Abstract
Setting:
A rural paediatric hospital in Bo, Sierra Leone.
Objectives:
To assess the level of adherence to standard treatment guidelines among clinicians prescribing treatment for children admitted with a diagnosis of malaria and/or lower respiratory tract infection (LRTI), and determine the association between (non) adherence and hospital outcomes, given that non-rational use of medicines is a serious global problem.
Design:
Secondary analysis of routine programme data.
Results:
Data were collected for 865 children admitted with an entry diagnosis of malaria and 690 children with LRTI during the period January to April 2011; some patients were classified in both categories. Non-adherence to guidelines comprised use of non-standard drug regimens, dosage variations, non-standard frequency of administration and treatment duration. Cumulative non-adherence to guidelines for LRTI cases was 86%. For malaria, this involved 12% of patients. Potentially harmful non-adherence was significantly associated with an unfavourable hospital outcome, both for malaria and for LRTI cases.
Conclusions:
Overall non-adherence to standard treatment guidelines by clinicians in a routine hospital setting is very high and influences hospital outcomes. This study advocates for the implementation of routine measures to monitor and improve rational drug use and the quality of clinical care in such hospitals.
Keywords: Sierra Leone, rational drug use, non-adherence, operational research, hospital
Abstract
Contexte:
Un hôpital pédiatrique rural à Bo, Sierra Leone.
Objectifs:
Evaluer le niveau d’adhésion aux directives standard de traitement parmi les cliniciens prescrivant des traitements aux enfants admis pour un diagnostic de malaria et/ou une infection du tractus respiratoire inférieur (LRTI), et déterminer l’association entre la (non-) adhésion et les résultats acquis à l’hôpital, étant donné que l’utilisation irrationnelle de médicaments constitue un grave problème mondial.
Schéma:
Analyse secondaire de données de routine du programme.
Résultats:
Pendant la période de janvier à avril 2011, on a colligé les données concernant 865 enfants admis avec un diagnostic d’entrée de malaria et 690 enfants atteints de LRTI, certains patients étant classés à la fois dans les deux catégories. La non-adhésion aux directives a compris l’utilisation d’un régime de médicaments non-standard, des variations de doses, une fréquence non-standardisée d’administration ainsi que la durée du traitement. La non-adhésion cumulative aux directives pour les cas de LRTI a été de 86% ; pour la malaria, elle concernait 12% des dossiers de patients. Une non-adhésion potentiellement néfaste a été en association significative avec un résultat défavorable à la sortie de l’hôpital tant pour les cas de malaria que de LRTI.
Conclusions:
Globalement, la non-adhésion aux directives par les cliniciens dans un contexte hospitalier de routine est très élevée et a une influence sur les résultats obtenus à l’hôpital. L’étude plaide en faveur de la mise en œuvre de mesures de routine pour suivre et améliorer l’utilisation rationnelle des médicaments ainsi que la qualité des soins cliniques dans ces hôpitaux.
Abstract
Marco de referencia:
Un hospital pediátrico en la zona rural de Bo, en Sierra Leona.
Objetivos:
Evaluar la observancia de las directrices de tratamiento normalizado por parte de los médicos que deciden el tratamiento de los niños hospitalizados con diagnóstico de malaria o infecciones de las vías respiratorias inferiores (LRTI) y se determinó la asociación entre el incumplimiento de las normas y los desenlaces clínicos en el momento del alta hospitalaria, visto que la utilización incorrecta de los medicamentos constituye un grave problema a escala mundial.
Métodos:
Se llevó a cabo un análisis secundario de los datos corrientes del programa.
Resultados:
Se recogieron los datos de los niños hospitalizados entre enero y abril del 2011 con diagnóstico de ingreso de malaria en 865 casos y de LRTI en 690 casos; algunos pacientes se clasificaron en ambas categorías. El incumplimiento de las directrices consistió en el uso de un régimen terapéutico diferente o en la modificación de las dosis terapéuticas, la frecuencia de administración o la duración del tratamiento. El incumplimiento acumulado de las directrices relacionadas con las LRTI fue de 86%. En los casos de malaria, se encontró una falta de observancia en el 12% de los casos. Se observó que un incumplimiento terapéutico posiblemente nocivo se asocia de manera significativa con desenlaces hospitalarios desfavorables en ambas enfermedades.
Conclusiones:
En general, el estudio puso en evidencia un alto grado de incumplimiento médico con las directrices de tratamiento normalizado en un entorno hospitalario corriente, con repercusiones notables en los desenlaces de la hospitalización. Se promueve la introducción de medidas sistemáticas que permitan supervisar y mejorar la utilización de los medicamentos y asegurar la calidad de la atención clínica en estos entornos hospitalarios.
