Abstract
Setting:
Cape Town, South Africa.
Objective:
To assess the completeness and accuracy of electronic recording of drug-resistant tuberculosis (DR-TB) in children.
Design:
Retrospective cohort study. All children aged <15 years treated for DR-TB during 2012 were included, with clinical data collected from routine health services. Matching was performed between clinical data and an extracted data set from an electronic register for DR-TB (EDR.web), and data sources were compared.
Results:
Seventy-seven children were identified clinically, of whom only 49 (64%) were found in EDR.web. Most data in EDR.web were complete and accurate, but there were some internal inconsistencies for confirmed TB. Only 4.4% of all EDR.web entries were children.
Conclusion:
Only two thirds of children clinically treated for DR-TB were recorded in the electronic reporting system, suggesting under-reporting. We also found a lower than expected prevalence of childhood DR-TB, probably suggesting both under-diagnosis and under-recording of DR-TB in children. Clinicians at facility level should be able to access the electronic reporting system, and data transfer between clinical paper-based and electronic sources should be simplified. Cross-linking between electronic registers for drug-susceptible and DR-TB or consolidation of registers could improve the accuracy of recording. Improved recording and reporting of DR-TB in children is needed.
Keywords: children, TB, surveillance, drug resistance, registration
Abstract
Context:
Cape Town, Afrique du Sud.
Objectif:
Evaluer le caractère complet et la précision de l’enregistre-ment électronique de la tuberculose à germes résistants (TB-DR) chez les enfants.
Schéma:
Etude rétrospective de cohorte. On a inclus tous les enfants <15 ans traités pour TB-DR pendant l’année 2011 au moyen des données cliniques provenant des services de santé de routine. On a comparé les données cliniques et un ensemble de données extraites d’un registre électronique pour TB-DR (EDR.web), et on a comparé les sources des données.
Résultats:
Des 77 enfants identifiés sur le plan clinique, 49 seulement (64%) ont été retrouvés dans le EDR.web. La plupart des données du EDR-web étaient complètes et précises mais avec certaines incohérences internes pour les TB confirmées ; 4,4% seulement des cas entrés dans l’EDR.web correspondaient à des enfants.
Conclusion:
Deux tiers seulement des enfants traités cliniquement pour TB-DR ont été enregistrés dans le système de déclaration électronique, ce qui suggère une insuffisance de déclaration. Nous avons également trouvé une prévalence plus faible qu’attendue de la TB-DR chez l’enfant, ce qui suggère probablement à la fois l’insuffisance du diagnostic et une insuffisance des déclarations de la TB-DR chez les enfants. Au niveau des services, les cliniciens devraient être capables d’accéder aux déclarations électroniques, et il y a lieu de simplifier le transfert des données entre les dossiers-papier cliniques et les sources électroniques. Une précision meilleure de l’enregistrement pourrait être obtenue en croisant les données provenant des registres électroniques pour les TB à germes sensibles ou résistants aux médicaments ou encore en consolidant les registres. Une amélioration de l’enregistrement et des déclarations des TB-DR s’impose chez les enfants.
Abstract
Marco de referencia:
La Ciudad del Cabo en Sudáfrica.
Objetivo:
Evaluar el carácter integral y la precisión del registro electrónico de casos de tuberculosis farmacorresistente (TB-DR) en los niños.
Método:
Fue este un estudio retrospectivo de cohortes. Se inclu-yeron en el estudio todos los niños <15 años de edad tratados por TB-DR en el 2012, cuyos datos clínicos se obtuvieron a partir de los servicios corrientes de salud. Se aparearon los datos clínicos con los datos de un registro electrónico de TB-DR (EDR.web) y se compararon las fuentes de información.
Resultados:
Se reconocieron clínicamente 77 casos de niños, de los cuales solo 49 (64%) aparecían en el registro EDR.web. La mayor parte de los datos del registro estaba completa y era precisa, pero se observaron algunas discordancias internas en los casos de TB confirmada. De todas las entradas presentes en el EDR.web, solo 4,4% correspondía a casos pediátricos.
