Abstract
Setting:
Tuberculosis (TB) clinic in Eldoret, Kenya.
Objective:
To identify TB exposed children through the implementation of a child contact register (CCR). To assess the demographics of children exposed to TB and the potential for initiation of isoniazid preventive therapy (IPT) in this cohort.
Methods:
A CCR was implemented in routine care with health care workers querying index cases regarding child contacts. Data were retrospectively analyzed.
Results:
In 12 months, the CCR revealed 580 children exposed to TB. Of these, 58% were exposed to smear-positive TB and 30% were aged <5 years. Of those exposed to smear-positive TB, 15% may have qualified for IPT initiation. Only 6 (1%) child contacts were screened for TB disease. More than 50% of the children with human immunodeficiency virus (HIV) positive mothers had not been HIV tested.
Conclusion:
Implementation of a CCR is a possible first step in child contact identification and management, which requires minimal resources and identifies children at risk for TB and HIV. Child contact screening and IPT initiation remain a challenge, and additional strategies are urgently needed.
Keywords: tuberculosis household contacts, isoniazid preventive therapy, pediatric tuberculosis
Abstract
Contexte:
Dispensaire de tuberculose (TB) à Eldoret, Kenya.
Objectif:
Identifier les enfants exposés à la TB grâce à la mise en œuvre d’un registre de contacts des enfants (CCR). Evaluer les données démographiques d’enfants exposés à la TB et les potentialités de mise en route d’un traitement préventif à l’isoniazide (IPT) dans cette cohorte.
Méthodes:
On a mis en œuvre un CCR dans les soins de routine avec l’aide des travailleurs de soins de santé qui questionnent les cas-index concernant leurs contacts avec des enfants. Les données ont été analysées rétrospectivement.
Résultats:
Sur 12 mois, le CCR a mis en évidence 580 enfants exposés à la TB. Parmi ceux-ci, 58% ont été exposés à une TB à frottis positif et 30% étaient âgés de <5 ans. Parmi ceux exposés à une TB à frottis positif, 15% pourraient avoir répondu aux critères de mise en route de l’IPT. Six seulement des enfants-contact (1%) avaient fait l’objet d’un dépistage d’une maladie TB. Plus de 50% des enfants dont les mères sont séropositives pour le virus de l’immunodéficience humaine (VIH) n’avaient pas été testés pour le VIH.
Conclusion:
La mise en route d’un CCR est une première étape possible pour l’identification et la prise en charge des enfants-contact ; elle exige des ressources minimales et identifie des enfants encourant le risque de TB et de VIH. Le dépistage des enfants-contact et la mise en route de l’IPT restent un défi, et des stratégies supplémentaires sont nécessaires et urgentes.
Abstract
Marco de referencia:
Un consultorio de atención de la tuberculosis (TB) en Eldoret, Kenia.
Objetivo:
Reconocer a los niños expuestos a la TB mediante la introducción de un registro de contactos pediátricos (CCR). Se buscó evaluar las características demográficas de los niños expuestos a la enfermedad y las posibilidades de iniciar un tratamiento preventivo con isoniazida (IPT) en esta cohorte de pacientes.
Métodos:
Se introdujo un CCR en la atención corriente, que exigía a los profesionales de salud investigar los casos nuevos de TB con respecto a la existencia de contactos pediátricos. Los datos se analizaron de manera retrospectiva.
Resultados:
En 12 meses el registro reveló la existencia de 580 niños expuestos. De estos, 58% estaban expuestos a un caso de TB con baciloscopia positiva y 30% eran <5 años de edad. De los niños expuestos a casos bacilíferos, 15% cumplían con los requisitos del IPT. Solo en seis contactos (1%) se practicó la detección de la enfermedad tuberculosa. Más del 50% de los niños con madres positivas frente al virus de la inmunodeficiencia humana (VIH) no contaban con una prueba diagnóstica esta infección.
Conclusión:
La introducción de un CCR constituye un primer paso viable en la detección y el manejo de los niños expuestos a la TB, exige mínimos recursos y permite reconocer a los niños con riesgo de padecer la TB y la infección por el VIH. Existen impedimentos a la detección sistemática de la TB en los contactos pediátricos y la iniciación del IPT, por lo cual se precisan con urgencia nuevas estrategias.