Non-rational use of medicines is a huge global problem.1 It may have serious consequences in terms of poor patient outcome, adverse drug reactions, increasing antimicrobial resistance and wasted resources. Rational use of medicines requires that patients take medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time and at the lowest cost to themselves and their community.2
Standard treatment guidelines are designed to ensure that medications are administered in a safe, effective and economic manner, and are very powerful tools in promoting the rational use of medicines. Standard treatment guidelines help prescribers make decisions about appropriate treatments for specific clinical conditions. When medicines are not prescribed in accordance with standard treatment guidelines, it constitutes non-rational use of medicines.3,4
In developing countries, the proportion of patients treated according to clinical standard treatment guidelines in primary care is less than 40% in the public sector and 30% in the private sector.1 For hospital settings, very limited published information is available. Such information in relation to malaria and lower respiratory tract infections (LRTI)—two of the most common diseases—would be particularly useful to assess rational medicine use.
The present study was undertaken to assess the level of adherence to standard treatment guidelines in prescriptions for children admitted with a diagnosis of malaria and/or LRTI in a rural hospital in Sierra Leone. We assessed 1) non-adherence to drug regimen, dosage, frequency of administration and treatment duration, and 2) the association between (non) adherence and hospital outcome.
METHODS
Design
A cross-sectional study of routine programme data.
Study setting
The study took place in the Gondama Referral Cen-tre (GRC) in Bo town, the second largest city in the small West African country of Sierra Leone. Since 2004, Médecins Sans Frontières (MSF) has run the GRC hospital, a second-level referral hospital of approximately 200 functioning beds providing paediatric, in-patient therapeutic feeding and emergency obstetrical services. The hospital targets children aged <15 years and pregnant women with obstetrical complications.
Patient sample
The eligible population included consecutive admissions to the paediatric wards at the MSF Gondama Referral Centre, Bo, Sierra Leone, from 1 January to 30 April 2011, with an entry diagnosis of malaria or LRTI. These two diseases were selected as they represent the most common diseases in the hospital setting, accounting for approximately 73% of all cases (unpublished MSF data). Those eligible for admission to the wards were aged 1 month to 15 years. Study participants were identified through the review of consecutive routine records.
Measuring adherence to standard treatment guidelines
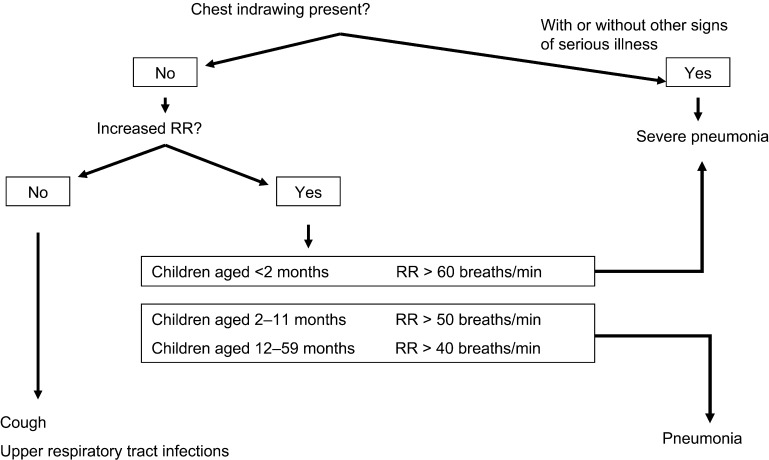
‘Prescription adherence to standard treatment guidelines’ was defined as follows: anti-infective medication prescribed within the first 24 h of admission that is adherent to MSF treatment protocols in terms of treatment regimen (drug type and combination of anti-infective drugs), drug dosage (correct dosage per weight, with an acceptable error margin of 10%), treatment frequency (number of administrations per day) and treatment duration (number of days of treatment). If one of the above conditions was not met, the prescription was classified as ‘non-adherent to standard treatment guidelines’. The standard guidelines in use are shown in Figure 1 and Tables 1 and 2.
FIGURE 1.
Standard treatment guidelines for LRTI. Diagnosis of pneumonia in children aged <5 years presenting with cough or difficulty breathing.5 RR = respiratory rate; LRTI = lower respiratory tract infection.
TABLE 1.
