Abstract
Setting:
Sex Workers Outreach Programme Clinic, Korogocho, Nairobi, Kenya.
Objective:
In a cohort of sex workers, to determine 1) the prevalence of cervical intra-epithelial neoplasia (CIN) and its association with human immunodeficiency virus-1 (HIV-1) infection, and 2) the incidence rate of CIN during the 3-year follow-up from December 2009 to December 2012.
Design:
Prospective nested cohort study.
Results:
Of the 350 women enrolled, the median age was 29 years (range 18–49); 84 (24%) were HIV-1-infected. At enrollment, 54 (15%) had an abnormal cytology, 39 (11%) had low-grade intra-epithelial lesions (LSIL) and 15 (4%) high-grade intraepithelial lesions (HSIL). HIV-1-infected women were 2.7 times (95%CI 1.7–4.4) more likely to have CIN than non-HIV-1-infected women. Among HIV-1-infected women, the prevalence of LSIL and HSIL was 2.5 times (95%CI 1.2–5.1) and seven times (95%CI 2.3–23.3) greater than among non-HIV-infected women. During the follow-up period, 39 (11%) women had incident CIN (6.6/100 person years [py]), with no difference by HIV status, i.e., respectively 7.9/100 py and 6.3/100 py in HIV-1-infected and non-HIV-1-infected women.
Conclusion:
The prevalence and incidence of CIN among HIV-1-infected sex workers was high; early, regular screening and follow-up of this life-threatening condition is therefore recommended.
Keywords: CIN, HIV, HPV, cervical cancer
Abstract
Contexte:
Clinique du programme de proximité à l’intention des travailleurs du sexe, Korogocho, Nairobi, Kenya.
Objectif:
Déterminer dans une cohorte de travailleuses du sexe 1) la prévalence des néoplasies intra-épithéliales du col de la matrice (CIN) et son association avec l’infection par le virus-1 de l’immunodéficience humaine (VIH-1), et 2) le taux d’incidence de la CIN au cours d’un suivi de 3 ans de décembre 2009 à décembre 2012.
Schéma:
Etude prospective de cohorte nichée.
Résultats:
Sur les 350 femmes recrutées, l’âge médian était de 29 ans (extrêmes 18–49) et 84 d’entre elles (24%) étaient infectées par le VIH. Au recrutement, la cytologie était anormale chez 54 (15%), des lésions intra-épithéliales de faible degré (LSIL) existaient chez 39 (11%) et des lésions intra-épithéliales de degré élevé (HSIL) chez 15 (4%). Les femmes infectées par le VIH-1 étaient 2,7 fois (IC95% 1,7–4,4) plus susceptibles d’être atteintes de CIN que les femmes non-infectées par le VIH-1. Parmi les femmes infectées par le VIH-1, la prévalence des LSIL et des HSIL était respectivement 2,5 fois (IC95% 1,2–5,1) et 7 fois (IC95% 2,3–23,3) plus élevée que chez les femmes non-infectées par le VIH-1. Au cours de la période de suivi, on a noté une incidence de la CIN chez 39 femmes (11% ; 6,6/100 années/personne [ap]) sans différence en fonction du statut VIH, ce qui correspond à 7,9/100 ap chez les femmes infectées par le VIH-1 et à 6,3/100 ap chez les femmes non-infectées par le VIH-1.
Conclusion:
La prévalence et l’incidence de la CIN chez les travailleuses du sexe infectées par le VIH-1 sont élevées et dès lors, un dépistage précoce et régulier ainsi que le suivi de ces conditions menaçant la survie est recommandé.
Abstract
Marco de referencia:
El consultorio del programa de extensión hacia los profesionales del sexo en Korogocho, Nairobi, en Kenia.
Objetivo:
Determinar los siguientes aspectos en una cohorte de mujeres profesionales del sexo: 1) la prevalencia de neoplasias intraepiteliales del cuello uterino (CIN) y su asociación con la infección por el virus de la inmunodeficiencia humana-1 (VIH-1) y 2) la tasa de incidencia de CIN durante el seguimiento de 3 años de diciembre del 2009 a diciembre 2012.
Métodos:
Fue este un estudio prospectivo anidado de cohortes.
