Abstract
Objectives:
To assess the acceptability of a ready-to-use therapeutic food (Plumpy’nut® [PPN]) among 1) care givers of malnourished children and 2) community health workers (CHWs) at a nutrition rehabilitation centre in an urban slum in Dhaka, Bangladesh.
Methods:
This was a cross-sectional semi-structured questionnaire survey conducted between April and June 2011 as part of a nutritional programme run by Médecins Sans Frontières. The study population included care givers of malnourished children aged 6–59 months who received PPN for at least 3 weeks, and CHWs.
Results:
Of the 149 care givers (93% female) interviewed, 60% expressed problems with PPN acceptability. Overall, 43% perceived the child’s dissatisfaction with the taste, 31% with consistency and 64% attributed side effects to PPN (nausea, vomiting, loose motion, diarrhoea, abdominal distension and pain). It is to be noted that 47% of children needed encouragement or were forced to eat PPN, while 5% completely rejected it after 3 weeks. Of the 29 CHWs interviewed, 48% were dissatisfied with PPN’s taste and consistency, and 55% with its smell. However, 91% of the care givers and all CHWs still perceived a therapeutic benefit of PPN for malnourished children.
Conclusion:
Despite a therapeutic benefit, only 4 in 10 care givers perceived PPN as being acceptable as a food product, which is of concern.
Keywords: Plumpy’nut, ready-to-use therapeutic food, acceptability, malnutrition, Bangladesh
Abstract
Objectifs:
Evaluer à Dacca, Bangladesh, au sein d’un centre de réhabilitation nutritionnelle d’un bidonville, l’acceptabilité d’une alimentation thérapeutique prête à l’emploi (Plumpy’nut®, [PPN]) par 1) les soignants d’enfants en état de malnutrition et 2) par les travailleurs de la santé dans la collectivité (CHW).
Méthodes:
Il s’agit d’une enquête transversale par questionnaire semi-structuré menée entre avril et juin 2011 dans un programme nutritionnel de Médecins Sans Frontières. La population de l’étude a comporté les soignants d’enfants en état de malnutrition âgés de 6 à 59 mois qui avaient reçu du PPN pendant au moins 3 semaines, ainsi que les CHW.
Résultats:
Sur les 149 soignants (93% de femmes) interviewés, 60% ont exprimé des problèmes en ce qui concerne l’acceptabilité du PPN. Au total, 43% d’entre eux avaient perçu une non-satisfaction de l’enfant concernant le goût et 31% la consistance, et 64% avaient attribué des effets collatéraux au PPN (nausées, vomissements, selles liquides, diarrhée, distension abdominale et douleurs). Il faut noter que 47% des enfants devaient être encouragés ou forcés à manger le PPN alors que 5% d’entre eux l’ont rejeté complètement après 3 semaines. Sur les 29 CHW interviewés, 48% n’étaient pas satisfaits du goût et de la consistance du PPN et 55% n’étaient pas satisfaits de son odeur. Il est important de noter que 91% des soignants et l’ensemble des CHW percevaient néanmoins encore l’avantage thérapeutique du PPN pour les enfants en état de malnutrition.
Conclusion:
En dépit d’un avantage thérapeutique, seuls quatre soignants sur 10 ont perçu le PPN comme un aliment acceptable, ce qui constitue une préoccupation.
Abstract
Objetivos:
Evaluar la aceptabilidad de un alimento terapéutico preparado (Plumpy’nut®, [PPN]) en un centro de rehabilitación nutricional de una zona urbana de viviendas precarias de Dhaka en Bangladesh por parte de las personas que prestan atención a los niños desnutridos y de los trabajadores comunitarios de salud (CHW).
Métodos:
Se llevó a cabo una encuesta transversal con un cuestionario semi-estructurado entre abril y junio del 2011 en el marco de un programa nutricional de Médicos Sin Fronteras. La población del estudio consistió en las personas a cargo de niños desnutridos cuya edad oscilaba entre 6 y 59 meses y que habían recibido PPN como mínimo durante 3 semanas y los CHW.