Standard treatment guidelines for LRTI, Gondama Hospital, Sierra Leone
| Age | Type of anti-infective | Dosage | Treatment frequency | Treatment duration |
| A Severe LRTI | ||||
| First-line | ||||
| 1–2 months | Ceftriaxone IM/IV | 50 mg/kg/day | Once daily | Minimum 3 days |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| 2 months–5 years | Ampicillin IM/IV | 100 mg/kg/day | 3 or 4×/day | Until no more serious illness |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| + Gentamicin IM | 3–6 mg/kg/day | Once daily | 7 days | |
| Chloramphenicol IM/IV | 100 mg/kg/day | 3×/day | Minimum 5 days | |
| Followed by chloramphenicol PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| >5 years | Benzylpenicillin procaine + benzylpenicillin IM | 100 000 IU/kg/day | Once daily | 2–3 days |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| Ampicillin IM/IV | 100 mg/kg/day | 3×/day | Until no more serious illness | |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7 days | |
| Second-line | ||||
| 1–2 months | Ampicillin IM/IV | 100 mg/kg/day | 3 or 4×/day | Until no more serious illness |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| + Gentamicin IM | 3–6 mg/kg/day | Once daily | 7 days | |
| 2 months–5 years | Ceftriaxone IM/IV | 50 mg/kg/day | Once daily | Minimum 3 days |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| >5 years | Chloramphenicol IM/IV | 100 mg/kg/day | 3×/day | 2-3 days |
| Followed by chloramphenicol PO | 100 mg/kg/day | 3×/day | Complete 7 days | |
| Ceftriaxone IM/IV | 50 mg/kg/day | Once daily | Minimum 3 days | |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| Persistent pneumonia | ||||
| First-line | Erythromycin PO | 50 mg/kg/day | 2 or 3×/day | 10 days |
| Second-line (>8 years only) | Doxycycline PO | 4 mg/kg/day | 2×/day | 10 days |
| Staphylococcus pneumonia | ||||
| First-line | Cloxacilline IV | 200 mg/kg/day | 3×/day | Until improvement |
| + Gentamicin IM/IV | 7.5 mg | Once daily | ||
| Followed by cloxacillin PO | Complete 10–14 days of treatment | |||
| Alternative (>2 months only) | Chloramphenicol IV | 100 mg/kg/day | 3×/day | Until improvement |
| Chloramphenicol PO | 100 mg/kg/day | 3×/day | Complete 10–14 days of treatment | |
| B Non-severe LRTI | ||||
| 1–2 months | Ceftriaxone IM/IV | 50 mg/kg/day | Once daily | Minimum 3 days |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| 2 months–5 years | Amoxicillin PO | 100 mg/kg/day | 3×/day | 5 days |
| >5 years | Benzylpenicillin procaine + benzylpenicillin IM | 100 000 IU/kg/day | Once daily | 5 days |
| Amoxicillin PO | 100 mg/kg/day | 3×/day | 5 days | |
| Second-line | ||||
| 1–2 months | Ampicillin IM/IV | 100 mg/kg/day | 3 or 4×/day | Until no more serious illness |
| Followed by amoxicillin PO | 100 mg/kg/day | 3×/day | Complete 7–10 days | |
| + Gentamicin IM | 3–6 mg/kg/day | Once daily | 7 days | |
| 2 months–5 years | Chloramphenicol PO | 100 mg/kg/day | 3×/day | 5 days |
LRTI = lower respiratory tract infection; IM = intramuscular; IV = intravenous; PO = oral; IU = international unit.
TABLE 2.
Standard treatment guidelines for malaria, Gondama Hospital, Sierra Leone
| Age | Type of anti-infective | Dosage | Treatment frequency | Treatment duration |
| A Severe malaria | ||||
| First-line | Artemether IM/IV | 3.2 mg/kg | Single dose | Loading dose D1 |
| 1.6 mg/kg | Once daily | Until patient is able to take oral treatment | ||
| Infant (2–11 months) | AS/AQ | AS 25 mg/AQ 67.5 mg (1 tablet) | Once daily | 3 days |
| Child (1–5 years) | AS/AQ | AS 50 mg/AQ 135 mg (1 tablet) | Once daily | 3 days |
| Youth (6–13 years) | AS/AQ | AS 100 mg/AQ 270 mg (1 tablet) | Once daily | 3 days |
| Alternative | Quinine IV | 20 mg/kg/4 h | Infusion | Loading dose D1 |
| 10 mg/kg/8 h | Infusion | Until patient is able to take oral treatment | ||
| Infant (2–11 months) | …then or AS/AQ | AS 25 mg/AQ 67.5 mg (1 tablet) | Once daily | 3 days |
| Child (1–5 years) | AS/AQ | AS 50 mg/AQ 135 mg (1 tablet) | Once daily | 3 days |
| Youth (6–13 years) | AS/AQ | AS 100 mg/AQ 270 mg (1 tablet) | Once daily | 3 days |
| …or quinine PO | Complete 7 days of treatment | |||
| B Non-severe malaria | ||||
| Infant (2–11 months) | AS/AQ | AS 25 mg/AQ 67.5 mg (1 tablet) | Once daily | 3 days |
| Child (1–5 years) | AS/AQ | AS 50 mg/AQ 135 mg (1 tablet) | Once daily | 3 days |
| Youth (6–13 years) | AS/AQ | AS 100 mg/AQ 270 mg (1 tablet) | Once daily | 3 days |
IM = intramuscular; IV = intravenous; AS/AQ = artesunate-amodiaquine.