Childhood tuberculosis (TB) remains a neglected aspect of TB control programs, given the traditional programmatic emphasis on transmission reduction. Children present primarily with non-contagious forms of disease, therefore contributing little to TB transmission. In addition, smear microscopy (a test to detect infectiousness, but with little value in pediatric TB disease) remains the major diagnostic test for TB in low-resource settings. These programmatic priorities exist in stark contrast to international guidelines that emphasize the vulnerability of children to tuberculous infection when exposed, and the efficacy of low-cost prevention of TB disease in children through isoniazid preventive therapy (IPT). Globally, the pediatric TB burden is likely to be underestimated.
Kenya is a resource-constrained high-burden TB setting, with 5.8% of the 99 272 registered cases of TB in 2010 occurring in children.1
Children exposed to pulmonary TB (PTB) in the household are at greater risk of both TB infection and disease compared to adults. The risk of severe TB disease and progression to death is particularly increased if primary infection occurs at a young age (<5 years) or in a human immunodeficiency virus (HIV) infected child.2–4 A Brazilian study demonstrated that >60% of children with TB disease report a recent TB contact.5 Similar results were found by Du Preez et al. in South Africa, where 56.8% of children aged <5 years reported a TB contact.6 Thus, identification of children living in PTB households represents an important first step to implementing screening and preventive efforts for pediatric TB disease.
Despite World Health Organization (WHO) guidelines recommending that child household contacts be identified and screened for TB disease with the goal of initiation of IPT or treatment of active disease, TB control programs report operational challenges and child contact management is rarely implemented.7–9 Our site, the United States Agency for International Development/Academic Model Providing Access to Healthcare (USAID/AMPATH) Partnership at Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya, faces similar barriers. At the TB clinic, there was no structured approach for identifying and tracking children exposed prior to this study. Health care workers (HCW) sporadically asked index cases at the primary visit to bring children back to the clinic for screening. However, few index cases did so, and HCWs were unable to follow up because children in the index case households were not tracked or recorded.
The objective of this study was to identify TB exposed children through the implementation of a child contact register (CCR), a register modeled on the WHO recommended IPT register, with additional symptom-based questions. The secondary aim was to assess, for the first time at our site, the demographics of children exposed and gauge the number of children who could potentially qualify for IPT initiation.
METHODS
Design
Retrospective review of a programmatic intervention.
Setting
Kenya is one of the 22 high-burden TB countries. MTRH, associated with Moi University School of Medicine and USAID/AMPATH, located in Eldoret, Kenya, is the second largest referral hospital serving the western half of the country. MTRH has a TB and an HIV clinic caring for adults and children. HIV care, including antiretroviral therapy, is supported through USAID/AMPATH. Although TB care and medications are free for TB diagnosed patients through the Kenyan Division of Leprosy, TB and Lung Disease, the patient must pay for pre-TB diagnosis ancillary testing and registration.