Resultados:
De los 149 cuidadores entrevistados (93% de mujeres), el 60% expresó problemas de aceptabilidad del PPN. En general, el 43% de los cuidadores percibía insatisfacción del niño por causa del sabor, el 31% por la consistencia y el 64% atribuía al PPN reacciones adversas (náuseas, vómito, heces blandas, diarrea y distensión o dolor abdominal). Cabe anotar que el 47% de los niños precisó estímulos para recibir el PNN o lo aceptó por la fuerza y el 5% de ellos lo rechazó totalmente después de 3 semanas de administración. De los 29 CHW que respondieron a la encuesta, el 48% se manifestó insatisfecho con el sabor y la consistencia del PPN y el 55% desaprobó su olor. Sin embargo, es importante anotar que el 91% de los cuidadores y todos los CHW percibieron una ventaja terapéutica con el uso del PPN en los niños desnutridos.
Conclusión:
Es fuente de preocupación el reconocer que pese a la aceptación de una ventaja terapéutica del PPN, solo cuatro de los 10 cuidadores de niños desnutridos lo consideraron como un producto alimentario aceptable.
The World Health Organisation (WHO) recently endorsed the use of ready-to-use therapeutic food (RUTF) for the community-based management of children with uncomplicated severe acute malnutrition (SAM).1 Reliance on RUTF has a number of operational advantages: it reduces the logistic load for the end user, allows rapid roll-out and access to treatment, permits home-based ambulatory care and is cost-effective.2,3 One of the most widely used RUTFs in Africa is Plumpy’nut® (PPN; Nutriset, Malaunay, France; http://www.nutriset.fr/en/product-range/produit-par-produit/plumpynut-ready-to-use-therapeutic-food-rutf.html), which is a peanut-based mixture of milk powder, sugar, vegetable oil, minerals and vitamins. It does not require cooking or dilution with water, and is thus practical for use where such resources are limited. An important operational advantage is that it is a microbiologically safe product, meaning that it can be stored for several months in routine household conditions.4,5
Médecins Sans Frontières (MSF) has been involved with a community-based nutrition programme that has been using PPN for the management of acute malnutrition among children in a slum setting in Dhaka, Bangladesh. Bangladesh has one of the highest prevalence rates of childhood malnutrition in the world:6,7 almost 46% of children aged < 5 years are stunted (low height for age) and 15% are wasted (low weight for height).8
During the implementation of this nutrition programme, several care givers of children complained of difficulties in feeding their children with PPN and attributed it to the taste and smell of PPN. The programme also faced challenges in terms of a high loss-to-follow-up rate (15–17%) and relatively low nutritional recovery rates (59–65%) compared to those reported in the literature.9 A relatively long average length of stay in the programme (60–63 days) has also been observed. We are concerned that these findings might be related to the overall acceptability of PPN, which is a key determinant in ensuring favourable outcomes of nutritional rehabilitation. While some studies in Africa have shown that peanut-based RUTF has good acceptability and compliance among severely malnourished children,10–12 other studies have demonstrated barriers to its use and inadequate compliance, mainly due to sharing within the households.13,14 There is, however, limited published literature on PPN acceptability in South Asia.
In the present study, we assessed the acceptability of PPN among 1) care givers of malnourished children and 2) community health workers (CHWs), in Kamrangirchar slum, Dhaka, Bangladesh.
METHODS
Design
This was a cross-sectional semi-structured questionnaire survey.
Study setting and study population
The study was conducted between April and June 2011 in Kamrangirchar, an urban slum setting in Dhaka, Bangladesh, with about 400 000 inhabitants living in an area of 3.1 km2. It is not officially considered as part of Dhaka City, and all health services are outsourced to non-governmental organisations, who are the main providers.
MSF started its nutrition programme for children in May 2010. All services are provided free of charge through two primary health care (PHC) clinics. The management protocols are in line with MSF15 and WHO guidelines.¹ The study population included two groups: 1) direct care givers of all consecutive malnourished children (aged 6–59 months) who had completed at least 3 weeks of PPN and were still in the programme (a direct care giver was defined as the family member who was feeding the child during the day and at the time of the follow-up visit); and 2) all CHWs who were involved with PPN distribution and follow-up of the children during the study period. The rationale of choosing at least 3 weeks of PPN as a study inclusion criterion was based on the programme observation that it takes at least 2 weeks (and often 3 weeks) for both a care giver and child to become used to PPN in terms of how to give it and its taste and smell, etc. Acceptability before this time point might thus tend to be more negative, and our study thus represents a better case scenario.
Nutritional rehabilitation of malnourished children
The nutritional programme was centred on an ambulatory community management approach. Children were screened at the two MSF PHCs using both mid-upper arm circumference measurement (MUAC) and weight for height, expressed in Z-scores (WHZ). The latter is derived by measuring the weight and height and comparing them to standard values. Screening was also done door-to-door in the community by a team of CHWs using an MUAC tape.