Conclusión:
Solo dos tercios de los niños tratados clínicamente por TB-DR aparecían consignados en el sistema electrónico de notificación, lo cual indica un problema de infranotificación. Se observó además una prevalencia de TB-DR inferior a la prevista, lo cual puede corresponder a una deficiencia en el diagnóstico y en la notificación de los casos pediátricos de TB-DR. Los médicos deberían tener acceso a la notificación electrónica en los centros de atención, y sería importante simplificar la transferencia de los datos del registro clínico en papel al sistema electrónico. La precisión de los registros se podría mejorar mediante la vinculación de los registros electrónicos de TB normosensible y farmacorresistente o la integración de los registros. Es preciso perfeccionar la notificación y el registro de los casos de TB-DR en los niños.
Complete and accurate data are necessary for the effective management and planning of paediatric tuberculosis (TB) health services, including hospital and primary care resource allocation and the ordering of medications. They are also necessary for monitoring the effectiveness of both the childhood TB programme and the TB programme as a whole, as childhood TB acts as a sentinel marker for transmission within the community. The South African National Tuberculosis Programme (SANTP) recommends decentralised care, with routine diagnosis and treatment of TB patients at community-based clinics. In Western Cape Province (WCP), an electronic TB register (ETR.net) has routinely been used for reporting provincial TB data since 2004, with a separate electronic register for drug-resistant (DR) TB (EDR.web) since 2009. All children diagnosed with DR-TB are first entered into a paper-based register for DR-TB at the clinic or hospital where they are treated. Data from the paper register are captured and collated at subdistrict level into EDR.web before submission to centralised DR-TB units and the provincial TB directorate.1 Provincial data are reported to the SANTP and then to the World Health Organization (WHO).
Electronic reporting aims to improve the quality and completeness of data, save time and improve the security of personal information. It also aims to facilitate more complex data analysis.2 However, the quality of electronic data depends on the quality of data collection, data entry and the transfer of data onto the electronic system. In a study of TB clinics in Cape Town from 2003 to 2004, 12% of children treated for TB were not registered in the facility-based paper TB register, including 75% of children with disseminated TB disease.3 In addition, a recent study from South Africa found that only 62% of children with culture-confirmed, drug-susceptible TB diagnosed at a tertiary referral hospital in Cape Town from 2007 to 2009 were recorded in the provincial ETR.Net.4 Children with the most severe forms of disease, and those who had died in hospital, were least likely to be recorded. These results raise concerns that children with DR-TB may also be inaccurately recorded. Children with DR-TB often experience considerable diagnostic delay and are frequently admitted to hospital for the first few months of treatment.4 These factors may influence recording and reporting within the existing TB surveillance systems.
The ‘onion’ model provides a framework for assessing the proportion of TB cases found in notification data.5 A number of layers are identified, each of which can result in TB cases being missed: lack of access to health care, cases not accessing available health services, cases accessing health care but not being diagnosed, and cases being diagnosed but not reported. All of these can lead to under-reporting in electronic registers.
Although SANTP guidelines suggest that recording should occur at the local clinic, it is unclear how many children with DR-TB are recorded in the provincial electronic DR-TB register. In addition, some children may be included in ETR.net before a diagnosis of DR-TB is made. It is unknown how many children are added to EDR.web once a diagnosis has been changed. No studies have previously assessed the completeness or accuracy of paediatric DR-TB recording and reporting in our setting. The aim of this operational study was to determine the completeness and accuracy of the electronic recording of children diagnosed and treated for all forms of DR-TB in the WCP, South Africa.
METHODS
Study setting
The 2011 national census recorded the WCP population as 5 287 863, of whom approximately 31% were aged <15 years.6 In 2011, 389 974 cases of TB were notified nationally.7 Approximately 7386 TB cases had confirmed multidrug-resistant (MDR) TB, of whom 1422 were in the WCP.1 Prospective surveillance in Cape Town from 2007 to 2009 demonstrated that 15% of children with culture-confirmed TB who had drug susceptibility testing (DST) results had some form of DR-TB.8 Routine provincial data indicate that 17% of all TB cases occur in children.
