Abstract
Setting:
Fourteen primary health care facilities in Cape Town, South Africa.
Objective:
To determine the proportion and characteristics of infectious adult tuberculosis (TB) cases that identify children aged <5 years who qualify for isoniazid preventive therapy (IPT), and to determine the proportion of children who initiate and complete IPT.
Design:
A retrospective clinical record review conducted as a stratified cluster survey.
Results:
Of 1179 records of infectious adult cases, 33.3% had no documentation of contacts. Of the remaining 786 records, 525 contacts aged <5 years were identified, representing 0.7 child contacts per infectious adult case. Older age, male, human immunodeficiency virus (HIV) positive, smear-negative and retreatment TB cases were all associated with no documentation of contacts. Of the 525 child contacts identified, less than half were screened for TB, 141 initiated IPT and 19 completed it.
Conclusion:
Less than 67% of infectious TB case records had documentation of contacts. Younger, female, HIV-negative and new smear-positive TB cases were more likely to have had contacts identified. Less than 14% of children already initiated on IPT completed 6 months of treatment.
Keywords: IPT, child contacts, completion, index case
Abstract
Contexte:
Quatorze services de soins de santé primaires à Cape Town, Afrique du Sud.
Objectif:
Déterminer la proportion et les caractéristiques des cas de tuberculose (TB) contagieuse chez l’adulte qui permettent d’identifier les enfants âgés <5 ans pouvant être considérés pour un traitement préventif à l’isoniazide (IPT), et déterminer la proportion des enfants mis sur l’IPT et qui le terminent.
Schéma:
Revue rétrospective des dossiers cliniques menée sous forme d’une enquête stratifiée en grappes.
Résultats:
Parmi les 1179 dossiers de cas adultes contagieux, il n’y a eu aucune documentation des contacts chez 33,3%. Sur les 786 dossiers restants, 525 contacts âgés <5 ans ont été identifiés, ce qui représente 0,7 enfant-contact par cas contagieux chez l’adulte. Un âge plus avancé, le sexe masculin, la positivité pour le virus de l’immunodéficience humaine (VIH), la négativité du frottis ainsi que le retraitement de la TB sont tous en association avec l’absence de documentation concernant les contacts. Sur les 525 enfants-contact identifiés, moins de la moitié ont fait l’objet d’un dépistage de la TB, 141 ont commencé un IPT et 19 l’ont achevé.
Conclusion:
Une documentation concernant les contacts fait défaut chez près de 67% des cas de TB contagieuse. Les sujets plus jeunes, les femmes, les sujets négatifs pour le VIH ainsi que les nouveaux cas de TB à frottis positif sont plus susceptibles d’être accompagnés d’un dépistage des contacts. Moins de 14% des enfants déjà mis sous IPT ont achevé les 6 mois de traitement.
Abstract
Marco de referencia:
Catorce centros de atención primaria de salud en la Ciudad del Cabo en Sudáfrica.
Objetivo:
Evaluar las características y determinar la proporción de casos de tuberculosis (TB) contagiosa en los adultos que permiten reconocer a los niños <5 años de edad que satisfacen las condiciones del tratamiento preventivo con isoniazida (IPT). Una meta posterior fue determinar la proporción de estos niños que inician el IPT y lo completan.
Método:
Se llevó a cabo un examen retrospectivo de las historias clínicas en una encuesta con muestreo estratificado y por conglomerados.
Resultados:
De los 1179 expedientes clínicos examinados de casos de TB contagiosa en adultos, el 33,3% carecía de documentación sobre contactos. En los 786 casos restantes, se detectaron 525 contactos <5 años de edad, lo cual representa 0,7 contactos pediátricos por cada caso contagioso de un adulto. La edad avanzada, el sexo masculino, la serología positiva frente al virus de la inmunodeficiencia humana (VIH), la baciloscopia negativa y los casos de retratamiento de TB se asociaron todos con la falta de documentación de contactos. De los 525 contactos pediátricos reconocidos, en menos de la mitad se investigó el diagnóstico de TB, solo 141 niños comenzaron el IPT y 19 de ellos lo completaron.
Conclusión:
Menos del 67% de las historias clínicas de casos de TB contagiosa contaba con documentación de contactos. Fue más probable haber investigado contactos en los casos de TB en jóvenes, mujeres, personas negativas frente al VIH y en los casos nuevos con baciloscopia positiva. Menos del 14% de los niños que había comenzado el IPT completó 6 meses de tratamiento.
