Abstract
Setting:
Tertiary care hospital for diabetes mellitus (DM) in Tamil Nadu, South India.
Objective:
To compare the socio-demographic, clinical and biochemical characteristics in DM patients with and without tuberculosis (TB).
Design:
A descriptive study involving a review of routinely maintained records to capture the results of screening of DM patients for TB between March and December 2012. DM patients were first asked whether they already had TB, and if not they were screened for TB symptoms, followed by investigations for and possible diagnosis of TB.
Results:
Of 7083 DM patients, 38 already had TB. Of the remainder, 125 (1.8%) had TB symptoms; 71 were investigated and 12 were newly diagnosed with TB. Of the 50 TB patients, 64% had smear-positive pulmonary TB (PTB). DM-TB patients were older, and had lower education level and economic status, a higher frequency of alcohol use, lower body mass index, a longer duration of DM, a greater likelihood of receiving insulin and poorer glycaemic control.
Conclusion:
Screening of DM patients for TB was feasible in a tertiary care hospital. The yield of new TB cases was low and merits further investigation. Socio-demographic and clinical characteristics were different in patients with DM and TB compared to those with DM only.
Keywords: DM, TB, India, screening
Abstract
Contexte:
Hôpital de soins tertiaires pour le diabète sucré (DM) à Tamil Nadu, Inde du Sud.
Objectif:
Comparer les caractéristiques socio-démographiques, cliniques et biochimiques chez les patients DM atteints ou non de tuberculose (TB).
Schéma:
Etude descriptive impliquant une révision des dossiers entretenus en routine entre mars et décembre 2012 pour y prélever les résultats du dépistage chez les patients DM à la recherche d’une TB. On a d’abord demandé aux patients DM s’ils avaient déjà été diagnostiqués avec une TB et dans la négative, on les a dépistés à la recherche de symptômes de TB, puis investigués et diagnostiqués en matière de TB.
Résultats:
Sur 7083 patients DM, 38 souffraient déjà de TB. Parmi les restants, 125 (1,8%) étaient atteints de symptômes de TB, 71 ont été investigués et 12 nouveaux cas de TB ont été diagnostiqués. Une TB pulmonaire à frottis positif existait chez 64% de l’ensemble des 50 patients TB. Les patients DM-TB étaient plus âgés, bénéficiaient d’une éducation et d’un statut socio-économique moins favorables, connaissaient une fréquence plus élevée d’utilisation d’alcool, avaient un indice de masse corporelle plus bas et une durée plus longue du DM ainsi qu’une susceptibilité plus grande de recevoir de l’insuline et un contrôle moins bon de la glycémie.
Conclusion:
Le dépistage de la TB chez les patients DM est réalisable dans un hôpital de soins tertiaires. Le rendement en nouveaux cas de TB est bas et mérite d’être investigué davantage. Les caractéristiques socio-démographiques et cliniques sont différentes chez les patients atteints de DM-TB par comparaison avec ceux atteints de DM.
Abstract
Marco de referencia:
Un hospital de atención terciaria de la diabetes (DM) en Tamil Nadu, en el sur de la India.
Objetivo:
Comparar las características sociodemográficas, clínicas y bioquímicas entre los pacientes con diagnóstico de DM que padecen tuberculosis (TB) y los pacientes que sufren exclusivamente de DM.
Métodos:
Fue este un estudio descriptivo, que consistió en examinar los expedientes clínicos corrientes, a fin de investigar los resultados del cribado de la TB en los pacientes con diagnóstico de DM entre marzo y diciembre del 2012. Inicialmente se preguntó a los pacientes DM sobre el antecedente personal de TB y en los casos negativos, se buscaron los síntomas indicativos de la enfermedad y se practicó la investigación y el diagnóstico de la TB.
Resultados:
De los 7083 pacientes con diagnóstico de DM, 38 tenían un antecedente de TB y del resto de pacientes, 125 presentaban síntomas indicativos de la enfermedad (1,8%), se investigaron 71 pacientes y se detectaron 12 casos nuevos de TB. De los 50 pacientes que padecían TB, el 64% correspondió a una TB pulmonar con baciloscopia positiva. Los pacientes con ambas enfermedades eran mayores, poseían un menor grado de instrucción, presentaban una situación económica menos favorable, mayor frecuencia de consumo de alcohol, un índice de masa corporal más bajo, una evolución más prolongada de la DM, mayor probabilidad de estar recibiendo insulina y exhibían un equilibrio menos eficaz de la glucemia.