Child contact register
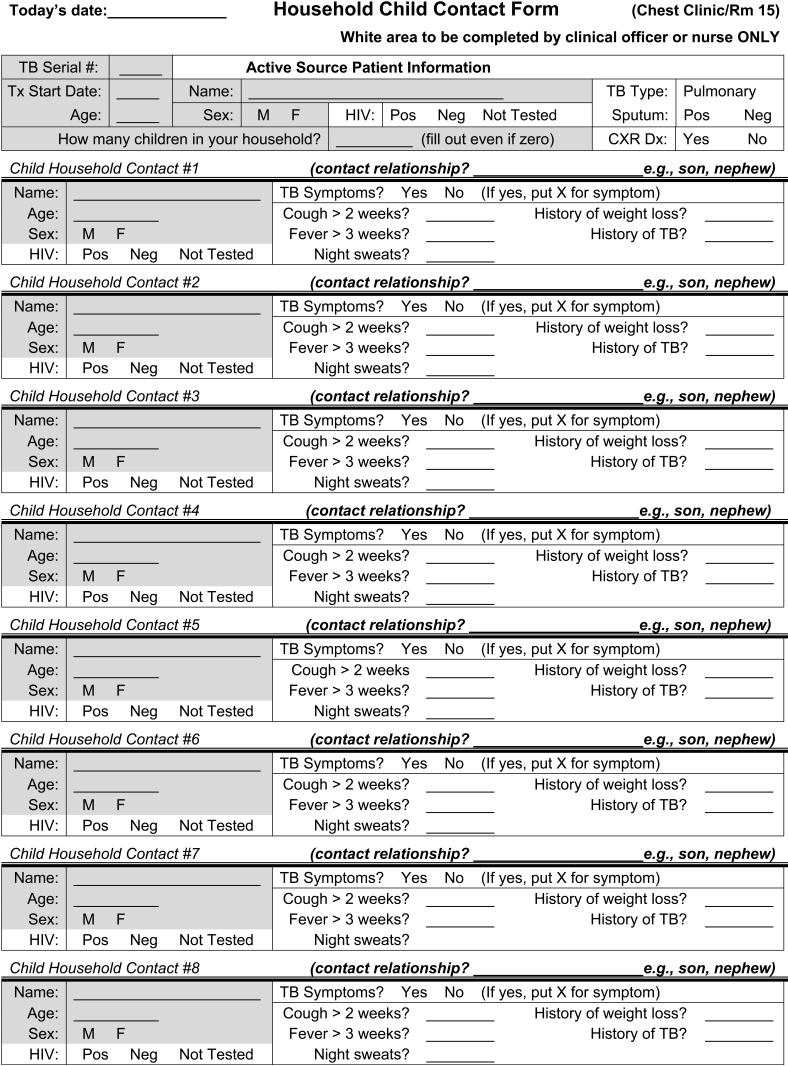
The CCR was implemented in routine care in December 2010. An HCW queried any registered PTB index case once (preferably at the time of diagnosis) concerning the existence of household child contacts. Forms were linked by the index case TB registration number. A child contact information form consisting of the exposed children’s names and demographics was generated for each index case.
The following information was collected for each index case: age, sex, HIV status, relationship to child and pulmonary infectiousness status (sputum smear-positive or -negative). The following information was collected for each exposed child: age, sex, HIV status as verbally reported by index case, symptoms (cough >2 weeks, fever >3 weeks, night sweats, weight loss/failure to thrive, history of TB) as reported subjectively by the index case (Figure). The CCR cost less than US$20 to implement.
FIGURE.
Household child contact form.
Interviews were typically conducted immediately before or after the index case visit, and lasted <10 minutes. HCWs periodically validated information by asking patients about their child contacts on subsequent visits.
Data collection and analysis
Data from the CCR were collected between January and December 2011, and de-identified data were organized and analyzed in a password-protected Microsoft Excel document. The child contacts exposed were stratified by age (<5 years or ≥5 years), sputum smear exposure of the index case, HIV status, and symptoms. The proportion of child contacts in each group was calculated.
Ethics approval
This study was approved by the Moi University College of Medicine Institutional Research Ethics Committee and the Lifespan Institutional Review Board.
RESULTS
Over a 12-month period, 1309 TB index cases received TB care; 974 (74%) had PTB, 280 had extra-pulmonary TB, 11 died and 44 transferred care. While 449 (46%) PTB index cases were queried for the CCR, 418 (93%) index cases (242 [58%] smear-positive and 176 [42%] smear-negative) were included for analysis. The median age of the index cases was 33 years, almost half were female, and more than half were HIV-infected (63%). Sixty-six per cent of the index cases reported child contacts in the home. There were on average two (standard deviation 1.26) children per household (Table 1).
TABLE 1.
Characteristics of the index cases
| Smear-positive n (%) | Smear-negative n (%) | Total n (%) | |
| Index cases | 242 (58) | 176 (42) | 418 |
| Index cases with children | 162 (67) | 112 (64) | 274 (66) |
| Number of children in households, mean ± SD | 2 ± 1.26 | 2 ± 1.26 | 2 ± 1.26 |
| Median age of index case, median [IQR] | 32 [26–39] | 35 [26–43] | 33 [26–40] |
| HIV-infected index case | 138 (56) | 125 (71) | 263 (63) |
| Index case females | 115 (48) | 86 (49) | 202 (48) |
SD = standard deviation; IQR = interquartile range; HIV = human immunodeficiency virus.