Tygerberg Children’s Hospital (TCH) is a tertiary referral hospital in Cape Town to which children with DR-TB exposure or disease are referred from regional clinics and hospitals for initial investigation and management. Once stable, children with DR-TB are often transferred to Brooklyn Hospital for Chest Diseases (BHCD), a specialised TB hospital in Cape Town, for the first few months of treatment.9 Children with DR-TB receive first-line drugs to which the isolate is susceptible. Depending on the DST of the isolate, a fluoroquinolone, ethionamide and terizidone are added to build a regimen of four active drugs. In most MDR cases, a second-line injectable anti-tuberculosis agent is also included for the first few months of treatment. Additional drugs, such as para-aminosalicylic acid and linezolid, are used in cases with more extensive drug resistance. Most children are discharged from BHCD to complete treatment at their local clinic. Some children with less severe forms of disease, and with reliable care givers, are treated solely at their local clinic and receive ambulatory treatment. Children at both TCH and BHCD are managed by a single experienced paediatric infectious diseases consultant for admission and follow-up. Children aged ≥13 years are more likely to receive ambulatory treatment, and some are not referred to hospital for assessment or treatment.
Design
We used a retrospective cohort study design. Using WHO definitions for paediatric TB, all children aged <15 years, diagnosed with and treated for any form of DR-TB at BHCD or TCH and its outreach clinics from 1 January to 31 December 2012 were included.
Data sources
Data from routine health services were used. A list of all children from BHCD treated for any form of DR-TB was compiled from the paediatric ward register, review of in-patient folders and discharge summaries. Additional children were identified from out-patient clinic attendance lists from TCH and outreach clinics. Where diagnoses were unclear, clinical records were reviewed. Variables collected for the clinical data set were those entered into EDR.web: first name, family name, date of birth, age, sex, human immunodeficiency virus (HIV) and antiretroviral treatment status, facility or clinic, year of treatment, type of DR-TB, certainty of diagnosis (confirmed by DST of child’s culture or presumed, i.e., contact of DR-TB source case or failing adherent first-line TB treatment), disease classification, treatment start date and any bacteriological results (culture and DST). Treatment outcome was not available for most children because the majority were still receiving treatment at the time of the study. Mycobacterial culture, DST and genotypic results were obtained from the National Health Laboratory Service or other laboratories (private or research), where relevant.
Matching
A list of all children registered on EDR.web as starting treatment for DR-TB from 1 January to 31 December 2012 was extracted. The data were received in Microsoft Excel (Palisade Corp, Newfield, NY, USA) format and were password-protected. Electronic matching was performed between the compiled list of children treated and the data set extracted from EDR.web using Link Plus (Centers for Disease Control and Prevention, Atlanta, GA, USA) probabilistic record linking software.10,11 Four of the participants’ personal identifiers (first name, family name, sex and date of birth) were used for matching. Names were converted to the New York State Identification and Intelligence System, a phonetic coding system that reduces the possibility that variations in the spelling of first names or family names result in non-matching records. If there was agreement on any three of the four demographic variables, the records were reviewed manually.
Study
For children in the clinical cohort for whom a matching entry was found in EDR.web, data were compared between the routine clinical records and EDR.web data. For all variables where there were discrepant results, the clinical records were retrieved and reviewed. Each matched entry in EDR.web was also reviewed for internal consistency between data entered in different categories, such as confirmation and DST results. For mycobacterial culture and DST results, laboratory results were used to audit the electronic register. A diagnosis of DR-TB was considered as confirmed either if the culture was positive and DST confirmed drug resistance, or if drug resistance was identified by genotypic testing such as the Hain GenoType® MTBDRplus line probe assay (LPA; Hain Lifescience, Nehren, Germany) or Xpert® MTB/RIF (Cepheid, CA, USA).
The drug regimen was compared between the clinical data set and EDR.web. As TB treatment regimens may change during the course of treatment and because data were cross-sectional from two different points in time, the following considerations were taken into account when deciding whether the EDR.web entry was accurate: 1) all second-line oral medications needed to be recorded; 2) if the child was treated with a fluoroquinolone, any of the three used to treat children (ofloxacin, levofloxacin or moxifloxacin) were accepted as interchangeable; 3) amikacin and kanamycin were considered to be interchangeable; and 4) omission of first-line anti-tuberculosis drugs was not considered incorrect.