Resultados:
De las 350 mujeres que participaron en el estudio, la mediana de la edad fue 29 años (entre 18 y 49 años) y 84 sufrían de infección por el VIH-1 (24%). Al comienzo del estudio 54 mujeres presentaron resultados anormales de la citología (15%) como sigue: 39 lesiones intraepiteliales de bajo grado (LSIL; 11%) y 15 lesiones intraepiteliales de alto grado (HSIL; 4%). Las mujeres infectadas por el VIH-1 exhibieron una probabilidad de presentar una CIN 2,7 veces mayor que las mujeres exentas de esta infección (IC95% 1,7–4,4). En las mujeres infectadas, la prevalencia de LSIL fue 2,5 veces mayor (IC95% 1,2–5,1) y la prevalencia de HSIL fue siete veces más alta (IC95% 2,3–23,3), que en las mujeres sin infección. Durante el período de seguimiento, 39 mujeres (11%) presentaron casos nuevos de lesiones intraepiteliales (6,6 por 100 años-persona [py]), sin diferencia significativa con respecto a su situación frente al VIH (7,9 por 100 py en las mujeres infectadas por el VIH-1 y 6,3 por 100 py en las mujeres sin infección).
Conclusión:
La prevalencia y la incidencia de CIN en las mujeres profesionales del sexo infectadas por el VIH-1 son altas y por lo tanto se recomienda una detección sistemática periódica y el seguimiento de esta entidad que representa un peligro vital.
Cervical cancer is the second most common cancer among women worldwide, with an estimated 529 409 new cases and 274 883 deaths in 2008. About 86% of cases occur in developing countries.1 Worldwide, the highest incidence rates are in eastern, western and southern Africa, with age-standardized rates of respectively 34.5, 33.7 and 26.8 cases per 100 000 population.2 In resource-poor settings, it is estimated that <5% of women are screened for cervical cancer compared to 40–50% in high-income countries, where there has been a marked reduction in disease incidence and prevalence.3
Cervical intra-epithelial neoplasia (CIN) is the pre-cancerous condition of the cervix, which if left untreated can progress to cervical cancer. CIN is detectable and curable. The most common subtypes of CIN are low-grade intra-epithelial lesions (LSIL) and high-grade intra-epithelial lesions (HSIL). Human immunodeficiency virus (HIV) induced immunodeficiency appears to predispose to CIN and cervical carcinoma by facilitating the expression of the causal agent, human papillomavirus (HPV), which, like HIV, is also sexually transmitted.4 HIV-induced immunosuppression exacerbates HPV-mediated cervical cytologic abnormalities.5 Studies have shown that HIV-infected women have a higher incidence and prevalence of HPV than non-HIV-infected women.6,7
In Kenya, hospital-based registry data for the period 1981–1990 indicated that cervical cancer accounted for 70–80% of all cancers of the genital tract and 8–20% of all cancer cases.8 Among women in Kenya, cervical cancer ranks as the second most frequent cancer.1 In the capital, Nairobi, HIV-infected women in the general population have been found to have a notably higher prevalence of cervical HPV infection than non-HIV-infected women (49% vs. 17%), together with a higher prevalence of LSIL (21% vs. 6.9%) and HSIL and cancer (5.8% vs. 0.6%).9
Sex workers are a population subgroup at high risk for HIV and HPV infections10 due to their participation in high-risk sexual practices (e.g., unprotected vaginal and/or anal sex), sex with multiple partners, and higher levels of symptomatic or untreated sexually transmitted infections (STIs). Longitudinal data from the East African region on cervical neoplasia among sex workers are scarce. Such information may better inform decisions around cervical screening strategies for these women.
In a cohort of young sex workers attending a Sex Workers Outreach Programme Clinic (SWOP) in Nairobi, Kenya, we aimed to determine the prevalence and incidence of CIN and the association with HIV-1 infection.
METHODS
Study design
This was a nested-cohort study involving the analysis of data from an ongoing prospective cohort study entitled ‘Investigation of the natural history of human papillomavirus DNA and cervical neoplasia in a cohort of female sex workers in Kenya’.
Study population
The study population included all 350 sex workers enrolled between December 2009 and December 2012 for the prospective cohort study. The cohort was established through peer leader recruitment efforts in the community. Inclusion criteria for the study included willingness to participate and age between 18 and 50 years.