All children found with SAM (WHZ < −3 and/or MUAC < 115 mm and/or nutritional oedema) or moderate acute malnutrition (MAM; WHZ > −3 to < −2) associated with medical complications are admitted into the nutrition programme.
PPN is the main RUTF used in the nutrition programme. Children with uncomplicated SAM have to pass an appetite test for PPN. This test verifies whether or not the child readily accepts to eat PPN. If the child ingests PPN readily, treatment is started and continued at home. On the other hand, if the mother faces problems with PPN feeding (the child refuses to ingest PPN for any reason), an initial period of supervised feeding is done at the PHC until the child is able to take PPN readily. This is then followed by home-based management supervised by CHWs. Provision of PPN to the child’s care giver is done on a weekly basis in the community according to standard guidelines.15 Children who completely reject PPN during the prescribed period of intake are shifted to BP-100 (high-energy biscuit; G C Rieber, Bergen, Norway).
The MSF’s CHWs are directly involved with subsequent follow-up assessments, the distribution of PPN and continuing health education. Eight CHW teams, composed of two persons per team, along with three supervisors, cover all community-related activities. Team members are allocated to specific geographic areas of the slum and circulate using an adapted rickshaw.
Children are discharged from the programme when they attain a WHZ of >-2 (maintained on two consecutive weighings 1 week apart), have no oedema or acute medical complications and have adequate food intake. Children found to have severe medical complications are referred to a private hospital in-patient therapeutic feeding centre for specialised medical management.
Plumpy’nut acceptability among children according to care givers and community health workers
Two different semi-structured questionnaires were developed, translated into the local language (Bengali; Appendix A and B), and used to gather socio-demographic information and perceptions on PPN. The interviewed care givers and CHWs responded ‘Yes’ or ‘No’ to questions related to taste, smell, consistency, colour and side effects of PPN. The questionnaires also included open-ended questions. The questionnaires were pre-tested among 10 care givers and four CHWs, after which improvements were made to the initial questionnaire. Three trained female interviewers, who were independent from the CHWs team, conducted the interviews in the local language. The households of children recorded in a pre-defined list from the programme register were visited, and participants were reassured that names and responses would be anonymised and treated with full confidentiality so as to limit responder bias.
PPN acceptability among children was assessed through their care givers in two ways: 1) perception in terms of taste, smell, colour, consistency or attributed side effects at any time during the course of intake; and 2) feeding acceptability by the child, graded in a standardised manner. PPN was considered acceptable if the care givers did not perceive any problems with taste, smell, colour, consistency, attributed side effects due to PPN intake or if the child ingested PPN readily. PPN acceptability among CHWs was also assessed in terms of their own perceptions of taste, smell, consistency, colour and packaging.
Ethics approval was received from the MSF Ethics Review Board, Geneva, Switzerland, and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. Verbal and written informed consent was sought and obtained from all study participants.
Data analysis
The data obtained from the semi-structured questionnaires were coded, and Epi Info 6.04d (Centers for Disease Control and Prevention, Atlanta, GA, USA) was used for data entry and analysis.
RESULTS
Characteristics of the study population
At the time of the study, a total of 149 children receiving PPN for ≥3 weeks were available in the database. The median age was 26 months (interquartile range 16–48) and 84 (57%) children were males. Overall, 121 (81%) children had SAM and 28 (19%) had MAM. Nearly 51% of the included children had received PPN for 3 weeks and the remainder had received it for >3 weeks (range 3–22).
A total of 149 care givers were interviewed: 138 (93%) were mothers and housewives, three were fathers and eight were female family members. Overall, 58 (39%) care givers were illiterate and the rest had received at least 1 year of primary education. Interviews were also conducted with 29 CHWs, of whom 69% were women. The average educational level of the CHWs was 11 years of schooling.