Conclusión:
Es posible practicar la detección de la TB en los pacientes DM en un hospital de atención terciaria. Se obtuvo un bajo rendi-miento diagnóstico de casos nuevos de TB, lo cual justifica nuevas investigaciones. Al comparar los pacientes que padecen DM únicamente con los pacientes que padecen ambas enfermedades se observa-ron diferencias en las características sociodemográficas y clínicas.
Diabetes mellitus (DM) has become a global epidemic, especially in low- and middle-income countries, where 80% of DM-related mortality is estimated to occur.1 Currently, there are more than 61 million people living with DM in India.2 In a similar vein to DM, about one third of the world’s population is currently infected with Mycobacterium tuberculosis, and approximately 8.8 million new cases of active TB are identified globally each year.3 India also has a huge TB burden, with an estimated 2.3 million new cases every year.4
There is good evidence that the risk of TB among people with DM is three times higher than in those without DM,5 and patients with both DM and TB have poorer TB treatment outcomes.6–8 India, which has a high dual burden of DM and TB,9,10 could benefit if patients were screened early for either disease. The World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease (The Union) launched the ‘Collaborative framework for the Care and Control of Diabetes and Tuberculosis’, with one of several important recommendations being the routine implementation of bidirectional screening of the two diseases.11 However, screening methods, recording and reporting for the two diseases in routine health care settings have not been well determined, and operational research is needed to provide better information in this area.12 Based on these recommendations, a standardised procedure for bi-directional screening, a monitoring tool and a quarterly system of recording and reporting were recently developed and implemented in eight tertiary centres and more than 60 peripheral health facilities across India. Our hospital was one of the eight tertiary health care facilities that participated in the pilot screening of DM patients for TB. Evaluation of this pilot in 2012 by the India Diabetes Mellitus–Tuberculosis Study Group showed that it is feasible to screen DM patients for TB within the routine setting, resulting in high rates of detection of TB.13
Despite these good results, there is a paucity of information on the association of socio-demographic characteristics and clinical features in DM patients with TB. In the present study, we therefore aimed to describe the screening of DM patients for TB and the socio-demographic characteristics, clinical features and biochemical variables of DM patients in relation to the diagnosis of TB in a tertiary care hospital for DM in South India.
METHODS
Study design
This was a descriptive study involving a review of rec-ords maintained during the pilot screening of DM patients for TB.
Setting
The study was conducted at the MV Hospital for Diabetes, a 100-bed tertiary care hospital for DM in the state of Tamil Nadu, South India. More than 200 000 DM patients have been registered in care at the hospital since its opening, and 100–200 patients visit the hospital every day on an out-patient basis. Patients attending the hospital and suspected of having DM are screened using the 2 h 75 g oral glucose tolerance test. The diagnosis of DM is based on previous DM history or on the WHO’s criteria for the classification of glucose intolerance.14 Fasting and postprandial samples are collected from known cases of DM.
For the TB screening, all DM patients presenting to the out-patient department were asked whether they had already been diagnosed with TB and were on TB treatment. If the answer was yes, this was recorded and the patient was not asked again about TB until completion of TB treatment. If the answer was no, the patient was screened for symptoms by trained staff, based on the Revised National TB Control Programme (RNTCP) guidelines.13,15 Briefly, patients with cough for ≥2 weeks or any suspicion of active pulmonary TB (PTB) or extra-pulmonary TB were categorised as having presumptive TB and were further investigated to confirm the disease. Two same-day sputum specimens from presumptive TB patients were collected in the DM clinic and transported to the government-run microscopy centre (1.5 km away) for sputum smear microscopy by Ziehl-Neelsen staining.15 Patients with negative sputum smears or extra-pulmonary TB suspects underwent appropriate investigations such as chest radiography to confirm TB. Those subsequently diagnosed with TB were referred to the RNTCP for TB treatment. All patient data were recorded on treatment cards and captured in an electronic database.