According to the World Health Organization (WHO) and South African national policy, all children aged <5 years exposed to an infectious (smear- and/or culture-positive) pulmonary tuberculosis (PTB) case should be identified and screened for tuberculosis (TB), with subsequent provision of isoniazid preventive therapy (IPT) for 6 months for those without TB disease.1,2 Isoniazid (INH) monotherapy, with good adherence, is effective in preventing incident TB in children infected with susceptible strains of Mycobacterium tuberculosis.3
This policy is seldom implemented in high TB burden settings, however. Schaaf et al. showed missed opportunities (no previous anti-tuberculosis treatment despite a known contact) for chemoprophylaxis in 64% of children aged <5 years admitted to hospital in Cape Town with culture-confirmed TB.4 Reasons for the programmatic failure to put child contacts at high risk of developing TB on IPT are not well documented.
Cape Town serves an estimated population of 3.4 million, with >29 000 TB cases documented in 2009 and 51% of TB patients co-infected with the human immunodeficiency virus (HIV).5 Approximately 1% of children aged <5 years are recorded with TB disease annually.6 The City of Cape Town, a municipal health service, is managed as a single TB reporting district with a number of primary health care facilities.
The primary aim of this study was to determine the proportion of infectious TB cases in primary health care facilities in the City of Cape Town with routine documentation of child contacts for TB screening. Secondary aims were to determine what proportion of these children start and complete 6 months of IPT and to assess TB case risk factors for no documentation of contacts.
METHODS
This was a retrospective clinical record review of provision of IPT among child contacts aged <5 years by the routine TB programme in the City of Cape Town.
Setting
This study was conducted at 14 primary health care facilities located at randomly selected sites across the City of Cape Town, South Africa.
Data collection
Design
This was a stratified cluster survey with case records sampled over a 6-month period.
Sample size calculation and sampling
The 91 City of Cape Town primary health care facilities were ranked according to their total TB case load and categorised into two strata: high-burden and low-burden facilities, using the median TB case load of 240 cases per annum as a cut-off. A sampling period of 6 months was used and was intended to be conducted at random primary health care facilities within each of the two defined strata. The City of Cape Town Health directorate calculates the proportion of infectious TB cases with child contacts <5 years identified in the clinical records as part of quality assurance. In the 2010 audit, it was estimated that 75% of infectious TB cases have child contacts identified. For this study, a precision of 3.5% and 95% confidence interval (95%CI) was required. Using simple random sampling, 590 cases were required to estimate the proportion of clinical records that identify child contacts <5 years. Assuming a design effect of two for the cluster survey design, considering the two strata, the total sample needed would be 1180. Seven randomly selected facilities from each stratum were sampled, 14 facilities in total. The expected yield from this cluster sampling was 1680 cases within 6 months (half of the median annual case load of 240 per annum for 14 facilities). This indicated 40% oversampling, which would make provision for records not found or incomplete recording.
Electronic tuberculosis register (ETR.net)
Data on each TB diagnosis and outcomes are routinely captured into an electronic database, ETR.net. The following data were extracted from ETR.net for each record of smear and or culture-positive PTB: sex, age, history of TB treatment (new or retreatment TB case), HIV status recorded as positive, negative or unknown, smear status recorded as smear-positive, smear-negative or no smear, culture result recorded as positive, negative or unknown.
Clinical record reviews
Between 1 April and 30 September 2010, all infectious (smear- and/or culture-positive) PTB patients recorded in the electronic TB register were identified from the 14 facilities. A standardised search algorithm was applied to ensure that the clinical records were located and reviewed.7 A detailed review of each clinical record was conducted and the number of records with documentation of child contacts was determined.7 The number of child contacts screened for TB (documentation found on recording of TB symptoms and/or tuberculin skin test and/or chest X-ray and/or sputum mycobacterial investigation), initiated on IPT (documentation of start date of IPT or prescription of INH) and who completed IPT (documentation of IPT completion or INH prescriptions for 6 months) was noted.
Statistical analysis
Data collected were captured into an EpiData database version 3.1 (EpiData Association, Odense, Denmark), then exported into Microsoft Excel (Microsoft Excel, Palisade Corp, Newfield, NY, USA) and STATA release 12 (Stata Corporation, College Station, TX, USA).
Infectious TB cases were stratified by age; all cases aged ≤12 years were excluded. Initial descriptive statistics comprised means and standard deviations for continuous variables and frequency distributions and proportions for categorical variables. Documentation of contacts was defined as present if there was any record of contacts (e.g., ‘no contacts aged <5 years’) and absent if there was no record in the relevant space in the TB case file.