Care givers’ perception of PPN acceptability among malnourished children
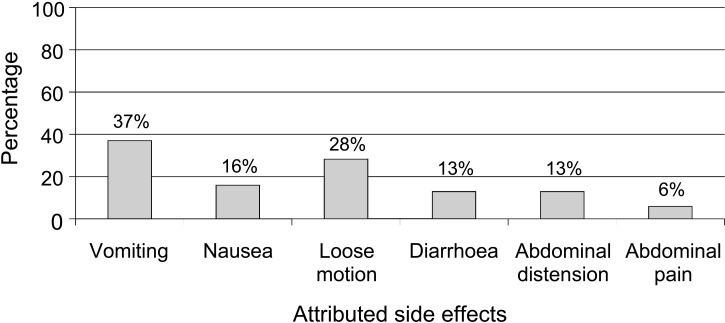
Of all care givers interviewed, 60 (40%) said that PPN was completely accepted by their children, while the majority (n = 89, 60%) expressed problems with its acceptability (Table 1). Problems included perceived child’s dissatisfaction with taste (43%) and consistency (31%). At least one attributed side effect was reported by 64% of the care givers (Figure). In terms of the child’s intake of PPN, 72 (48%) children took PPN readily, while 70 (47%) needed encouragement or had to be forced. Seven (5%) children completely rejected PPN after 3 weeks (Table 1). Nearly 78 (52%) of the care givers mixed PPN with water and/or other food in an attempt to facilitate intake. Importantly, 79 (53%) care givers did not understand the instructions on the PPN package; 64 (81%) because the instructions were inconspicuous and incomprehensible, and 15 (19%) because they were illiterate.
TABLE 1.
PPN acceptability among malnourished children according to their care givers in Kamrangirchar, Dhaka, Bangladesh
| Variable | n (%) |
| Total care givers | 149 |
| Taste | |
| Unacceptable | 64 (43) |
| Too sweet | 34 (53) |
| Too salty | 29 (45) |
| Unfamiliar taste | 1 (2) |
| Smell | |
| Unacceptable | 19 (13) |
| Strong smell of peanut | 12 (63) |
| Medicine-like smell | 3 (16) |
| Unwelcome smell | 4 (21) |
| Consistency | |
| Unacceptable | 46 (31) |
| Sticky | 32 (70) |
| Thick | 14 (30) |
| Colour | |
| Unacceptable (faeces-like) | 3 (2) |
| PPN ingestion among children | |
| Accepted readily | 72 (48) |
| Encouragement and effort needed | 20 (13) |
| Forced to eat PPN | 50 (34) |
| Rejected completely | 7 (5) |
PPN = Plumpy’nut®.
FIGURE.
Side effects of Plumpy’nut® intake reported by care givers of malnourished children in Kamrangirchar, Dhaka, Bangladesh (n = 149).
Despite these problems with acceptability, 135 (91%) care givers still perceived PPN to be beneficial for their children in terms of weight gain, increased activity of the child, improved general health and improved appetite (Table 2).
TABLE 2.
Perceived benefits of PPN for malnourished children by care givers in Kamrangirchar, Dhaka, Bangladesh (N = 149)
| Variable | n (%) |
| Perceived benefits of PPN* | 135 (91) |
| Weight gain | 112 (83) |
| Increased activity | 52 (39) |
| Improved general health | 50 (37) |
| Improved appetite | 42 (31) |
| Improved milestones | 16 (12) |
| Increased length/height | 9 (7) |
More than one response by the same care giver.
PPN = Plumpy’nut®.
PPN acceptability among community health workers
Of the 29 CHWs interviewed, 14 (48%) were dissatisfied with the taste and consistency of PPN, 16 (55%) with its smell and 6 (21%) with the colour. The majority (93%) of the CHWs found the package easy to open, and only one found the package instructions incomprehensible. Despite these concerns, all CHWs felt that malnourished children receiving PPN did benefit from the food product.
Suggestions on how to improve PPN acceptability
All care givers were asked for their opinions on how to improve PPN acceptability among children; 101 (67%) had specific suggestions. Of these, 75 (74%) suggested making improvements to taste (37% that it should be less salty and 44% that it should be less sweet), 31% felt that it should be less sticky, and 21% asked for the peanut-based smell to be changed to a different smell more adapted to the local setting.
Of the 29 CHWs, 28 (96%) made suggestions for improvement of PPN. Seventeen (61%) suggested that taste should be changed (94% to be less sweet, 6% to add a flavour), 15 (54%) felt that the consistency needed to be more fluid to facilitate feeding, while 11 (39%) suggested that the peanut-based smell should be changed to a different smell.
DISCUSSION
In this study, 6 in 10 care givers of malnourished children expressed dissatisfaction with their child’s acceptability of PPN as a food product. Half of the children required encouragement or needed force-feeding with PPN. Surprisingly, about half of the CHWs supporting the programme also perceived problems with PPN acceptability. These findings notwithstanding, 91% of the care givers and all of the CHWs perceived PPN to be of therapeutic benefit for malnourished children.