The TB screening process started in March 2012 and was performed when the patient visited the clinic. Screening was done on every patient visit. For the purpose of this study, however, we only describe the results of screening on the first visit.
Study population
All DM patients aged ≥ 15 years attending the MV hospital for their routine DM care and screened for TB between March and December 2012 were included in the study.
Data variables and sources of data
Data variables included: 1) socio-demographic characteristics: DM registration number, age, sex, residence, education, occupation and socio-economic status, smoking (current smoker was defined as a history of smoking in the last 3 months) and alcohol consumption (60 ml of alcohol daily). Occupation status was classified as skilled (carpenter, painter, electrician, fitter, etc.), unskilled (farmer, labourer), business, and ‘others’ for categories such as retired people, homemakers and the unemployed. Economic status was classified as low (family income <US$200 per month), middle (family income US$200–400 per month) and high (family income >US$400 per month); 2) clinical features: family history of DM, weight and height (for body mass index [BMI]), duration of DM and current medication for DM; and 3) blood glucose measurements performed at the time of TB screening, including fasting and postprandial glucose in mg/dl and glycosylated haemoglobin (HbA1c) in %. Plasma glucose was estimated using the glucose oxidase peroxidase method. HbA1c was estimated using the high-performance liquid chromatography method with Bio-Rad Variant Turbo equipment (Bio-Rad Laboratories, Hercules, CA, USA; Appendix Table A). Data were also collected on the TB screening process, diagnosis of TB, type of TB and referral for TB care.
Analysis and statistics
Data were extracted from the electronic database and analysed using SPSS (Statistical Package and Service Solutions, version 16.0, SPSS Inc, Chicago, IL). The flow of patients from screening to diagnosis of TB was described, and the socio-demographic characteristics, clinical features and biochemical variables of DM patients without TB symptoms (DM only) and with TB (previously known and newly diagnosed DM-TB) were evaluated. Patients with symptoms of TB who were either not investigated or not diagnosed with TB and patients with missing data were not included in this comparative analysis. Mean and standard deviations (SD) were calculated. Continuous variables such as age, BMI and duration of DM were converted to categorical variables and compared using the χ² test where appropriate. Levels of significance were set at 5%.
Ethics approval
Ethics approval for the study was obtained from the Institutional Ethics Committee of MV Diabetes Research Centre and The Union Ethics Advisory Group.
RESULTS
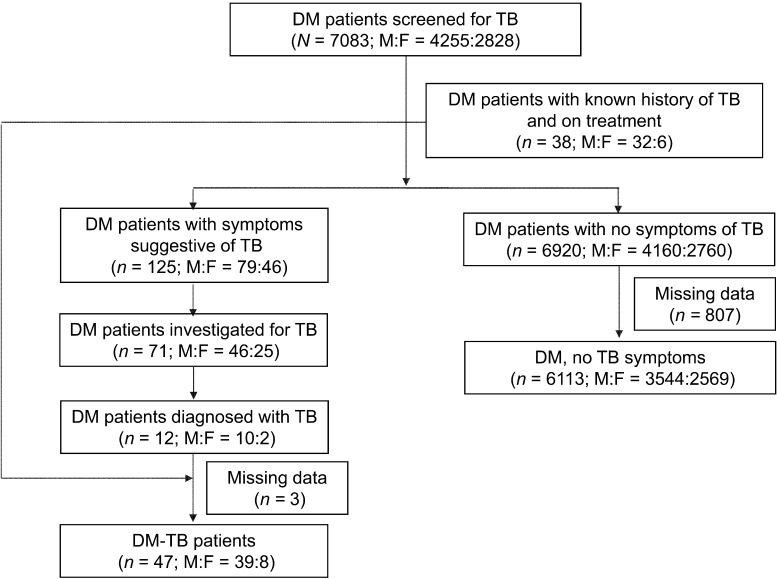
The TB screening process is shown in the Figure. Of 7083 DM patients screened for TB, (mean age 54 [SD 11.3] years, 4255 [60%] male), 38 had a known history of TB and were on treatment (26 smear-positive PTB, 8 smear-negative PTB, 2 extra-pulmonary TB and 2 with no information on TB type; 33 new and 5 retreatment patients). There were 6920 patients with no symptoms of TB and 125 (1.8%) who screened positive for TB symptoms. Of these 125 patients, 71 (57%) submitted two sputum specimens for smear examination and were investigated for TB, and 12 were diagnosed with TB (6 new smear-positive PTB, 4 new smear-negative PTB and 2 extra-pulmonary TB). The clinical and socio-demographic characteristics of the 71 patients examined for TB did not differ substantially from those of the 54 patients not investigated (Table A). In all, there were 50 patients (0.7%) with TB among the DM patients; the categories and types of TB are shown in Table 1. Notably, 32 patients (64%) had smear-positive PTB.