Setting
The study site was the SWOP Clinic, located in one of the largest informal settlements in Nairobi, Korogocho, with a high population of sex workers. The SWOP Clinic offers free HIV/STI screening services to over 3000 sex workers in Korogocho.
Screening and follow-up
Sex workers agreeing to participate in the study were requested to attend the clinic on regularly scheduled visits. At enrollment and every 3 months, they were offered 1) HIV counseling and testing, and 2) screening for STIs using polymerase chain reaction based testing. Women who were non-HIV-infected underwent HIV enzyme-linked immunosorbent assay (ELISA) testing every 6 months. HIV-1-infected women had CD4+ T-cell counts measured every 6 months. Cervical specimens were collected every 6 months for conventional Pap smear and liquid-based cytology. The Pap smear was reported using the 2001 Bethesda Classification System. The Pap smear test was independently reviewed and reported on by two pathologists during the course of the study.
Management of CIN
Women diagnosed with LSIL and atypical squamous cells of undetermined significance (ASC-US) were followed up with a repeat cytological smear at 6 months after initial diagnosis, while those with HSIL or ‘atypical squamous cells, cannot exclude HSIL’ (ASC-H) had a cervical biopsy taken during colposcopy for definitive diagnosis. Women confirmed with HSIL using histology received standard care with loop electrosurgical excision procedure. Women diagnosed with invasive cervical cancer were referred for care to Kenyatta National Hospital.
Data collection
Data on demographic and reproductive characteristics, HIV status and Pap smear test results were obtained from participant case report forms and electronic databases.
Data analysis
Data were entered and analyzed using Epi-Data software, version 3.1 (The EpiData Association, Odense, Denmark). All women were initially subcategorized into HIV-1 infected and non-HIV-1-infected. Associations between HIV status and demographic variables were tested using the χ2 test. All women with LSIL, ASC-US, ASC-H and HSIL at enrollment were taken as prevalent CIN cases. Women without CIN at enrollment who developed LSIL, ASC-US, ASC-H or HSIL during the course of follow-up, were considered incident cases. Time to regression from HSIL or LSIL was measured from the index visit (i.e., the first instance of an abnormal cytologic result) to the follow-up visit at which transition to a normal or less severe cytologic smear was first detected.
To calculate the denominators for estimating incidence and regression rates, person-years (py) of follow-up were calculated. Relative risks and 95% confidence intervals (CIs) were calculated and used to study the association between HIV infection and CIN.
Ethics approval
The study protocol was approved by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease. The ongoing prospective cohort study protocol was approved by the Kenyatta National Hospital/University of Nairobi Ethics Review Committee.
RESULTS
Characteristics of the study population
A total of 350 female sex workers were enrolled in the study, of whom 84 (24%) were HIV-1-infected, with a mean CD4+ T-cell count of 566 cells/mm3 and 15 (18%) were on antiretroviral treatment (ART) at enrollment. The median age of the participants was 29 years (range 18–49). Their baseline demographic and reproductive characteristics are shown in Table 1. HIV-1-infected women were comparable to non-HIV-1-infected women except that they were older, had been younger at their first pregnancy and were more likely to use condoms.
TABLE 1.