The strengths of this study are that 1) it is one of the first to assess PPN acceptability among malnourished children in South Asia, 2) it was conducted within the framework of a routine nutrition programme and is thus likely to reflect the ground reality, 3) acceptability was assessed among care givers and CHWs, whose perceptions can directly influence adherence to RUTF and therapeutic response, and 4) the study was conducted in a setting with a high burden of malnutrition and among a vulnerable and mobile population, which makes it unique.
Study limitations include the fact that questions about perception were posed to the care givers of the children and the reported perception might understandably not reflect those of the children themselves. Important cultural and behavioural factors might also play a role in the acceptance of a therapeutic nutritional product, and such factors were not assessed in this study. We were unable to include children who were lost to follow-up in the survey due to the practical difficulties of tracing a mobile and migrant slum population. We do not know if this introduced a bias in the results. Furthermore, the assessment of children’s adherence to PPN intake was not within the scope of our study, and this will require further research. The impact of adult perceptions of actual adherence in children is thus not known. Given the relatively small sample size and geographic location of our study, similar studies would be useful in other contexts in Bangladesh and other South Asian countries.
Despite these limitations, a number of interesting findings have been revealed and merit attention. First, about 4 in 10 care givers expressed dissatisfaction with PPN, mainly relating to taste and consistency. Care givers also found the product either too sweet or too salty. These perceptions of a food product need to be taken into consideration and addressed, as they might negatively influence adherence. In a study from Malawi, mothers of malnourished children suggested that the sweetness of a similar RUTF should be reduced, as they believed it was unsuitable to be given to children with cough.11
Second, a surprisingly high proportion of care givers (6/10) attributed side effects to PPN intake, the most common being vomiting and loose stool. Diarrhoea was also reported as a side effect in our study; this is in line with the results reported by Manary et al.16 Similar findings have also been reported with other alternatives, such as the therapeutic milk F-100 (Nutriset, Malaunay, France) used for in-patient rehabilitation of SAM.17 Although we are unable to substantiate any direct relation between the attributed side effects and PPN intake, this link is of concern, as unwarranted blame placed on PPN is likely to have implications on adherence. This issue merits further assessment and research.
Third, only half of all the care givers actually found the package instructions comprehensible. Understanding package instructions is critical, and adapting them in a suitable manner is essential, particularly in settings where there is a high illiteracy rate.
Fourth, it is surprising that about half of the CHWs who were in charge of promoting and encouraging PPN among care givers perceived problems themselves with its acceptability. This information was not available before the study, and it may be a reflection of inadequate interaction and discussions between the medical and community teams and/or over-confidence in PPN acceptability among the clinical team. Involving the community early in the course of implementation and being vigilant with regard to aspects of acceptability in similar contexts is essential if perceptions are to be better understood and addressed.
Despite the perceived limitations of the product in terms of acceptability, it is encouraging that more than 9 in 10 care givers and all CHWs still felt that PPN had therapeutic benefits for malnourished children. This finding is also supported by a systematic review that demonstrated the efficacy and effectiveness of peanut-based RUTF in the management of SAM among children.9 It is thus logical to believe that if we improved aspects that currently hinder its acceptability, there would be a positive effect on the overall perceived ‘value’ of PPN among beneficiaries.
Despite the product-related limitations in acceptability, the present reality is that there are few alternative RUTFs that use local foods, such as sesame, soya and chickpeas. A practical consideration is that accessibility of these alternatives may not be the same as that of PPN and there is thus a need to continue supporting the continued use of PPN in this setting.
Meanwhile, we call on nutritional agencies and food manufacturers to intensify efforts towards developing more RUTF alternatives that are better adapted and accessible to the local context and that have the required therapeutic contents for the management of children with SAM.
Acknowledgments
The authors thank the Médecins Sans Frontières (MSF) team working in the Kamrangirchar nutrition programme for their collaboration and support in implementing the study. They are particularly grateful to the care givers and community health workers who participated in the survey. They also thank the interviewers, N Rahman, K F Runa and A Akter.
The study was funded by the Brussels Operational Centre of MSF and the Ministry of Foreign Affairs, Luxembourg.
Conflict of interest: none declared.
