomes is to initiate ART much earlier than is currently the case. There is growing evidence from observational studies that early ART initiation at much higher CD4 counts than is currently the case can reduce HIV-related morbidity.15–17 We attempted to explore this question specifically for KS patients in our subanalysis of the timing of ART and VCR initiation. Our study results show that patients who started VCR after the start of ART were less likely to die than those who started VCR before or at the same time as ART, highlighting a significant protective effect of earlier ART on mortality among HIV-infected KS patients. There may have been bias here due to less sick patients being administered a delayed VCR regimen and very sick patients being started simultaneously on both VCR and ART, although baseline characteristics suggest no large differences between these groups. However, we are aware that our sample was small and retrospective and that these groups may have differed in their disease course in other important ways. This question is nevertheless important and warrants further specific studies.
While several other studies have been carried out assessing the use of chemotherapy and other interventions for HIV-associated KS in sub-Saharan Africa, these have mainly been randomised trials comparing combination chemotherapy regimens, radiotherapy and supportive care.11,12,18–21 Most of the regimens studied require clinical resources that are simply not available in our setting. In one study in Zimbabwe, single-agent oral Etoposide therapy resulted in better survival outcomes than radiotherapy and combination chemotherapy.11 As the main requirement for use is relatively simple laboratory monitoring, this may be a potentially feasible option in the treatment of KS in resource-constrained settings, and warrants further study.
Data from resource-rich settings suggest that systemic chemotherapy can improve outcomes in advanced cases, and that chemotherapeutic agents such as newer liposomal anthracyclines and taxanes have improved efficacy and tolerability compared to older generation drugs such as bleomycin, doxorubicin, VCR, vinblastine or adriamycin.19–21 However, these newer drugs are not available in most programmes in sub-Saharan Africa, mainly because of their high cost. Some of the agents, such as liposomal doxorubicin, are not only expensive, they can also cause haematological and gastrointestinal side effects which would require intensive laboratory monitoring that is not available in our setting. Moreover, a recent systematic review of the literature showed that the evidence for efficacy of any particular intervention for KS was of low quality,21 and findings did not support the recommendation of any particular therapeutic strategy. The authors concluded that further studies are required, and stressed the importance of standardising the assessment of disease activity and clinical response.
In our programme, the second and third courses of single-agent VCR had no added clinical benefits once the first course had failed. According to national ART guidelines, combined bleomycin and VCR therapy are the second-line option for patients who respond poorly to VCR monotherapy. In a Médecins Sans Frontières cohort in Thyolo, Malawi, it was found that although treatment with single-agent bleomycin reduced mortality by half, mortality was still high.22 However, although recommended by the national protocol, bleomycin is not available in most government hospitals. We would recommend that bleomycin be made available in the national programme to avoid continuation of VCR monotherapy in patients who fail to show satisfactory response to the first course of VCR.
Given the high mortality and morbidity rates observed, it is important to ensure that palliative care, better treatment for infected KS and pain relief are available for patients with KS. However, these services need first to be strengthened throughout the country, and they need to function better in collaboration with ART services.
The strengths of this study are the large number of patients, the long-term follow-up and the standardised monitoring and recording system in place at the Zomba Central Hospital. Limitations relate to the operational nature of the study, with information missing from records and lack of laboratory monitoring during follow-up. There was also a lack of information about important morbidity such as peripheral neuropathy due to VCR and stavudine, which might have compromised the acceptance of further VCR treatment by patients. We acknowledge the lack of histopathological diagnosis of KS which might have led to underdiagnosis of visceral and lymph node KS, and the lack of detailed data on the clinical response to VCR therapy, including the impact of treatment on the number and size of KS lesions, as well as lack of data on other WHO Stage 3 or Stage 4 diseases and records. Finally, the structure of our retrospective clinical records does not allow us to reliably identify those patients for whom disease progression after starting ART was the reason for delaying the initiation of VCR. As such, we cannot definitively identify cases of immune reconstitution inflammatory syndrome.23,24
In conclusion, less than optimal outcomes were achieved in the treatment of KS in HIV-infected patients receiving single-agent VCR and a first-line ART regimen consisting of d4T/3TC/NVP in Malawi. Better and more feasible chemotherapy regimens and other interventions are urgently needed for patients with advanced disease in addition to ART. It is possible that early initiation of ART may prevent KS, reduce morbidity and mortality, and improve treatment outcomes in this population. Meanwhile, strengthening palliative care services and the collaboration between palliative and ART programmes, decentralising VCR chemotherapy to ART facilities and making bleomycin available as a second-line treatment option could ease both patient suffering and the disease burden.
Acknowledgments
The authors thank P Chaula and N Kwenje working with Dignitas International, Zomba, Malawi, as data and registration clerks, respectively, and C Joshua of College of Medicine, Malawi, for their support in data collection. They also thank M Chibwe, data officer, College of Medicine, Welcome Trust, Zomba, Malawi, for assistance with the analysis of the data.
This research was supported through an operational research course that was jointly developed and run by the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease, Paris, France, and the Operational Research Unit, Médecins Sans Frontières (MSF), Brussels–Luxembourg. Additional support for running the course was provided by the Institute for Tropical Medicine, Antwerp, Belgium; the Center for International Health, University of Bergen, Bergen, Norway; and the University of Nairobi, Nairobi, Kenya.
Funding for the course was from an anonymous donor, the Department for International Development, London, UK, and MSF, Operational Center Brussels, MSF–Luxembourg.
Conflict of interest: none declared.