FIGURE.
Flow chart showing the results of screening DM patients for TB in a tertiary care hospital in South India, March–December 2012. DM = diabetes mellitus; TB = tuberculosis; M = male; F = female.
TABLE 1.
Types and categories of TB in DM patients screened for TB in a tertiary care hospital between March and December 2012
| Category and type of TB | Patients n |
| New | 45 |
| Smear-positive PTB | 27 |
| Smear-negative PTB | 12 |
| Extra-pulmonary TB | 4 |
| Not recorded | 2 |
| Retreatment | 5 |
| Relapse smear-positive PTB | 1 |
| Failure smear-positive PTB | 1 |
| Return after default smear-positive PTB | 3 |
TB = tuberculosis; DM = diabetes mellitus; PTB = pulmonary tuberculosis.
The socio-demographic characteristics of DM-TB and DM-only patients are shown in Table 2. A significantly higher proportion of DM-TB patients were male, older and had a lower education level and socio-economic status than the DM-only patients. More patients in the DM-TB group consumed alcohol compared to the DM-only group. The percentage of subjects defined as current cigarette smokers was also higher in the DM-TB group than that in the DM-only group, but the difference was not statistically significant.
TABLE 2.
Socio-demographic characteristics of DM patients with and without TB* in a tertiary care hospital in South India, March–December 2012
| Characteristic | DM patients with TB n (%) | DM patients with no TB* n (%) | P value |
| Total | 47 (100) | 6113 (100) | |
| Sex | |||
| Male | 39 (83) | 3544 (58) | <0.001 |
| Female | 8 (17) | 2569 (42) | |
| Age, years, mean ± SD | 57.4 ± 12.7 | 53.8 ± 11.2 | 0.03 |
| Age, years | |||
| 15–44 | 5 (10.6) | 1135 (18.6) | 0.23 |
| 45–64 | 32 (68.1) | 3956 (64.7) | 0.74 |
| ≥65 | 10 (21.3) | 1022 (16.7) | 0.52 |
| Residence | |||
| Urban | 27 (57.4) | 3383 (55.3) | 0.77 |
| Rural | 20 (42.6) | 2730 (44.7) | |
| Educational status | |||
| No school | 8 (17) | 1182 (19.3) | 0.83 |
| Primary/high school | 31 (66) | 2555 (41.8) | <0.001 |
| Technical/diploma | 2 (4.3) | 964 (15.8) | 0.05 |
| University degree | 6 (12.8) | 1412 (23.1) | 0.13 |
| Occupation | |||
| Skilled | 5 (10.6) | 741 (12.1) | 0.93 |
| Unskilled | 16 (34) | 517 (8.5) | <0.001 |
| Business | 3 (6.4) | 974 (15.9) | 0.11 |
| Other | 23 (48.9) | 3881 (63.5) | 0.05 |
| Economic status | |||
| Low | 13 (27.7) | 1783 (29.2) | 0.95 |
| Middle | 33 (70.2) | 3433 (56.2) | 0.07 |
| High | 1 (2.1) | 897 (14.7) | 0.03 |
| Current smoking | 7 (14.9) | 589 (9.6) | 0.22 |
| Alcohol consumption | 13 (27.7) | 627 (10.3) | <0.001 |
Those with no symptoms suggestive of TB.
DM = diabetes mellitus; TB = tuberculosis; SD = standard deviation.
The clinical features of the DM-TB and DM-only patients are shown in Table 3. There were significant differences between the DM-TB and DM-only groups: there was a stronger family history of DM in the DM-only group; the mean BMI was lower in the DM-TB group; and the DM-TB group had a higher proportion of patients with a duration of DM >10 years, and who were on combined oral medication and insulin.