APPENDIX A. PPN acceptability questionnaire among care givers
APPENDIX B. PPN acceptability questionnaire among community health workers
References
- 1.World Health Organization . Community-based management of severe acute malnutrition. Geneva, Switzerland: WHO; 2007. http://whqlibdoc.who.int/publications/2007/9789280641479_eng.pdf Accessed April 2013. [Google Scholar]
- 2.Collins S, Sadler K, Dent N, et al. Key issues in the success of community-based management of severe malnutrition. Food Nutr Bull. 2006;27(Suppl):S49–S82. doi: 10.1177/15648265060273S304. [DOI] [PubMed] [Google Scholar]
- 3.Briend A, Lacsala R, Prudhon C, Mounier B, Grellety Y, Golden M H. Ready-to-use therapeutic food for treatment of marasmus [letter] Lancet. 1999;353:1767–1768. doi: 10.1016/S0140-6736(99)01078-8. [DOI] [PubMed] [Google Scholar]
- 4.Golden K, Khara T, Sadler K, et al. Case studies from Ethiopia, Malawi and Sudan. Field Exchange 2004. Special Supplement. 2(13) http://fex.ennonline.net/102/chapter3.aspx Accessed April 2013. [Google Scholar]
- 5.Manary M J. Local production and provision of ready-to-use therapeutic food (RUTF) spread for the treatment of severe childhood malnutrition. Food Nutr Bull. 2006;27(Suppl):S83–S89. doi: 10.1177/15648265060273S305. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO Bangladesh Country Cooperation Strategy 2008–2013. New Delhi, India: WHO; 2007. http://www.who.int/countryfocus/cooperation_strategy/ccs_bgd_en.pdf Accessed April 2013. [Google Scholar]
- 7.National Institute of Population Research and Training . Bangladesh Demographic and Health Survey 2007. Dhaka, Bangladesh: NIPORT, Mitra and Associates & Macro International; 2009. http://www.measuredhs.com/pubs/pdf/FR207/FR207%5BApril-10-2009%5D.pdf28 Accessed April 2013. [Google Scholar]
- 8.Bangladesh Bureau of Statistics/UNICEF . Child and Mother Nutrition Survey 2005. Dhaka, Bangladesh: BBS and UNICEF; 2007. http://www.unicef.org/bangladesh/Child_and_Mother_Nutrition_Survey.pdf Accessed April 2013. [Google Scholar]
- 9.Gera T. Efficacy and safety of therapeutic nutrition products for home-based therapeutic nutrition for severe acute malnutrition: a systematic review. Indian Pediatr. 2010;47:709–718. doi: 10.1007/s13312-010-0095-1. [DOI] [PubMed] [Google Scholar]
- 10.Flax V L, Maleta K, Ashorn U, Manary M J, Briend A, Ashorn P. Intake of lipid-based nutrient supplements during illness and convalescence among moderately-underweight Malawian children. J Health Popul Nutr. 2008;26:468–470. [PMC free article] [PubMed] [Google Scholar]
- 11.Flax V L, Thakwalakwa C, Phuka J, et al. Malawian mothers’ attitudes towards the use of two supplementary foods for moderately malnourished children. Appetite. 2009;53:195–202. doi: 10.1016/j.appet.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Hess S Y, Bado L, Aaron G J, Ouedraogo J B, Zeilani M, Brown K H. Acceptability of zinc-fortified, lipid-based nutrient supplements (LNS) prepared for young children in Burkina Faso. Matern Child Nutr. 2011;7:357–367. doi: 10.1111/j.1740-8709.2010.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maleta K, Kuittinen J, Duggan M B, et al. Supplementary feeding of underweight, stunted Malawian children with a ready-to-use food. J Pediatr Gastroenterol Nutr. 2004;38:152–158. doi: 10.1097/00005176-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Ickes S B, Jilcott S B, Myhre J A, et al. Examination of facilitators and barriers to home-based supplemental feeding with ready-to-use food for underweight children in western Uganda. Matern Child Nutr. 2012;8:115–129. doi: 10.1111/j.1740-8709.2010.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Médecins Sans Frontières . Ambulatory feeding protocol and practical guide: Operational Centre Brussels. Brussels, Belgium: MSF; 2011. [Google Scholar]
- 16.Manary M J, Ndkeha M J, Ashorn P, Maleta K, Briend A. Home-based therapy for severe malnutrition with ready-to-use food. Arch Dis Child. 2004;89:557–561. doi: 10.1136/adc.2003.034306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talbert A, Thuo N, Karisa J, et al. Diarrhoea complicating severe acute malnutrition in Kenyan children: a prospective descriptive study of risk factors and outcome. PLoS ONE. 2012;7:e38321. doi: 10.1371/journal.pone.0038321. [DOI] [PMC free article] [PubMed] [Google Scholar]