Potentially harmful non-adherence
Prescriptions classified as ‘non-adherent to standard treatment guidelines’ were assessed for their potential harmfulness. Drug regimen and duration were included in this assessment. A potentially harmful regimen was defined as ‘no treatment prescribed; or treatment prescribed without any antimalarial agent (for a malaria-positive case) or without any antibiotic (for an LRTI case)’. A potentially harmful duration was defined as an antimalarial agent (artesunate-amodiaquine) prescribed for <2 days (for the malaria population) or an antibiotic (amoxicillin or gentamicin) prescribed for <5 days (for the LRTI population). If either regimen or duration were categorised as potentially harmful, the prescription was classified as potentially harmful non-adherence to guidelines. The association with hospital outcome (death or loss to follow-up) was examined. Potential drug toxicity (dosage and frequency) was not included in the assessment of potentially harmful non-adherence.
Variables, data collection and analysis
Records of all anti-infective treatment regimens for malaria and LRTI prescribed in the first 24 h after admission were entered into an Excel database (Microsoft, Redmond, WA, USA) and data entry file developed using EpiData Entry software version 2.2.1.171 (EpiData Association, Odense, Denmark). Data validation of the Excel database involved 100% double checking of entered data by a second person.
The characteristics of the treatment prescribed (type of drug, duration of treatment) depend on the severity of the disease. Severity of disease was assessed based on clinical parameters available in the case files. Variables collected included admission number, patient characteristics (sex, age, weight), treatment characteristics (dose, frequency and duration of anti-infectives prescribed in the first 24 h), a set of clinical parameters (respiratory rate, chest in-drawing [intercostal recession], malaria rapid diagnosis test [paracheck] result, haemoglobin, convulsions, assessment of impaired consciousness, inability to drink, shock). Results were recorded as positive (present), negative (not present) or not recorded.
Data screening for possible non-adherence was performed by an automated search algorithm. Cases flagged as possibly non-adherent were audited independently by two general practitioners. In case of discordance between the two clinicians, a final judgment was made by an experienced paediatrician. Non-adherent cases were classified as potentially harmful according to the above definition.
Ethics
Ethics approval was obtained from the Sierra Leone Ethics and Scientific Review Committee. This study also met the MSF Ethics Review Board-approved criteria for analysis of routinely collected programme data and was approved by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France.
RESULTS
Characteristics of the study population
Data on study subjects with an entry diagnosis of malaria and/or LRTI were extracted from 1602 case files for the period January to April 2011. Of the total 1602 case files, 865 patients (54%) had an entry diagnosis of malaria and 690 (43%) LRTI; some patients (n = 308) were classified in both categories, as both pathologies were diagnosed at the time of admission. The remaining 355 patients had an entry diagnosis other than malaria or LRTI.
Based on the set of clinical parameters assessed, at least 530 (61%) of the malaria cases and at least 514 (74%) of the LRTI cases were classified as severe. The LRTI population included 296 (43%) females and the median age was 11 months (interquartile range [IQR] 6–21). The malaria population included 377 (44%) females; the median age was 24 months (IQR 12–36).
Prescription characteristics
A total of 864 anti-malarial treatments were prescribed for the 865 patients admitted with malaria, the most frequent being artemether (73%), followed by the co-formulation artesunate/amodiaquine (14%) and quinine (13%). Some children did not receive a prescription, while others received multiple anti-malarial prescriptions (e.g., artemether loading dose, followed by artesunate/ amodiaquine).
The 690 patients admitted with LRTI were prescribed 1141 antibiotic treatments in total, consisting of gentamicin (45%) and ampicillin (44%), and to a lesser extent amoxicillin (3%), ceftriaxone (1%) or other antibiotics (6%). As combined intravenous (IV) or intramuscular (IM) ampicillin and gentamicin IM are the first-line treatment for pneumonia in children aged 2 months–5 years, the majority of the LRTI patients (73%) were prescribed two antibiotics per patient in the first 24 h following admission. The remaining LRTI patients were prescribed 0 (15%), 1 (8%), 3 (3%) or 4 (0.4%) antibiotics within the first 24 h of admission.