References
- 1.Msyamboza K P, Dzamalala C, Make C, et al. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res Notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis H, Bates M J, Kalilani L. Prospective study assessing tumor response, survival, and palliative care outcomes in patients with HIV-related Kaposi’s sarcoma at Queen Elizabeth Central Hospital. Blantyre, Malawi. AIDS Res Treat 2012. 2012:312564. doi: 10.1155/2012/312564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leach-Lemens C. KS in Africa: people with low CD4 counts still at risk despite ART. NAM Aidsmap. London, UK: NAM Publications; 2012. http://www.aidsmap.com/KS-in-Africa-people-with-low-CD4-counts-still-at-risk-despite-ART/page/2284968/ Accessed April 2013. [Google Scholar]
- 4.Mlombe Y. Management of HIV associated Kaposi’s sarcoma in Malawi. Malawi Med J. 2008;20:129–132. doi: 10.4314/mmj.v20i4.10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makombe S D, Harries A D, Yu J K, et al. Outcomes of patients with Kaposi’s sarcoma who start antiretroviral therapy under routine program conditions in Malawi. Trop Doct. 2008;38:5–7. doi: 10.1258/td.2007.060023. [DOI] [PubMed] [Google Scholar]
- 6.Government of Malawi. Malawi HIV and AIDS monitoring and evaluation report 2008–2009. 2010 Country progress report. Lilongwe, Malawi: Government of Malawi; 2012. p. 15. Section 1.3.1. http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2010countries/malawi_2010_country_progress_report_en.pdf Accessed April 2013. [Google Scholar]
- 7.HIV Unit, Ministry of Health of Malawi. Antiretroviral therapy. Quarterly HIV programme report 2011Q4. Lilongwe, Malawi: Government of Malawi; 2011. http://www.hivunitmohmw.org/uploads/Main/Quarterly_HIV_Programme_Report_2011_Q4.pdf Accessed June 2013. [Google Scholar]
- 8.Malawi Ministry of Health, HIV Unit. Malawi integrated guidelines for clinical management of HIV 2011. 1st ed. Lilongwe, Malawi: Malawi Ministry of Health; 2011. http://www.hivunitmohmw.org/uploads/Main/Malawi%20Integrated%20Guidelines%20for%20Clinical%20Management%20of%20HIV%202011%20First%20Edition.pdf Accessed April 2013. [Google Scholar]
- 9.American Hospital Association, Health Research & Educational Trust & Institute for Safe Medication Practices. Pathways for medication safety. Guideline for maximum adult dose limits of parenteral chemotherapy. Chicago, IL, USA: 2002. http://www.medpathways.info/medpathways/tools/content/1_F.pdf Accessed April 2013. [Google Scholar]
- 10.Daily Med Current Medication Information. Vincristine sulfate injection solution. Lake Forest, IL, USA: Hospira Worldwide; 2011. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=57958 Accessed April 2013. [Google Scholar]
- 11.Gudza I, Borok M, Olweny M L, et al. Treatment of AIDS-associated Kaposi’s sarcoma in Zimbabwe: results of a randomized quality of life focused clinical trial. Int J Cancer. 2005;113:632–639. doi: 10.1002/ijc.20606. [DOI] [PubMed] [Google Scholar]
- 12.Krown S E. Treatment strategies for Kaposi sarcoma in sub-Saharan Africa: challenges and opportunities. Curr Opin Oncol. 2011;23:463–468. doi: 10.1097/CCO.0b013e328349428d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz R A. Kaposi sarcoma staging—Medscape reference. New York, NY, USA: WebMD; 2011. [Google Scholar]
- 14.Harris J E, editor. Kaposi sarcoma staging. New York, NY, USA: WebMD; 2011. http://emedicine.medscape.com/article/2007127-overview Accessed April 2013. [Google Scholar]
- 15.Mosam A, Hurkchand H P, Cassol E, et al. Characteristics of HIV-1-associated Kaposi’s sarcoma among women and men in South Africa. Int J STD AIDS. 2008;19:400–405. doi: 10.1258/ijsa.2008.007301. [DOI] [PubMed] [Google Scholar]
- 16.Kitahata M M, Gange S J, Abraham A G, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.When To Start Consortium. Sterne J A, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen M S, Chen Y Q, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Eng J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosam A, Shaik F, Uldrick T S, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012;60:150–157. doi: 10.1097/QAI.0b013e318251aedd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Lorenzo G, Konstantinopoulos P A, Pantanowitz L, Di Trolio R, De Placido S, Dezube B J. Management of AIDS-related Kaposi’s sarcoma. Lancet Oncol. 2007;8:167–176. doi: 10.1016/S1470-2045(07)70036-0. [DOI] [PubMed] [Google Scholar]
- 21.Vanni T, Fonseca B A L, Polanczyk C A. Cost-effectiveness analysis comparing chemotherapy regimens in the treatment of AIDS-related Kaposi’s sarcoma in Brazil. HIV Clin Trials. 2006;7:194–202. doi: 10.1310/hct0704-194. [DOI] [PubMed] [Google Scholar]
- 22.Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2012;68:313–331. doi: 10.1016/j.jaad.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Chu K, Misindec D, Massaquoi M, et al. Risk factors for mortality in AIDS-associated Kaposi sarcoma in a primary care antiretroviral treatment program in Malawi. Int Health. 2010;2:99–102. doi: 10.1016/j.inhe.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Bower M, Nelson M, Young A M, et al. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J Clin Oncol. 2005;23:5224–5228. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]