TABLE 3.
Clinical features in DM patients with and without TB* in a tertiary care hospital in South India, March–December 2012
| Characteristic | DM patients with TB n (%) | DM patients with no TB* n (%) | P value |
| Total | 47 (100) | 6113 (100) | |
| Family history of DM | 22 (46.8) | 4740 (77.5) | <0.001 |
| BMI, kg/m2, mean ± SD | 22.8 ± 5.03 | 27.3 ± 4.45 | <0.001 |
| <18 | 8 (17.0) | 49 (0.8) | 0.001 |
| 18–23 | 19 (40.4) | 865 (14.2) | <0.001 |
| >23 | 20 (42.6) | 5199 (85) | <0.001 |
| Duration of DM, years, mean ± SD | 10.9 ± 8.7 | 5.93 ± 5.99 | <0.001 |
| <5 | 17 (36.2) | 3793 (62.0) | <0.001 |
| 5–10 | 6 (12.8) | 1135 (18.6) | 0.41 |
| >10 | 24 (51.1) | 1185 (19.4) | <0.001 |
| Current DM treatment | |||
| Diet only | 0 | 184 (3.0) | NA |
| Oral medication only | 8 (17.0) | 3465 (56.7) | <0.001 |
| Insulin only | 4 (8.5) | 98 (1.6) | <0.01 |
| Oral plus insulin | 35 (74.5) | 2366 (38.7) | <0.001 |
Those with no symptoms suggestive of TB.
DM = diabetes mellitus; TB = tuberculosis; BMI = body mass index.
Biochemical variables in DM-TB patients and DM-only patients are shown in Table 4. Although there were no differences in fasting and postprandial plasma glucose between the two groups, more individuals had HbA1c ≥9% in the DM-TB than the DM-only group.
TABLE 4.
Biochemical variables in DM patients with and without TB* in a tertiary care hospital of South India, March–December 2012
| Characteristic | DM patients with TB (N = 47) mean ± SD or n (%) | DM patients with no TB* (N = 6920) mean ± SD or n (%) | P value |
| Fasting plasma glucose, mg/dl | 189.4 ± 75.1 | 170.4 ± 69.7 | 0.17 |
| Postprandial plasma glucose, mg/dl | 302.9 ± 124 | 265 ± 111 | 0.05 |
| Glycosylated haemoglobin† | 9.2 ± 2.1 | 8.5 ± 2.1 | 0.03 |
| <7 | 7 (15.9) | 1234 (24.4) | 0.26 |
| 7–8.9 | 13 (29.5) | 1988 (39.3) | 0.24 |
| ≥9 | 24 (54.5) | 1834 (36.3) | 0.02 |
Those with no symptoms suggestive of TB.
Data available for 44 DM patients with TB and 5056 DM patients without TB.
TB = tuberculosis; DM = diabetes mellitus.
DISCUSSION
This study shows that the first-time screening of DM patients for TB is feasible in a routine health care setting of a tertiary care hospital for DM in South India. Over a period of 9 months, during which DM patients attending the clinic were screened, we detected 50 patients with TB, giving a case rate of 706 per 100 000 screened patients. Moreover, 64% of the patients diagnosed had smear-positive disease and were therefore highly infectious. Most of the patients had been diagnosed outside the DM clinic and were already on treatment at the time of screening. This is probably due to the very good geographical coverage of the RNTCP and good access to community TB case finding services in India.16 The relatively low yield in detecting new TB cases merits further investigation. However, if we exclude those already diagnosed with TB, the case rate for newly diagnosed cases would be 169 per 100 000 patients, which is still higher than the total TB case notification rate in India.