Proportions of IPT delivery were calculated as follows: the proportion of records with documentation of contacts aged <5 years among records of infectious TB cases; the number of contacts screened of those documented; the number of contacts initiated on IPT of those screened; and the number of contacts who completed IPT of those who initiated IPT along with their 95%CIs.
The following potential risk factors were measured for non-documentation of contacts: age, sex, HIV status, new or retreatment case and smear status of adult TB case.
Initial tests of association were completed using t-test for means and the χ2 test for proportions. For further analyses, continuous variables were dichotomised using the median as the cut-off. Crude odds ratios were measured and a multivariable logistic regression model was built using forward regression analyses.
Any documentation of contacts was further categorised into ‘contacts aged <5 years’ (details of contacts aged <5 years documented in TB case folder), ‘only contacts aged ≥5 years’ (details of contacts aged ≥5 years documented in TB case folder) and ‘no contacts aged <5 years’ (a specific phrase ‘no contacts aged <5 years’ documented in TB case folder). These categories were also compared with regard to age, sex, HIV status, new or retreatment case and smear status of adult TB case.
For all the above analyses, survey analyses were also conducted where each value within a variable was weighted depending on whether the specific record came from a high- or low-burden clinic. Weightings were calculated using the number of records that were found and reviewed within each stratum (high- or low-burden) of the total number of records within each stratum, according to ETR.net.
Records with missing data were included in descriptive statistics, but were excluded with further analyses. Statistical significance was considered at a P value of 0.05. Analyses were performed using STATA.
Ethics approval was received from the Stellenbosch University and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease. Permission was obtained from the City of Cape Town Health Directorate for the use of data and access to facilities.
RESULTS
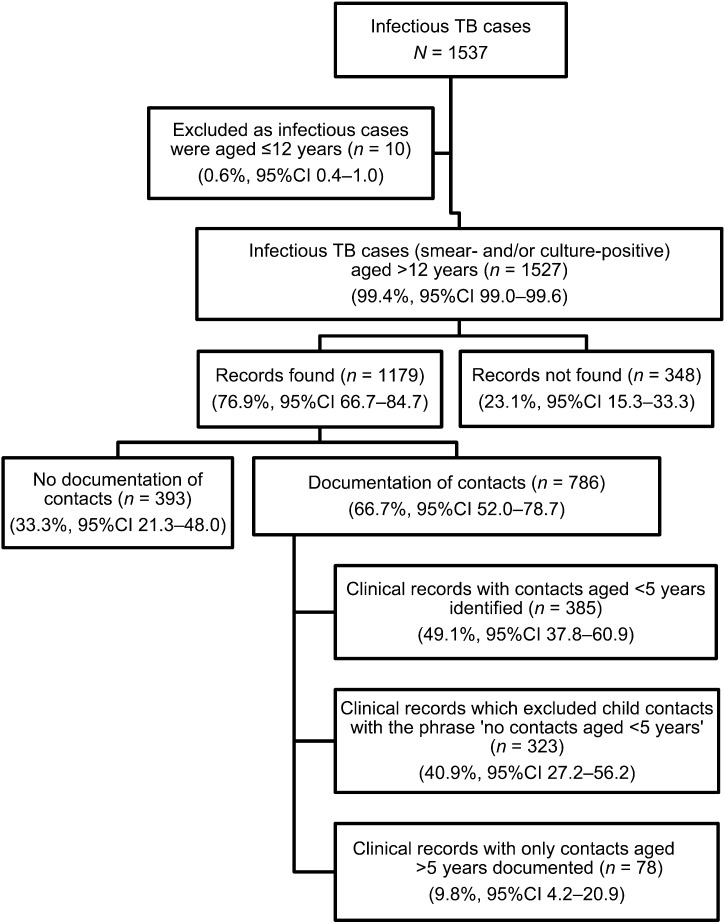
Between 1 April and 30 September 2010, a total of 1537 infectious TB cases were recorded in ETR.net in the 14 selected facilities. Of 1527 infectious cases aged >12 years recorded (Figure 1), 348 (22.8%) records could not be found. The mean age was 34 years (standard deviation [SD] 10.8), 54% were male, 60% were HIV-positive, 64% were new TB cases and 69% were smear-positive. For the remaining 1179 TB cases whose records were found, the mean age was 34 years (SD 11.6), 57% were male, 46% were HIV-positive, 68% were new TB cases and 71% were smear-positive.