Non-adherence to standard treatment guidelines
Table 3 shows the overall cumulative level of case files non-adherent to standard treatment guidelines. Treatment of some patients was non-adherent to more than one aspect of the guidelines (regimen-dosage-frequency-duration). Overall adherence to guidelines proved to be a bigger problem for LRTI cases, with 590 (86%) case files being non-adherent to at least one element of the guidelines, compared to 105 (12%) of the malaria case files. Non-adherence to regimen, dosage, frequency and duration were persistently higher for LRTI case files than malaria case files. Furthermore, overall non-adherence appeared to be higher in children aged ≥5 years, for both malaria and LRTI cases. Detailed analysis indicated that among non-adherent malaria cases, potential harm could have been caused in 48 (46%) cases. For LRTI, the potential for harm was identified in 104 of 590 (18%) cases with non-adherence.
TABLE 3.
Overall guideline non-adherence and types of non-adherence to standard treatment guidelines for children (age <15 years) admitted to Gondama Hospital, Sierra Leone, January–April 2011
| Patients with an initial diagnosis of malaria (n = 865) | Patients with an initial diagnosis of LRTI (n = 690) | |||||||
| Total (n = 865) n (%) | Potentially harmful (out of non-adherent) (n = 105) n (%) | Age <5 years (n = 804) n (%) | Age ≥5 years (n = 61) n (%) | Total (n = 690) n (%) | Potentially harmful (out of non-adherent) (n =590) n (%) | Age <5 years (n = 671) n (%) | Age ≥5 years (n = 19) n (%) | |
| Overall guideline non-adherence* | 105 (12.1) | 48 (45.7) | 83 (10.3) | 22 (36.1) | 590 (85.5) | 104 (17.6) | 573 (85.4) | 17 (89.5) |
| Non-adherent to regimen | 72 (8.4) | 47 (44.8) | 57 (7.1) | 15 (24.6) | 153 (22.2) | 102 (17.3) | 144 (21.5) | 9 (47.4) |
| No treatment | 43 (5.0) | 43 (41.0) | 33 (4.1) | 10 (16.4) | 91 (13.2) | 91 (15.4) | 83 (12.4) | 8 (42.1) |
| Incorrect treatment | 29 (3.4) | 4 (3.8) | 24 (3.0) | 5 (8.2) | 62 (9.0) | 11 (1.9) | 61 (9.1) | 1 (5.3) |
| Non-adherent to dosage | 29 (3.4) | NA | 24 (3.0) | 5 (8.2) | 170 (24.6) | NA | 166 (24.7) | 4 (21.1) |
| Non-adherent to frequency | 2 (0.2) | NA | 1 (0.1) | 1 (1.6) | 27 (3.9) | NA | 24 (3.6) | 3 (15.8) |
| Non-adherent to duration | 8 (0.9) | 1 (1.0) | 5 (0.6) | 3 (4.9) | 399 (57.8) | 2 (0.3) | 393 (58.6) | 6 (31.6) |
Number of patients with a prescription non-adherent to at least one of the elements of the standard treatment guidelines (regimen, dosage, frequency, duration).
LRTI = lower respiratory tract infection; NA = not assessed.
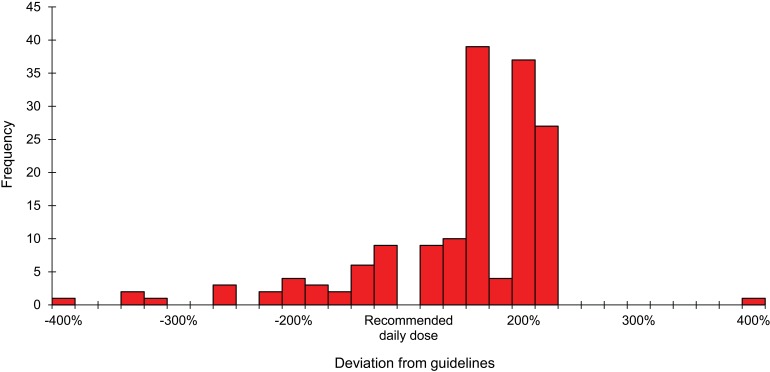
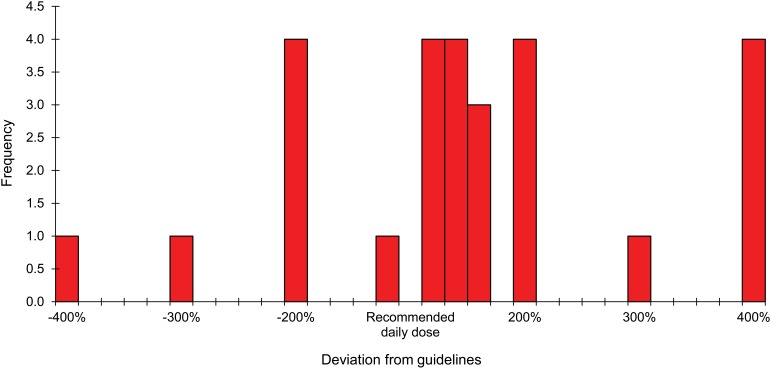
Table 4 shows the number of non-adherent prescriptions (individual drugs) for the above patients, stratified by dosage (detailed analysis also shown in Figures 2 and 3), frequency of administration and treatment duration. As evidenced, the three main problems for malaria involved no treatment being prescribed for 43 (5%) patients, incorrect treatment in 29 (3.4%) and over-dosage in 20 (2.3%). In case of LRTI, the main problems were excessively short treatment duration time for 399 (34.9%) patients, over-dosage in 139 (12.2%) and no treatment being prescribed for 91 (13.2%).