Statistical methods
Analyses were mostly descriptive; medians and interquartile ranges were used for continuous data, and numbers and percentages for categorical data. Associations between categorical variables were assessed using odds ratios with 95% confidence intervals and either χ² or Fisher’s exact tests. The Mann-Whitney U test was used to assess the difference in age at diagnosis and the delay in recording between children identified in EDR.web and those not identified. Statistical analysis was performed using Stata/IC 12.0 (Stata Corporation, College Station, TX, USA). STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines were used for reporting.12
Ethical considerations
Ethical approval was obtained from the Stellenbosch University Health Research Ethics Committee (Ref 2003/005), the Provincial Health Research Committee of the Western Cape (Ref 54/2012) and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Ref 122/12).
RESULTS
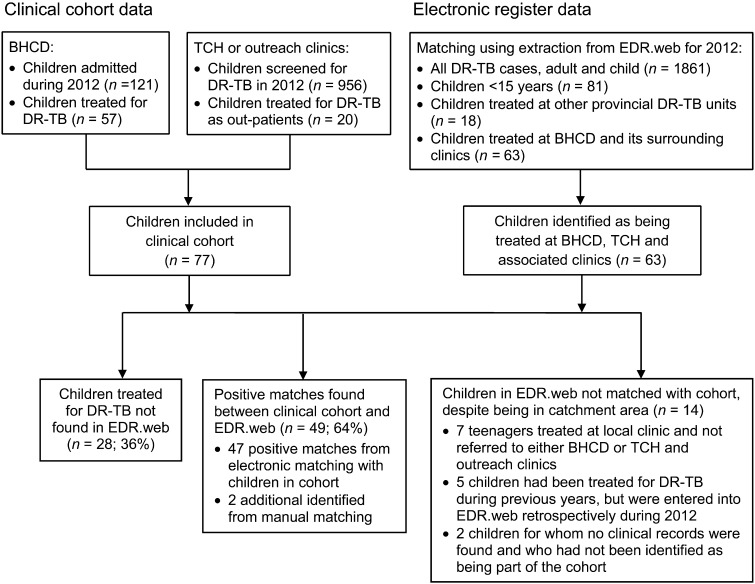
A total of 77 children were identified as being diagnosed and treated for DR-TB during the period reviewed. Of these, 49 (64%) were identified in EDR.web, and 28 (36%) were not found. The Figure demonstrates the identification and matching process. Table 1 indicates the demographic and clinical characteristics of children in the cohort. Of the 1861 entries in EDR.net in the WCP in 2012, 81 (4.4%) were children.
FIGURE.
Flow diagram of process for identifying children for inclusion in the clinical cohort and matching with electronic register data. BHCD = Brooklyn Hospital for Chest Disease; DR-TB = drug-resistant tuberculosis; TCH = Tygerberg Children’s Hospital.
TABLE 1.
Demographic and clinical data of children treated for drug-resistant tuberculosis included in the cohort comparing those identified with those not identified in EDR.web (N = 77 unless otherwise stated)
| In EDR.web (n = 49) n (%) | Not in EDR.web*(n = 28) n (%) | Total (N = 77) n (%) | Odds ratio (95%CI) | P value | |
| Age, median, years [interquartile range] | 3.8 [1.8–5.7] | 3.4 [1.5–4.9] | 3.6 [1.6–5.7] | — | 0.515 |
| Male sex | 27 (55) | 17 (61) | 44 (57) | 0.79 (0.31–2.04) | 0.632 |
| HIV-infected in those with status known (n = 69) | 10 (20) | 5 (18) | 15 (19) | 1.09 (0.28–4.67) | 1.000 |
| Type of TB | |||||
| PTB | 34 (70) | 20 (71) | 54 (70) | 0.91 (0.3–2.52) | 0.851 |
| EPTB | 6 (12) | 3 (11) | 9 (12) | — | — |
| Both | 9 (18) | 5 (18) | 14 (18) | — | — |
| Confirmed | 27 (55) | 10 (36) | 37 (48) | 2.21 (0.77–6.48) | 0.101 |
| DST only | 19 | 7 | |||
| DST and genotypic | 7 | 2 | |||
| Genotypic only | 1 | 1 | |||
| Type of DR-TB (confirmed cases)† | |||||
| XDR | 2 (1) | 0 | 2 (1) | — | |
| MDR | 31 (13) | 21 (5) | 52 (18) | 0.57 (0.20–1.61) | 0.293 |
| RMP-monoresistant | 6 (6) | 2 (1) | 8 (7) | — | |
| INH-monoresistant | 8 (7) | 5 (4) | 13 (11) | — | |
| Drug susceptible‡ | 2 (0) | 0 | 2 (0) | — | |
| DR-TB treatment unit | |||||
| BHCD | 37 (76) | 20 (71) | 57 (74)* | 1.23 (0.43–3.51) | 0.694 |
| Clinics | 12 (24) | 8 (29) | 20 (26) | ||
| Died | 1 (2) | 1 (4) | 2 (3) | 0.56 (0.01–5.77) | 0.685 |
Four of these children were recorded in EDR.web earlier in the year, but were not found in the final audit.