FIGURE 1.
Flow diagram indicating the sampling of infectious TB case records reviewed at 14 facilities using survey analyses and sample weightings. TB = tuberculosis; n = actual number of records; % = weighted sample proportion; CI = confidence intervals adjusted due to complex sample analysis.
Figure 1 shows that 786 (66.7%) TB case records had documentation of contacts and in 385 of those records contacts aged <5 years were identified.
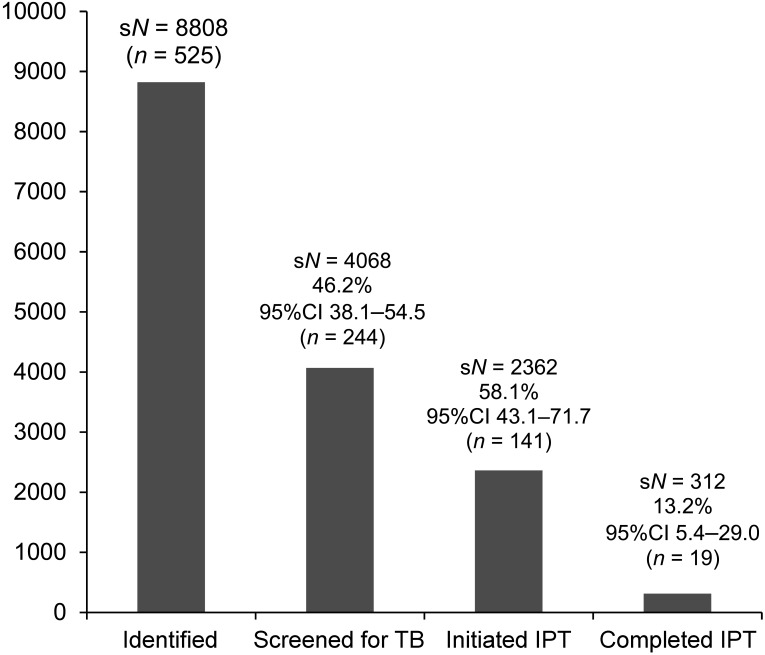
Figure 2 shows the analysis of the 525 child contacts aged <5 years; using the survey design and weighting, 8808 contacts aged <5 years were identified among the 13 144 records, representing 0.7 contacts per infectious TB case. Screening, initiation and completion of IPT are also demonstrated in Figure 2. The number starting on treatment for TB disease was not documented.
FIGURE 2.
Histogram demonstrating the sample weighted records of child contacts aged <5 years identified, screened for TB, initiated on IPT and who completed 6 months of IPT. sN = weighted sample population; % = weighted sample proportion; CI = confidence interval adjusted due to complex sample analysis; n = actual records; TB = tuberculosis; IPT = isoniazid preventive therapy.
Of the 1179 records of adult infectious TB cases reviewed, 68 (5.8%) had unknown HIV status and 8 (0.8%) had missing smear results; 1103 infectious adult case records were thus complete for TB case characteristics. The association between these characteristics and the documentation of contacts is shown in Table 1. Older age, male sex, HIV-positive status, smear-negative and retreatment TB cases were all associated with non-documentation of contacts. The associations remained significant with adjusted analyses.
TABLE 1.
Infectious TB case characteristics and their effect on documentation of contacts
| Infectious TB case characteristic | Records in category (% of all records*) n (%) | Records in category without documentation of contacts† (% of all records in category) n (%) | Crude OR (95% CI) | P value | Adjusted OR (95% CI)‡ | P value |
| Age, years | ||||||
| ≤33 | 583 (53) | 158 (27) | 1.00 (Reference) | –– | ||
| >33 | 520 (47) | 198 (38) | 1.65 (1.3–2.1) | 0.0001 | 1.01 (1–1.03) | 0.012 |
| Sex | ||||||
| Male | 616 (56) | 215 (35) | 1.00 (Reference) | –– | ||
| Female | 487 (44) | 141 (29) | 0.76 (0.6–0.9) | 0.0359 | 0.71 (0.5–0.9) | 0.012 |
| HIV status | ||||||
| Negative | 594 (54) | 165 (28) | 1.00 (Reference) | –– | ||
| Positive | 509 (46) | 191 (38) | 1.56 (1.2–2.0) | 0.0006 | 1.50 (1.1–2.0) | 0.003 |
| TB | ||||||
| Retreatment | 352 (32) | 138 (39) | 1.00 (Reference) | –– | ||
| New TB | 751 (68) | 218 (29) | 0.63 (0.5–0.8) | 0.0008 | 0.71 (0.5–0.9) | 0.018 |
| Smear status | ||||||
| Negative | 313 (28) | 131 (42) | 1.00 (Reference) | –– | ||
| Positive | 790 (72) | 225 (28) | 0.55 (0.4–0.7) | <0.0001 | 0.62 (0.5–0.8) | 0.001 |
Only complete records, n = 1103 (records with missing values excluded).