TABLE 4.
Types and patterns of non-adherence to standard treatment guidelines in prescriptions (individual drugs) for children admitted to Gondama Hospital, Sierra Leone (January–April 2011)
| Type of non-adherence | Malaria prescriptions (n = 864) | LRTI prescriptions (n = 1141) | |||||||
| Total n (%) | Age <5 years n (%) | Age≥ 5 years n (%) | Total n (%) | Age<5 years n (%) | Age ≥5 years n (%) | ||||
| Dosage | |||||||||
| Under-prescription | 10 (1.1) | 8 (0.9) | 2 (0.2) | 45 (4.0) | 43 (3.8) | 2 (0.2) | |||
| Over-prescription | 20 (2.3) | 17 (2.0) | 3 (0.3) | 139 (12.2) | 137 (12.0) | 2 (0.2) | |||
| Total | 30 (3.5) | 25 (2.9) | 5 (0.6) | 184 (16.2) | 180 (15.8) | 4 (0.4) | |||
| Frequency | |||||||||
| Under-prescription | 0 | 0 | 0 | 5 (0.4) | 5 (0.4) | 0 | |||
| Over-prescription | 2 (0.2) | 1 (0.1) | 1 (0.1) | 22 (2.0) | 19 (1.7) | 3 (0.3) | |||
| Total | 2 (0.2) | 1 (0.1) | 1 (0.1) | 27 (2.4) | 24 (2.1) | 3 (0.3) | |||
| Duration | |||||||||
| Under-prescription | 8 (0.9) | 6 (0.7) | 2 (0.2) | 399 (34.9) | 393 (34.4) | 6 (0.5) | |||
| Over-prescription | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) | 0 | |||
| Total | 8 (0.9) | 6 (0.7) | 2 (0.2) | 400 (35.0) | 394 (34.5) | 6 (0.5) | |||
LRTI = lower respiratory tract infection.
FIGURE 2.
Histogram: deviation from guidelines in prescribing daily dosages of anti-LRTI drugs, Gondama Hospital, Sierra Leone. LRTI = lower respiratory tract infection.
FIGURE 3.
Histogram: deviation from guidelines in prescribing daily dosages of anti-malaria drugs, Gondama Hospital, Sierra Leone.
Potentially harmful non-adherence
We assessed the putative association between an unfavourable hospital outcome (defined as death or loss to follow-up) and potentially harmful non-adherence to standard treatment guidelines (Table 5). Harmful non-adherence was significantly associated with an unfavourable outcome for both malaria and LRTI cases. This association was significant for prescription of an incorrect drug regimen; for potentially harmful non-adherent treatment duration, no association was observed.
TABLE 5.
Association of non-adherence to standard treatment guidelines with unfavourable hospital outcomes (death and loss to follow-up) for patients admitted to Gondama Hospital, Sierra Leone
| Unfavourable outcome n (row %) | Discharged n (row %) | OR (95%CI) | P value | |
| Malaria | ||||
| Adherent | 68 (9) | 687 (91) | — | — |
| Non-adherent (all) | 10 (22) | 36 (78) | 2.8 (1.2–6.1) | 0.0047 |
| Regimen | 10 (22) | 35 (78) | 2.8 (1.2–6.3) | 0.0048 |
| Duration | 0 | 1 (100) | NS | NS |
| LRTI | ||||
| Adherent | 8 (8) | 92 (92) | — | — |
| Non-adherent (all) | 22 (22) | 79 (78) | 3.2 (1.3–8.8) | 0.006 |
| Regimen | 21 (21) | 78 (79) | 1.7 (1.0–3.0) | 0.04 |
| Duration | 1 (50) | 1 (50) | NS | NS |
OR = odds ratio; CI = confidence interval; NS = non-significant; LRTI = lower respiratory tract infection.