From the index cases queried, 580 children (approximately 1.4 per PTB index case) exposed to TB were identified. Of these, 58% were exposed to smear-positive TB. The majority of the exposed children were aged ≥5 years (70%) and were asymptomatic (80%). Of the total children exposed, the most common child contacts’ symptoms reported were cough >2 weeks (7%), fever >3 weeks (4%), night sweats (3%), and weight loss (3%; Table 2). Two per cent of all children exposed were HIV-infected. The ratio of children aged <5 years to index cases queried was 0.41. Four children were reported to currently be on anti-tuberculosis medication, and 89 (15%) children were identified as potential IPT candidates (exposed to smear-positive TB, aged <5 years, asymptomatic OR exposed to smear-positive TB, HIV-infected and asymptomatic).
TABLE 2.
Characteristics of the child household contacts
| Smear-positive exposed n (%) |
Smear-negative exposed n (%) |
Total exposed n (%) |
|||||||
| Age <5 years | Age ≥5 years | Total | Age <5 years | Age ≥5 years | Total | Age <5 years | Age ≥5 years | Total | |
| Child contacts | 339 (58) | 241 (42) | 580 | ||||||
| Age, years | 101 (30) | 238 (70) | 339 | 71 (29) | 170 (71) | 241 | 172 (30) | 408 (70) | 580 |
| Symptomatic | 14 (14) | 17 (7) | 31 (9) | 9 (13) | 12 (7) | 21 (9) | 23 (13) | 29 (7) | 52 (9) |
| Cough >2 weeks | 10 (10) | 12 (5) | 22 (6) | 7 (10) | 10 (6) | 17 (7) | 17 (10) | 22 (5) | 39 (7) |
| Fever | 3 (3) | 8 (3) | 11 (3) | 5 (7) | 8 (5) | 13 (5) | 8 (5) | 16 (4) | 24 (4) |
| Night sweats | 3 (3) | 5 (2) | 8 (2) | 4 (6) | 6 (4) | 10 (4) | 7 (4) | 11 (3) | 18 (3) |
| Weight loss/failure to thrive | 6 (6) | 4 (2) | 10 (3) | 2 (3) | 4 (2) | 6 (2) | 8 (5) | 8 (2) | 16 (3) |
| HIV-infected | 3 (3) | 4 (2) | 7 (2) | 2 (3) | 3 (2) | 5 (2) | 5 (3) | 7 (2) | 12 (2) |
| Currently on TB treatment | 1 (0.9) | 1 (0.4) | 2 (0.5) | 1 (1) | 1 (0.6) | 2 (0.8) | 2 (1) | 2 (0.5) | 4 (0.7) |
| Children with HIV-infected index case | 55 (54) | 176 (74) | 231 (68) | 50 (70) | 126 (74) | 176 (73) | 105 (61) | 302 (74) | 407 (70) |
HIV = human immunodeficiency virus; TB = tuberculosis.
The register also revealed that 407 child contacts had an HIV-infected index case. Of these, more than half had an unknown HIV status, 20% of whom were aged <5 years. There were 44% children with an HIV-infected parent; in more than half of these the HIV-infected parent was the mother. Of the child contacts with an HIV-positive index case mother, 56% had an unknown HIV status, and again 20% were aged <5 years (Table 3).
TABLE 3.