Total number of records without documentation of contacts, n = 356.
Adjusted for all other patient characteristics (in first column).
TB = tuberculosis; OR = odds ratio; CI = confidence interval; HIV = human immunodeficiency virus.
Older age and male TB cases were associated with documentation of contacts aged <5 years (Table 2).
TABLE 2.
Comparison of demographics between records where contacts were documented (N = 747 complete records) with respect to identification or exclusion of contacts aged <5 years
| Infectious TB case characteristic | Contacts aged <5 years documented (n = 366) n (%) | ‘No contacts <5’ documented (n = 307) n (%) | Only contacts aged ≥5 years documented (n = 74) n (%) | P value |
| Age, years, mean [SD] | 32 [9.6] | 36 [12.7] | 33 [11.7] | <0.0001* |
| Male | 170 (46) | 189 (62) | 42 (57) | <0.0001 |
| HIV | 161 (44) | 129 (42) | 28 (38) | 0.601 |
| New TB | 260 (71) | 215 (70) | 58 (78) | 0.356 |
| Smear-positive | 276 (75) | 233 (76) | 56 (76) | 0.989 |
P value represents one-way ANOVA test. All other P values represent χ2 test. TB = tuberculosis; SD = standard deviation; HIV = human immunodeficiency virus.
With survey analyses (weight of 14.7 for low-burden compared to 17.2 for high-burden facilities), all results remained similar, with no change in significance.
DISCUSSION
This study of routine programmatic delivery of IPT among child contacts was conducted in a stratified cluster sample of primary health care facilities that adhere to national and local guidelines. It demonstrates poor implementation of existing IPT guidelines at multiple stages of the delivery process, and poor completion of IPT once initiated.
Inadequate and incomplete recording and identification of child contacts were noted. More than 20% of infectious TB case clinical records could not be retrieved despite the application of a detailed search strategy. ETR.net currently uses a facility-specific registration number for each case of TB and does not link this to the existing standard facility registration number. TB records are therefore not integrated into the routine facility service. The characteristics of those records that could not be found did not differ significantly from those records that were found.
There was no documentation of contacts in >30% of the clinical records that were found and should have had contacts <5 evaluated. Risk factors for non-documentation of child contacts have been outlined. Importantly, smear-positive TB cases, which represent higher risks for transmission to child contacts, were more likely to have contacts identified than smear-negative cases. Mandalakas et al. emphasised the importance of screening children exposed to maternal TB.8 Although the IPT policy has not been refined for targeting specific groups in Cape Town, it is noted that female index cases were more likely to have contacts identified than male cases. Older age, HIV positivity, or previous treatment for TB were also associated with a lower likelihood of contacts being identified. Failure to identify contacts or exclude the presence of contacts precludes the accurate assessment of the number of true eligible contacts per infectious TB case.
Excluding those cases with incomplete recording gave 0.7 child contacts per infectious TB case. Another study from Cape Town by Marais et al. used home visits and enumerated 1.3 child contacts per infectious TB case.9
Screening for and initiation of IPT among identified contacts represents a further obstacle to IPT implementation. Of the 525 child contacts identified, less than half were screened and only 141 initiated IPT. The current screening algorithm applied in these facilities relies on history and clinical assessment; skin testing and chest X-rays are not mandatory and should not be understood as obstacles to screening.
The proportion of those starting and completing IPT was 13.2%. Two other studies from Cape Town showed completion rates of 27% and 20% of unsupervised INH monotherapy; these studies were reviews of one and two facilities respectively.9,10 In a recent study from India, with the use of health care worker training and the introduction of specific tools (IPT card and register), the implementation of contact tracing and chemoprophylaxis for child contacts in a programme setting improved from 19% to 61%, although the study was limited to 87 children.11
This study was strengthened by the inclusion of a number of randomly selected primary health care facilities from different geographical areas, with varying TB case loads and programme conditions representative of all primary health care facilities in the City of Cape Town. Furthermore, data from more than 1500 infectious cases were extracted, applying a rigorous search algorithm for clinical case records with detailed review of each clinical record. This allowed for sample weighting and statistical analysis of a survey model including more than 25 000 cases, more than any previous review of IPT implementation. We further reviewed multiple steps in IPT management, from identification of child contacts to screening, initiation and completion of IPT. We note that a limitation of the study was records that could not be found and could not be assumed to contribute to child contact recording.