DM patients with TB tended to be male, older, have low BMI and were more likely to consume tobacco and alcohol than patients without TB. These findings might be expected given the epidemiology of TB. Rieder has reported that age and sex are strong determinants of TB, with the highest risks being found in elderly people,17 and this was evident in the current study. Systematic reviews have shown that undernutrition, smoking, diabetes and alcohol misuse are individual risk factors that can double or triple the risk of developing active TB.18 A mathematical modelling analysis of the effects of smoking on TB infection and mortality projected that smoking would produce an excess of 18 million TB cases and 40 million deaths from TB between 2010 and 2050.19 It was also reported that smoking could delay the attainment of the Stop TB Partnership target of reducing TB mortality by 50% from 1990 to 2015.19 A large part of the TB burden in India can be attributed to smoking (40%)20 and DM (15%).21 Alcohol misuse and DM are predicted to increase in low- and middle-income countries, and might be crucial factors in the coming decades.22,23 More patients with both DM and TB came from the unskilled workforce sector with lower socio-economic status, more had had their metabolic disease for >10 years, were on combination therapy with oral and insulin medication and their glycosylated haemoglobin levels were significantly higher, indicating poorer glycaemic control. These data all indicate that DM patients with TB were poorer and had long-standing, severe and uncontrolled disease, an association that has previously been reported as a risk factor for TB.24
The strengths of this study were that a large number of DM patients were screened, the registration of patients was consecutive and robust, allowing a denominator for the study, and the recording and reporting system using the established electronic database worked well. There were a number of limitations and challenges. First, over 40% of patients with symptoms suggestive of TB were reluctant to give sputum specimens for reasons that are currently unclear and may be related to stigma or disbelief that they might have TB, and this requires further prospective research using qualitative methods. Second, we are reporting only on the results of the first screening, and it is possible that patients returning to the clinic and being screened again may be found to have TB on subsequent occasions. This is also the subject of further research, as we will be prospectively following up this cohort of 7000 patients over several years to determine the yield of TB screening at each subsequent clinic visit. Third, we relied on symptom screening and sputum smear examination, both of which lack sensitivity for diagnosing TB. Screening by chest radiography might give a higher yield, but the cost and logistical challenges would also be greater. More research is needed to determine the most appropriate screening and diagnostic algorithm for DM patients attending clinics, and also whether it is cost-effective to use newer technology such as Xpert® MTB/RIF (Cepheid, Inc, Sunnyvale, CA, USA) for diagnosing TB.25 Advocacy and the political will to deploy more effective and affordable point-of-care diagnostics at DM clinics might assist in the diagnosis and management of both of these diseases. This is urgently required if we are to move forward in making TB screening routine in DM clinics. Fourth, the lower mean BMI in the DM-TB group compared to the DM-only group should be interpreted with caution, given the cross-sectional nature of the study, and it is not clear whether TB led to low BMI or whether TB occurred as a result of a low BMI. Fifth, a multiple regression model would have strengthened the conclusions of the study, but due to the small number of TB-DM cases, we felt it prudent to limit our analysis to bivariate associations.
In terms of policy, the screening of TB patients for DM is relatively straightforward, and in pilot studies in different states in India this has resulted in a high rate of detection of DM in TB patients (10–15%).26 A decision was thus made by the Ministry of Health to scale up screening of DM in TB patients countrywide, with a national training manual and revised registers and treatment cards to support this activity. However, screening of DM patients for TB is less easy, with pilot studies in India documenting challenges such as poor registration of DM patients, a reluctance by DM doctors to undertake this additional work and systematically record every screening event, and resistance of patients to submit sputum specimens.13 Our current study also confirms these findings. Importantly, an electronic recording system can be used to reliably collect and report on the necessary data. Paper-based systems could probably not work long-term in this type of setting with patients in chronic care, and there is a need for electronic health systems, as shown elsewhere in DM clinics in Africa and the Middle East.27,28 Further research is needed to better understand some of these challenges, and in particular the value of repeated screening and use of point-of-care TB diagnostics at each subsequent clinic visit. This study shows the importance of good collaboration between communicable and non-communicable disease programmes.
In conclusion, the study highlights that screening of DM patients for TB is feasible in the routine health care setting of a tertiary care hospital. DM-TB patients were older, had lower education levels and socio-economic status, a higher frequency of smoking and alcohol use, a longer duration of DM, a greater likelihood of being on oral medication and insulin and lower BMI and poorer glycaemic control.
Acknowledgments
The authors acknowledge the help rendered by R Priyadarshini, Krishna, M Rajalakshmi, V Arulmozhi and C Deepika for data collection. They also acknowledge Selvan and A Vigneswari for conducting data analysis. The authors thank all their patients for cooperating with them throughout the study.