HIV status of child contacts
| <5 years n (%) | ≥5 years n (%) | Total n (%) | |
| Children with HIV+ index case | |||
| Not tested | 48 (46) | 189 (63) | 237 (58) |
| Tested and positive | 5 (5) | 7 (2) | 12 (3) |
| Tested and negative | 52 (50) | 106 (35) | 158 (39) |
| Total | 105 | 302 | 407 |
| Children with HIV+ index case as parent | |||
| Not tested | 21 (46) | 88 (65) | 109 (60) |
| Tested and positive | 4 (9) | 3 (2) | 7 (4) |
| Tested and negative | 21 (46) | 44 (33) | 65 (36) |
| Total | 46 | 135 | 181 |
| Children with HIV+ index case as mother | |||
| Not tested | 11 (42) | 45 (61) | 56 (56) |
| Tested and positive | 2 (8) | 2 (3) | 4 (4) |
| Tested and negative | 13 (50) | 27 (36) | 40 (40) |
| Total | 26 | 74 | 100 |
HIV = human immunodeficiency virus.
Only six (1%) exposed children were brought to the TB clinic for screening and evaluation. No active TB cases were identified and two children were documented as having started IPT. Three HCWs who were informally queried reported that the major barrier for index cases bringing children to the TB clinic for screening and evaluation was cost (transport, registration, and diagnostic testing).
DISCUSSION
This is the first study in Kenya to demonstrate an implementation strategy for a TB clinic to identify and stratify child contacts exposed to TB in the household. Contrary to other programs, such as in South Africa, where HCWs routinely document child contacts on the back of the TB case folders,10 TB clinics in western Kenya do not have a recording card/system in place for identifying and tracking child contacts. Before implementing a CCR, HCWs at the USAID/AMPATH-MTRH TB clinic were unaware of the children exposed to TB in the household and were subsequently unable to identify high-risk children who should be prioritized for screening and IPT or active TB treatment.
In the presence of the Kenyan Division of Leprosy, TB and Lung Disease’s efforts to begin IPT initiation in exposed children aged <5 years, implementation of a CCR reveals a ‘denominator’ that is vital in determining programmatic resource needs. In this study, a large number of children were identified over a 12-month period, 30% of whom were aged <5 years. The CCR required minimal resources and time. HCWs were able to complete CCR evaluation as part of routine TB care for almost half of all PTB patients. This completion rate may be improved through increased educational training and programmatic political will.
HIV remains an important public health issue in Kenya and greatly impacts the burden of pediatric TB disease. An unanticipated result of our CCR initiation was the identification of children who, as reported by the index cases, had never been screened for HIV, even in families where the risk of pediatric infection is high (e.g., with an HIV-positive mother). International guidelines recommend HIV testing in any child with suspected HIV exposure. Although one might consider stigma as the obvious explanation for this lack of testing, the result should be viewed in the context of the local program. All of the co-infected index cases in this TB clinic were enrolled in the HIV care program, which is located in the building next to the TB clinic and has been in existence for over 10 years. It is also possible that HIV-infected index cases (including mothers) did not correctly report child contacts’ HIV status or were unaware that the child was tested when in fact he/she had been tested previously. This may indicate that the HIV care program needs to ensure that child contacts are tested and/or educate HIV-infected individuals of their child’s HIV status.
This study had several limitations. Data were collected in a routine clinic fashion and were prone to inaccuracies and missing elements. The data were also collected and analyzed retrospectively. The study relied on child contact information reported from the index cases. Some of the index cases, while living in the household, were not in the child contacts’ immediate family and may have reported incorrect information regarding a child’s demographics. Information on the child contacts’ symptoms, weight loss, and history of TB were also subjectively reported by the index case. Parents may underestimate their children’s weight status, as demonstrated in recent studies on parents’ perceptions of childhood obesity.11 Subjective child contact symptom reporting may not be specific for a child’s actual health status.
An important finding of this study is that very few of the exposed children underwent clinical evaluation for TB disease or preventive services. The results at our site are consistent with other sites globally. A passive case-finding strategy in Malawi similar to the one in this study did not yield any children for X-ray referral, and an Indian study found only 14% of child contacts screened for TB.8,12 The Malawi study reported that transport costs and chest X-ray reading delays were barriers for IPT. This is also similar to the informal interviews at our site, with staff members who found cost to be the major barrier for evaluation. In our region the cost of transport, facility registration, and chest radiograph is greater than the average daily wage for most of the population. Further evaluation of other barriers in western Kenya and beyond is urgently needed in the light of these findings of large numbers of children exposed to PTB.