It is important to note that the number of contacts identified does not represent all child contacts, as data included incomplete records and no home visits were done to validate the data recorded in the clinical records. The true denominator of contacts may therefore be significantly higher, and IPT delivery considerably overestimated in this study. The study is also limited as it did not evaluate the method of medication supervision and its contribution to adherence and completion of IPT or the number of child contacts who were diagnosed with TB disease and started on anti-tuberculosis treatment.
This study demonstrates poor IPT delivery to young children and the need for improvements in IPT delivery in primary health care facilities. In current practice, specific categories of infectious TB cases are more likely to identify child contacts; further research is recommended into the reasons why. It further highlights the low proportion of completion of IPT amongst those initiating IPT and recommends strategies to improve the follow-up of those initiating IPT as they are already within the primary health care system.
CONCLUSION
Suboptimal performance at multiple stages in IPT delivery in a programme setting resulted in less than 14% of child contacts initiated on IPT completing the 6 months of treatment. Specific categories of TB cases have been identified where contact identification needs to be improved.
Acknowledgments
The authors acknowledge the Desmond Tutu TB centre and the Operational Research Assistance Project staff and mentors, particularly Brenda Smuts and Pren Naidoo. The authors also acknowledge the City of Cape Town for access to the electronic databases, the staff at the facilities where clinical record reviews were conducted and the research support staff involved with data collection and entry. This research was supported by a United States Agency for International Development Cooperative Agreement (TREAT TB–Agreement no. GHN-A-00-08-00004-00). The contents are the responsibility of the author(s) and do not necessarily reflect the views of USAID.
Conflict of interest: none declared.
References
- 1.Department of Health, South Africa. National Tuberculosis Control Programme: national tuberculosis management guidelines. Pretoria, South Africa: Department of Health; 2009. [Google Scholar]
- 2.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. WHO/HTM/TB/2006.371. Geneva, Switzerland: WHO; 2006. [PubMed] [Google Scholar]
- 3.Smieja M J, Marchetti C A, Cook D J, Smaill F M. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev. 2000;2:CD001363. doi: 10.1002/14651858.CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaaf H S, Marais B J, Whitelaw A, et al. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Infect Dis. 2007;7:140. doi: 10.1186/1471-2334-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood R, Lawn S D, Caldwell J, Kaplan R, Middelkoop K, Bekker L G. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV status. PLOS ONE. 2011;6:e25098. doi: 10.1371/journal.pone.0025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood R, Lawn S D, Johnstone-Robertson S, Bekker L G. Tuberculosis control has failed in South Africa: time to reappraise strategy. S Afr Med J. 2011;101:111–114. doi: 10.7196/samj.4587. [DOI] [PubMed] [Google Scholar]
- 7.Van Wyk S S, Hamade H, Hesseling A C, Beyers N, Enarson D A, Mandalakas A M. Recording isoniazid preventive therapy delivery to children: operational challenges. Int J Tuberc Lung Dis. 2010;14:650–653. [PubMed] [Google Scholar]
- 8.Mandalakas A M, Kirchner H L, Lombard C, et al. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int J Tuberc Lung Dis. 2012;16:1033–1039. doi: 10.5588/ijtld.12.0027. [DOI] [PubMed] [Google Scholar]
- 9.Marais B J, Van Zyl S, Schaaf H S, Van Aardt M, Gie R P, Beyers N. Adherence to isoniazid preventive chemotherapy: a prospective community based study. Arch Dis Child. 2006;91:762–765. doi: 10.1136/adc.2006.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Zyl S, Marais B J, Hesseling A C, Gie R P, Beyers N, Schaaf H S. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis. 2006;10:13–18. [PubMed] [Google Scholar]
- 11.Rekha B, Jagarajamma K, Chandrasekaran V, Wares F, Sivanandham R, Swaminathan S. Improving screening and chemoprophylaxis among child contacts in India’s RNTCP: a pilot study. Int J Tuberc Lung Dis. 2013;17:163–168. doi: 10.5588/ijtld.12.0415. [DOI] [PubMed] [Google Scholar]