A workshop was convened in Delhi, India, for the purpose of writing the papers that are published in this supplement. The workshop was run by the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France; The Union South-East Asia Office, New Delhi, India; the Operational Research Unit, Médecins Sans Frontières, Luxembourg; the World Health Organization Country Office in India, New Delhi, India; the All India Institute of Medical Sciences, New Delhi, India; and ESIC Medical College, Bangalore, India.
Funding for the workshop and open access publication was received from the World Diabetes Foundation, Gentofte, Denmark.
Conflict of interest: none declared.
Appendix.
TABLE A.
Data variables
|
N = 125 (M:F 79:46) |
P value | ||
| DM patients with symptoms suggestive of TB who were investigated n = 71 (46:25) n (%) | DM patients with symptoms suggestive of TB who were not investigated n = 54 (32:22) n (%) | ||
| Socio-demographic characteristics | |||
| Age, years, mean ± SD | 57.1 ± 11.3 | 56 ± 11.1 | 0.58 |
| Residence | |||
| Rural | 24 (33.8) | 17 (31.5) | 0.85 |
| Urban | 47 (66.2) | 37 (68.5) | |
| Educational status | |||
| No schooling | 5 (7) | 4 (7.4) | 0.75 |
| Primary/high school | 51 (71.8) | 41 (75.9) | 0.76 |
| Technical/diploma | 6 (8.5) | 5 (9.3) | 0.87 |
| University degree | 9 (12.7) | 4 (7.4) | 0.51 |
| Occupation | |||
| Skilled | 6 (8.5) | 2 (3.7) | 0.48 |
| Unskilled | 14 (19.7) | 7 (13) | 0.45 |
| Business | 11 (15.5) | 12 (22.2) | 0.47 |
| Other | 40 (56.3) | 33 (61.1) | 0.72 |
| Socio-economic status (USD) | |||
| Low (<200) | 5 (7) | — | |
| Middle (200–400) | 60 (84.5) | 47 (87) | 0.89 |
| High (>400) | 6 (8.5) | 7 (13) | 0.60 |
| Current smoking | 7 (9.9) | 4 (7.4) | 0.76 |
| Alcohol consumption | 9 (12.7) | 4 (7.4) | 0.39 |
| Clinical features | |||
| Positive family history of DM | 52 (73.2) | 36 (66.7) | 0.44 |
| BMI, kg/m2, mean ± SD | 24.6 ± 4.8 | 27.3 ± 4.9 | 0.002 |
| Duration of DM | |||
| <5 years | 29 (40.8) | 21 (38.9) | 0.97 |
| 5–10 years | 16 (22.5) | 18 (33.3) | 0.25 |
| >10 years | 26 (36.6) | 15 (27.8) | 0.39 |
| Current DM treatment | |||
| Diet only | 1 (1.4) | — | |
| Oral medication only | 28 (39.4) | 24 (44.4) | 0.7 |
| Insulin only | 2 (2.8) | 2 (3.7) | 0.82 |
| Oral plus insulin | 40 (56.3) | 28 (51.9) | 0.75 |
| Biochemical variables | |||
| Fasting plasma glucose, mg/dl | 165.8 ± 67.6 | 172 ± 82 | 0.687 |
| Postprandial plasma glucose, mg/dl | 238.9 ± 90.1 | 265.6 ± 92.5 | 0.12 |
| Glycosylated haemoglobin % | 9.1 ± 2.3 | 8.6 ± 1.9 | 0.19 |
M = male; F = female; DM = diabetes mellitus; TB = tuberculosis; SD = standard deviation.