55/57 = 96.5% in paper register at BHCD. Both children not included in the paper register were in EDR.web.
Children initiated on DR-TB empirically, but subsequently changed to treatment for drug-susceptible TB following DST confirmation of drug-susceptible isolates.
CI = confidence interval; HIV = human immunodeficiency virus; TB = tuberculosis; PTB= pulmonary TB; EPTB= extra-pulmonary TB; DST = drug susceptibility testing; DR-TB = drug-resistant TB; XDR = extensively drug-resistant; MDR = multidrug-resistant; RMP = rifampicin; INH= isoniazid; BHCD = Brooklyn Hospital for Chest Diseases.
There were no statistically significant associations between EDR.web entry and age, sex, HIV status, type of TB, site of treatment initiation, confirmation of diagnosis or pattern of drug resistance. Two children died, one of whom was included in EDR.web. The other child who died had been treated at TCH, where children are not registered in EDR.web. According to SANTP guidelines, children are entered into EDR.web at the local TB hospital or clinic and not at the referral hospital, to avoid double entry. If children are not referred to the local TB hospital or clinic, there is currently no mechanism to ensure that these children are registered in EDR.web.
Most of the information included in EDR.web was correct (Table 2). There were some internal inconsistencies: eight children were indicated as not having a confirmed diagnosis, but mycobacteriological results entered into EDR.web (DST or LPA) indicated that the diagnosis of DR-TB had been confirmed. Completeness and accuracy were high for type of drug resistance (94%), indication that treatment had started (84%) and HIV status in HIV-infected children (100%). Overall, completeness and accuracy were moderate for disease spectrum, TB diagnosis confirmation, treatment start date within a week of the actual date, correct second-line anti-tuberculosis drugs and HIV status in non-HIV-infected children.
TABLE 2.
Completeness and accuracy of classification of children included in EDR.web (n = 49)
| n (%) | |
| Type of DR-TB | |
| Correct | 46 (94) |
| Incorrect | 1 (2) |
| Missing | 2 (4) |
| Confirmation of TB diagnosis | |
| Correct | 38 (78) |
| Incorrect | 9 (18) |
| Confirmed, indicated as not confirmed* | 8 |
| Not confirmed, indicated as confirmed | 1 |
| Missing | 2 (4) |
| Disease spectrum | |
| PTB (n = 34) | |
| Correct | 27 (79) |
| Incorrect | 4 (12) |
| Missing | 3 (9) |
| EPTB (n = 6) | |
| Correct | 5 (83) |
| Incorrect | 0 |
| Missing | 1 (17) |
| Both PTB and EPTB (n = 9) | |
| Indicated as PTB† | 5 (56) |
| Indicated as extra-pulmonary TB | 3 (33) |
| Missing | 1 (11) |
| Total correct | 37 (76) |
| Started on treatment (n = 49) | |
| Yes | 41 (84) |
| Missing | 8 (16) |
| Current treatment start date | |
| Within 1 week | 29 (59) |
| Within 1 month | 6 (12) |
| >1 month | 6 (12) |
| Missing (same as missing started on treatment) | 8 (16) |
| Current treatment | |
| Correct | 28 (57) |
| Some second-line drugs missing | 12 (24) |
| All missing | 9 (18) |
| HIV status | |
| Positive (n =10) | |
| Correct and all indicated as starting ART | 10 (100) |
| Negative (n = 35) | |
| Correct | 27 (77) |
| Incorrect (indicated ‘positive’ or ‘unknown’) | 2 (6) |
| Missing | 6 (17) |
| Missing in clinical data set (n = 4) | |
| Indicated as HIV-negative in EDR.web | 4 (100) |
6 of those indicated as not confirmed had culture/LPA results entered indicating confirmation of drug resistance.