CONCLUSION
In a low-resource setting TB clinic, child household contacts at risk for TB and HIV can be identified and stratified by implementing a CCR, which requires minimal resources and no additional staff. The institution of a CCR at our site serves as the first step towards identifying vulnerable children exposed to TB and HIV, and ultimately uncovers a large gap in pediatric TB care in a resource-constrained clinic. Child contact screening and IPT initiation remain a challenge, and additional strategies need to be investigated to reduce the burden of TB disease in children.
Acknowledgments
The authors acknowledge the support received from the United States Agency for International Development/Academic Model Providing Access to Healthcare (USAID/AMPATH) Partnership as part of the President’s Emergency Plan for AIDS Relief (PEPFAR), the tuberculosis clinic at Moi Teaching and Referral Hospital, and the Kenyan Division for Leprosy, Tuberculosis and Lung Disease. This work was supported by the National Institutes of Health (NIH) Office of the Director, Fogarty International Center Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental & Craniofacial Research, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, and NIH Office of Women’s Health and Research through the International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
The authors also thank and recognize A Koech, N Kemboi, and the other health care workers in the MTRH TB clinic for their diligent work with the child contact register.
DS was supported through the Fogarty International Clinical Research Scholars Program. EJC is the principal investigator for the Fogarty International Clinical Research Scholars Grant. She is also supported as the TB HIV technical consultant for USAID/AMPATH under funding for USAID/AMPATH (PEPFAR). PO and FO received no funding.
Conflict of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis control. WHO/HTM/TB/ 2011.16. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 2.Palme I B, Gudetta B, Bruchfeld J, Muhe L, Giesecke J. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J. 2002;21:1053–1061. doi: 10.1097/00006454-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. WHO/HTM/TB/2006.371. Geneva, Switzerland: WHO; 2006. [PubMed] [Google Scholar]
- 4.Marais B J, Gie R P, Schaaf H S, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 5.Franco R, Santana M A, Matos E, Sousa V, Lemos A C. Clinical and radiological analysis of children and adolescents with tuberculosis in Bahia, Brazil. Braz J Infect Dis. 2003;7:73–81. doi: 10.1590/s1413-86702003000100009. [DOI] [PubMed] [Google Scholar]
- 6.Du Preez K, Hesseling A C, Mandalakas A M, Marais B J, Schaaf H S. Opportunities for chemoprophylaxis in children with culture-confirmed tuberculosis. Ann Trop Paediatr. 2011;31:301–310. doi: 10.1179/1465328111Y.0000000035. [DOI] [PubMed] [Google Scholar]
- 7.Van Wyk S S, Reid A J, Mandalakas A M, et al. Operational challenges in managing isoniazid preventive therapy in child contacts: a high-burden setting perspective. BMC Public Health. 2011;11:544–549. doi: 10.1186/1471-2458-11-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banu Rekha V V, Jagarajamma K, Wares F, Chandrasekaran V, Swaminathan S. Contact screening and chemoprophylaxis in India’s Revised Tuberculosis Control Programme: a situational analysis. Int J Tuberc Lung Dis. 2009;13:1507–1512. [PubMed] [Google Scholar]
- 9.Hill P C, Rutherford M E, Audas R, van Crevel R, Graham S M. Closing the policy practice gap in the management of child contacts of tuberculosis cases in developing countries. PLOS Med. 2011;8:e1001105. doi: 10.1371/journal.pmed.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Wyk S S, Hamade H, Hesseling A C, Beyers N, Enarson D A, Mandalakas A M. Recording isoniazid preventive therapy delivery to children: operational challenges. Int J Tuberc Lung Dis. 2010;14:650–653. [PubMed] [Google Scholar]
- 11.Tschamler J M, Conn K M, Cook S R, Halterman J S. Underestimation of children’s weight status: views of parents in an urban community. Clin Pediatr (Phila) 2010;49:470–476. doi: 10.1177/0009922809336071. [DOI] [PubMed] [Google Scholar]
- 12.Zachariah R, Spielmann M-P, Harries A D, et al. Passive versus active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:1033–1039. [PubMed] [Google Scholar]