DISCUSSION
To the best of our knowledge, this is the first published study to assess the level of non-adherence to standard treatment guidelines by clinicians in a rural hospital setting in Africa. It shows a high level of non-adherence, the consequences of which could, at the very least, include adverse drug reactions, increasing antimicrobial resistance and poorly planned/wasted resources. In addition, an association was found between non-adherence, classified as potentially harmful, and unfavourable hospital outcomes of patients (deaths and losses to follow-up), suggesting that non-adherence to standard guidelines has a direct and detrimental effect on the quality of hospital care.
The strengths of the study are that 1) this assessment involved routine operational data, and the findings are thus likely to reflect clinical reality on the wards; 2) this is, as far as we know, one of the first published studies to assess adherence to standard treatment guidelines in a hospital setting; and 3) adherence was assessed independently by three experienced clinicians.
The study limitations are that our assessment was based purely on audits of case files and as such, under-reporting and errors in case files could have been sources of bias. For example, we were unable to verify whether the 13% of LRTI cases and 5% of malaria cases who were indicated as not having been prescribed treatment represent a genuine problem of no prescription or a recording error in the case files.
Moreover, based on the set of clinical parameters assessed, at least 61% of the malaria cases and at least 74% of the LRTI cases were classified as severe. Some severe cases may have been missed, as not all clinical variables could be collected, and these numbers therefore present the absolute minimum of severe cases. We also observed that patterns of non-adherence differed considerably between regimens: this would merit drug-specific analysis, which was outside the scope of this study. Finally, entry and exit dates were not collected for the patients included in the study; any effects of prescription non-adherence on length of hospital stay could therefore not be assessed. We could also not assess whether the negative outcomes associated with absence of treatment occurred early after hospitalisation and were thus the cause of the absence of treatment rather than the consequence. A negative hospital outcome could, in that case, explain the statistically significant association with potentially harmful non-adherence to guidelines.
Our findings nevertheless raise a number of issues that merit discussion. First, although we assessed overall non-adherence to be high, with inadequacies being seen in all aspects of prescriptions (drug regimen, dosage, frequency and duration), we do not know how these findings compare with other similar hospital settings due to the paucity of published literature on the subject. There is thus a need for similar studies in busy hospitals to routinely monitor and report on this important issue.
Second, adherence to a relatively straightforward treatment guideline (that for malaria) was better than that to more complex guidelines (such as those for LRTI). This might be related to the fact that malaria treatment is more standardised, there are fewer drug options than for LRTI and, as artemisinin-based combinations are used, monitoring of drug use is more rigorous. These factors may have restricted clinician flexibility in prescribing.
Third, the MSF diagnostic and treatment guidelines are well standardised.5 This manual is designed to respond in the simplest and most practical way possible to managing diseases by field medical staff. It is based on many years of MSF field experience, and is in line with World Health Organization (WHO) recommendations.4 From a public health and operational perspective, such standardisation is required as it facilitates the development of treatment guidelines, allows standardisation in training curricula—which also makes staff training easier, and facilitates drug forecasting. The disadvantage of such standardisation is that it introduces a form of rigidity. As such, our study based on the existing standard treatment guidelines will not capture nuances based on clinical judgment. For example, although the MSF guidelines recommend 100 mg/kg/day as the dose for an antibiotic such as ampicillin, a clinician could justifiably raise this to 200 mg/kg/day or more based on the severity of the child’s condition.
We are therefore likely to have overestimated ‘incorrect’ or non-rational use, as we considered any deviation from standard treatment guidelines to be non-rational. However, the problems we observed, such as no treatment having been recorded and variations in the frequency of administration and duration of treatment, are not subject to such clinical flexibility. One explanation for this could be that the Bo Hospital is staffed by doctors and senior clinicians who often rely on clinical judgment and are thus less adherent to treatment guidelines. For example, in Benin, higher percentages of children with diarrhoea received oral rehydration therapy, and more children with fever were appropriately treated with a recommended antimalarial drug by nursing aides than by nurses (intermediate) or physicians (worst performance).6,7 Furthermore, coordination staff, who are often junior clinicians, might feel reluctant to advise their more senior colleagues on prescription practice, as this is traditionally not done in medical practice. One way of tackling this problem would be to introduce regular auditing and reporting of rational drug use at monthly or quarterly hospital clinical meetings and open discussions between clinical staff, during which deviations from the protocol are explained. Non-adherence to guidelines might be justified in some cases, but clinicians should be expected to justify their actions when deviating from protocol.