WHO recommendation to report as PTB if both PTB and EPTB. Not possible to enter as both in EDR.web.
DR-TB = drug-resistant tuberculosis; PTB = pulmonary TB; EPTB = extra-pulmonary TB; HIV = human immunodeficiency virus; ART = antiretroviral treatment; LPA = line probe assay; WHO = World Health Organization.
Reviews of quarterly EDR.web reports showed that in 2012, five children who were treated for DR-TB that year were registered in EDR.web earlier in the year but did not appear in the final extraction. One child with presumed DR-TB was later confirmed to have drug-susceptible TB; the remaining four children were all treated for DR-TB, three of whom had culture-confirmed DR-TB. It is not clear why these children were removed from EDR.web.
Fourteen children were found in EDR.web who were not matched with the clinical cohort, despite being treated in the BHCD catchment area. Seven children aged 13–14 years were treated at local TB clinics, five children had been treated for DR-TB during previous years, but were entered in EDR.web during 2012, and two could not be identified and no clinical record was found. One of these was indicated in EDR.web as having confirmed MDR-TB, but died before starting DR-TB treatment.
DISCUSSION
Electronic TB registers are designed to allow the generation of useful reports on important TB programme indicators. Although electronic data can theoretically be more readily used to evaluate and improve programme management, data that are incomplete or inaccurate may lead to incorrect conclusions being reached. In addition, electronic data carry a greater risk of inadvertent data deletion.
As with drug-susceptible TB, most children with DR-TB do not receive a culture-confirmed diagnosis and are often initiated on treatment based on contact with a known adult DR-TB source case, suggestive symptoms and chest radiograph.7 Some of these cases are subsequently confirmed, some never have a confirmed diagnosis and a small number are later confirmed to have drug-susceptible TB. It is unclear how this should be reflected in EDR.web.
In EDR.web, 21% of children with confirmed DR-TB were entered as not confirmed, but had DST results indicating confirmed resistance. With an electronic register, there is the potential to perform internal consistency checks that prompt staff if inconsistent data are entered. For many children, regimens recorded in the EDR.web were inconsistent with those indicated in the clinical records, and they were not consistent with routine treatment regimens.
Only two thirds of the children diagnosed and started on treatment at the two studied facilities were recorded in the electronic register. This under-reporting is worrying, and has implications for both resource planning and burden estimates. In addition, although approximately 17% of TB cases occur in children in WCP, only 4.4% of EDR.web entries were for children. This is probably a result of under-diagnosis combined with under-reporting. The roll-out of molecular diagnostic tests may lead to increased detection of both adults and children with DR-TB. If more adults are diagnosed there may also be an associated increase in children with a presumed diagnosis of DR-TB (clinical TB disease and contact with an adult DR-TB source case).
Limitations
It was not possible to study treatment outcomes, as most children had not completed treatment at the time of the study. Reported outcomes in the EDR.web may be used to measure programme success, and it is important to assess their accuracy.
We did not cross-validate between facility paper registers and the electronic register. This would have been useful to identify transcription and data capture errors. Information on previous drug history and patient category was not found in many clinical records, preventing assessment of these data categories. This study only assessed the recording of paediatric TB patients referred through BHCD and its associated clinics, excluding other provincial TB units from the analysis. Because not all teenagers with DR-TB are referred to either BHCD or TCH, it is likely that not all eligible teenagers were identified and included in the cohort. Some children with isoniazid-monoresistant TB might not have been identified and included in the clinical cohort because some cases might be treated at TB clinics without referral to the hospital. We also did not assess whether any of these children were incorrectly included in ETR.net instead of EDR.web.