References
- 1.World Health Organization. Diabetes. Fact sheet no. 312. Updated March 2013. WHO Media Centre: WHO; 2013. http://www.who.int/mediacentre/factsheets/fs312/en/index.html Accessed August 2013. [Google Scholar]
- 2.International Diabetes Federation. IDF diabetes atlas. 2012 update. In: Unwin N, Whiting D, Guariguata L, et al., editors. 5th ed. Brussels, Belgium: International Diabetes Federation; 2012. http://www.eatlas.idf.org/diabetesatlas/5e/update2012 Accessed August 2013. [Google Scholar]
- 3.World Health Organization. Global tuberculosis control, 2011. WHO/HTM/TB/2011.16. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 4.World Health Organization. Global tuberculosis report, 2012. WHO/HTM/TB/2012.6. Geneva, Switzerland: WHO; 2012. http://www.who.int/tb/data Accessed August 2013. [Google Scholar]
- 5.Jeon C Y, Murray M B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLOS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alisjahbana B, Sahiratmadja E, Nelwan E J, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–435. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 7.Restrepo B I, Fisher-Hoch S P, Smith B, et al. Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg. 2008;79:541–544. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C S, Yang C J, Chen H C, et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009;137:203–210. doi: 10.1017/S0950268808000782. [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan V, Kumpatla S, Aravindalochanan A, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLOS ONE. 2012;7:e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balakrishnan S, Vijayan S, Nair S, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLOS ONE. 2012;7:e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization/International Union Against Tuberculosis and Lung Disease. Provisional collaborative framework for care and control of tuberculosis and diabetes. WHO/HTM/TB/2011.15. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 12.Harries A D, Murray M B, Jeon C Y, et al. Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Trop Med Int Health. 2010;15:659–663. doi: 10.1111/j.1365-3156.2010.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.India Diabetes Mellitus–Tuberculosis Study Group. Screening of patients with diabetes mellitus for tuberculosis in India. Trop Med Int Health. 2013;18:646–654. doi: 10.1111/tmi.12083. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. WHO/NCD/NCS/99.2. Geneva, Switzerland: WHO; 1999. http://whqlibdoc.who.int/hq/1999/who_ncd_ncs_99.2.pdf Accessed August 2013. [Google Scholar]
- 15.Central Tuberculosis Division. Directorate General of Health Services, Ministry of Health and Family Welfare. Technical and operational guidelines for tuberculosis control. New Delhi, India: Government of India; 2005. Revised National Tuberculosis Control Programme. [Google Scholar]
- 16.Sachdeva K S, Kumar A, Dewan P, Kumar A M V, Satyanarayana S. New vision for Revised National Tuberculosis Control Programme (RNTCP): universal access—‘Reaching the un-reached’. Ind J Med Res. 2012;135:690–694. [PMC free article] [PubMed] [Google Scholar]
- 17.Rieder H. International Union Against Tuberculosis and Lung Disease. Paris, France: 1999. Epidemiologic basis of tuberculosis control. [Google Scholar]
- 18.Lönnroth K, Castro K G, Chakaya J M, et al. Tuberculosis control and elimination 2010–50: cure, care and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Stuckler D, Bitton A, Glantz S A. Projected effects of tobacco smoking on worldwide tuberculosis control: mathematical modelling analysis. BMJ. 2011;343:d5506. doi: 10.1136/bmj.d5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassmiller K. The impact of smoking on population level tuberculosis outcomes. TSRU progress report, 2007. The Hague, The Netherlands: KNCV; 2007. [Google Scholar]
- 21.Stevenson C R, Forouhi N G, Roglic G, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dooley K E, Chaisson R E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health OrganizationDepartment of Mental Health and Substance Abuse. Global status report on alcohol 2004: country profiles Uganda. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 24.Restrepo B I, Fisher-Hoch S P, Pino P A, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–641. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boehme C C, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.India Tuberculosis–Diabetes Study Group. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health. 2013;18:636–645. doi: 10.1111/tmi.12084. [DOI] [PubMed] [Google Scholar]
- 27.Allain T J, van Oosterhout J J, Douglas G P, et al. Applying lessons learnt from the ‘DOTS’ tuberculosis model to monitoring and evaluating persons with diabetes mellitus in Blantyre, Malawi. Trop Med Int Health. 2001;16:1077–1084. doi: 10.1111/j.1365-3156.2011.02808.x. [DOI] [PubMed] [Google Scholar]
- 28.Khader A, Farajallah L, Shahin Y, et al. Cohort monitoring of persons with diabetes mellitus in a primary healthcare clinic for Palestine refugees in Jordan. Trop Med Int Health. 2012;17:1569–1576. doi: 10.1111/j.1365-3156.2012.03097.x. [DOI] [PubMed] [Google Scholar]