Finally, urgent measures need to be undertaken in the hospital setting to improve current prescription practices. Evidence and experience suggest that inappropriate training on the existing curriculum, poor supervision and weak regulatory mechanisms affect the quality of care provided by any cadre. Examples include the poor ability of medical and nursing graduates in Ghana and Tanzania to deliver quality family practice,8 and medical assistants persisting with unconventional treatment patterns after in-service training.9
A concerted approach is thus needed, involving enhanced training of clinical staff on existing standard treatment guidelines, which should be regularly updated on the basis of new evidence.10 As staff turnover is high, a system needs to be put in place to ensure that all new staff are systematically briefed on the guidelines, and that regular refresher trainings are conducted. Pocket handbooks highlighting standard treatment guidelines for common diseases might serve as a useful bedside reference tool for clinicians. On a monthly basis, a formal independent evaluation of 10 prescriptions for malaria and LRTI issued by each clinician would also be a way of assessing adherence to guidelines on a continuing basis and providing an alert when needed.
In 1993, the WHO and the International Network for Rational Use of Drugs produced a manual that defined core indicators and provided a methodology for measuring these indicators for general out-patients in health facilities.11 Similar standards do not exist for in-patient settings. We call upon the WHO and partners to establish similar indicators to measure pharmaceutical use by hospital in-patients, where medicine use patterns are far more complex.
In conclusion, this study highlights an important under-addressed problem in routine hospital care and advocates for the implementation of routine measures to monitor and improve rational drug use.
Acknowledgments
This research was supported through an operational research course that was jointly developed and run by the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease, Paris, France, and the Operational Research Unit, Médecins Sans Frontières, Brussels–Luxembourg. Additional support for running the course was provided by the Center for International Health, University of Bergen, Bergen, Norway.
The course was funded by an anonymous donor and the Department for International Development, London, UK.
Conflict of interest: none declared.
References
- 1.World Health Organization. The world medicines situation 2011. Rational use of medicines. 3rd ed. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 2.World Health Organization. The rational use of drugs: report of a conference of experts, Nairobi, 25–29 November 1985. Geneva, Switzerland: WHO; 1987. [Google Scholar]
- 3.Management Sciences for Health. Managing drug supply: managing access to medicines and other health technologies. 3rd ed. Cambridge, MA, USA: MSH; 2012. http://www.msh.org/resource-center/managing-drug-supply.cfm Accessed May 2013. [Google Scholar]
- 4.World Health Organization. Medicines: rational use of medicines. Fact sheet no. 338. Geneva: WHO; 2013. http://www.who.int/mediacentre/factsheets/fs338/en/ Accessed May 2013. [Google Scholar]
- 5.Médecins Sans Frontières. Clinical guidelines. Diagnosis and treatment manual. Geneva, Switzerland: MSF; 2010. [Google Scholar]
- 6.Wilson I B, Landon B E, Hirschhorn L R, et al. Quality of HIV care provided by nurse practitioners, physician assistants and physicians. Ann Int Med. 2005;143:729–736. doi: 10.7326/0003-4819-143-10-200511150-00010. [DOI] [PubMed] [Google Scholar]
- 7.Rowe A K, Onikpo F, Mama M, Cokou F, Deming M S. Management of childhood illness at health facilities in Benin: problems and their causes. Am J Public Health. 2001;91:1625–1635. doi: 10.2105/ajph.91.10.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dovlo D. Using mid-level cadres as substitutes for internationally mobile health professionals in Africa. A desk review. Human Res Health. 2004;2:1–12. doi: 10.1186/1478-4491-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ofori-Adjei D, Arhinful D. Effect of training on the clinical management of malaria by medical assistants in Ghana. Soc Sci Med. 1996;42:1169–1176. doi: 10.1016/0277-9536(95)00389-4. [DOI] [PubMed] [Google Scholar]
- 10.Chopra M, Munro S, Lavis J, Vist G, Bennett S. Effects of policy options for human resources for health: an analysis of systematic reviews. Lancet. 2008;371:668–674. doi: 10.1016/S0140-6736(08)60305-0. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. How to investigate drug use in health facilities: selected drug use indicators. WHO/DAP/93.1. Geneva, Switzerland: WHO; 1993. http://apps.who.int/medicinedocs/pdf/s2289e/s2289e.pdf Accessed May 2013. [Google Scholar]