Recommendations
Clinicians at facility level should be able to routinely access and edit data in the electronic TB registers. Practical guidelines are needed on how to record and report DR-TB in children at facility, local and national TB programme levels. The process of transferring data from the paper register at facility level to the electronic register should be simplified, with improved updating and verification systems and the inclusion of internal consistency checks. Cross-linking between different electronic TB registers could ensure the appropriate transfer of data when a child’s diagnosis changes. A mechanism needs to be put in place to ensure that children with TB who die in health facilities are included in the electronic TB register. Improved recording and reporting of paediatric DR-TB is needed to ensure that children with DR-TB are diagnosed and managed appropriately and also that children treated for DR-TB are entered in facility-based paper registers and in local and national electronic TB registers.
Acknowledgments
The authors sincerely thank the staff of Brooklyn Hospital for Chest Diseases, Tygerberg Children’s Hospital and the Western Cape Department of Health.
PCR is an operational research fellow at the Desmond Tutu TB Centre, partly supported by the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease, Paris, France. HSS is supported by the South African National Research Foundation.
This research was supported by a United States Agency for International Development (USAID) Cooperative Agreement (TREAT TB—agreement no. GHN-A-00-08-00004-00). The contents are the responsibility of the author(s) and do not necessarily reflect the views of USAID.
Conflict of interest: none declared.
References
- 1.Directorate: Multi-drug Resistant Tuberculosis, TB & HIV, Department of Health, South Africa. A policy framework on decentralised and deinstitutionalised management for South Africa. Pretoria, South Africa: Government of South Africa; 2011. p. 36. http://www.doh.gov.za/docs/policy/2011/policy_TB.pdf Accessed August 2013. [Google Scholar]
- 2.Nadol P, Stinson K W, Coggin W, et al. Electronic tuberculosis surveillance systems: a tool for managing today’s TB programs. Int J Tuberc Lung Dis. 2008;12(Suppl 1):S8–S16. [PubMed] [Google Scholar]
- 3.Marais B J, Obihara C C, Warren R M, Schaaf H S, Gie R P, Donald P R. The burden of childhood tuberculosis: a public health perspective. Int J Tuberc Lung Dis. 2005;9:1305–1313. [PubMed] [Google Scholar]
- 4.Du Preez K, Schaaf H S, Dunbar R, Swartz A, Bissell K, Hesseling A. Incomplete registration and reporting of culture-confirmed childhood tuberculosis diagnosed in hospital. Public Health Action. 2011;1:19–24. doi: 10.5588/pha.11.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Assessment of the fraction of cases being missed by routine TB notification data, based on the ‘onion’ model. Geneva, Switzerland: WHO; April 2009. http://www.who.int/tb/advisory_bodies/impact_measurement_taskforce/meetings/ie_apr09_fracion_missed_en.pdf Accessed August 2013. [Google Scholar]
- 6.Statistics South Africa. Statistical release. Mid-year population estimates. Pretoria, South Africa: SSA; 2012. [Google Scholar]
- 7.World Health Organization. Global tuberculosis report. 2012. WHO/HTM/TB/2012.6. Geneva, Switzerland: WHO; 2012. http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf Accessed August 2013. [Google Scholar]
- 8.Seddon J A, Hesseling A C, Marais B J, Jordaan A, Victor T, Schaaf H S. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. Int J Tuberc Lung Dis. 2012;16:928–933. doi: 10.5588/ijtld.11.0679. [DOI] [PubMed] [Google Scholar]
- 9.Seddon J A, Hesseling A C, Willemse M, Donald P R, Schaaf H S. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clin Infect Dis. 2012;54:157–166. doi: 10.1093/cid/cir772. [DOI] [PubMed] [Google Scholar]
- 10.Méray N, Reitsma J B, Ravelli A C J, Bonsel G J. Probabilistic record linkage is a valid and transparent tool to combine databases without a patient identification number. J Clin Epidemiol. 2007;60:883–891. doi: 10.1016/j.jclinepi.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Newcombe H B. Handbook of record linkage: methods for health and statistical studies, administration and business. Oxford, UK: Oxford University Press; 1988. [Google Scholar]
- 12.Von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45:247–251. doi: 10.1016/j.ypmed.2007.08.012. [DOI] [PubMed] [Google Scholar]