Socio-demographic and reproductive characteristics of sex workers enrolled in an HPV study in Nairobi, Kenya, from December 2009 for a period of 3 years
| Variable | Total n (%) | HIV-1-infected n (%) | Non-HIV-1-infected n (%) | P value* |
| Total | 350 (100) | 84 (24) | 266 (76) | — |
| Age, years | 0.006 | |||
| 18–35 | 276 (79) | 55 (65) | 221 (83) | |
| ≥36 | 74 (21) | 29 (35) | 45 (17) | |
| Age at first sexual intercourse, years (n = 349)† | 0.24 | |||
| 10–17 | 257 (73) | 66 (79) | 191 (72) | |
| ≥18 | 92 (26) | 18 (21) | 74 (28) | |
| Age at first pregnancy, years (n = 339)† | 0.01 | |||
| 10–17 | 140 (40) | 43 (51) | 97 (36) | |
| ≥18 | 199 (57) | 38 (45) | 161 (61) | |
| Parity (n = 339)† | 0.3 | |||
| 0 | 14 (4) | 2 (2) | 12 (5) | |
| 1–3 | 285 (81) | 66 (79) | 219 (82) | |
| ≥4 | 40 (11) | 13 (15) | 27 (10) | |
| Duration of sex work, years (n = 345)† | 0.08 | |||
| 1–5 | 216 (62) | 46 (55) | 170 (64) | |
| ≥6 | 129 (37) | 38 (45) | 91 (34) | |
| Any contraceptive use at baseline | 0.91 | |||
| Yes | 47 (13) | 11 (13) | 36 (14) | |
| Condom use (n = 349)† | <0.001 | |||
| Never/rarely | 21 (6) | 6 (7) | 14 (15) | |
| Not always | 240 (69) | 17 (20) | 186 (70) | |
| Always | 88 (25) | 61 (73) | 65 (25) | |
| Mean number of clients per day | 0.51 | |||
| 1–2 | 104 (30) | 24 (29) | 80 (30) | |
| 3–4 | 208 (59) | 48 (57) | 160 (60) | |
| ≥5 | 38 (11) | 12 (14) | 26 (10) | |
| History of cigarette smoking | 0.26 | |||
| Yes | 132 (38) | 36 (43) | 96 (36) | |
| Education level (n = 348)† | 0.15 | |||
| None | 5 (1) | 3 (4) | 2 (1) | |
| Primary | 261 (75) | 63 (75) | 198 (74) | |
| Secondary/technical school | 82 (23) | 18 (21) | 64 (24) |
χ2 test.
Excluding those with missing data.
HPV = human papillomavirus; HIV = human immunodeficiency virus.
Prevalence of CIN
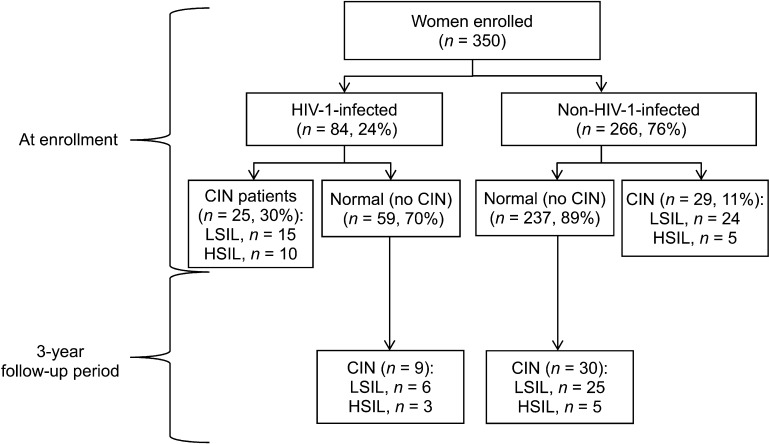
At enrollment, 54 women had CIN (15%), of whom 39 (11%) had LSIL/ASC-US and 15 (4%) had HSIL/ASC-H (Figure). HIV-1-infected women were 2.7 times (95%CI 1.7–4.4) more likely to have CIN than non-HIV-1-infected women, and the prevalence of LSIL/ASC-US and HSIL/ASC-H was respectively 2.5 times (95%CI 1.2–5.1) and 7 times (95%CI 2.3–23.3) higher among HIV-1-infected women. One case of invasive cervical cancer was reported in an HIV-1-infected woman.
FIGURE.
Flow chart showing the prevalence and incidence of CIN among HIV-1-infected and non-infected sex workers in Nairobi, Kenya, 2009–2012. HIV = human immunodeficiency virus; CIN = cervical intra-epithelial neoplasia; LSIL = low-grade squamous intra-epithelial lesion; HSIL = high-grade squamous intra-epithelial lesion.
Incidence of CIN
The incidence of CIN during the study for women without any cervical lesions at enrollment is shown in Table 2. Nine (15%) of 59 HIV-1-infected and 30 (13%) of 237 non-HIV-1-infected women developed incident CIN. The overall incidence of CIN was 6.6 cases per 100 py of follow-up. Stratified by HIV status, the incidence of CIN among HIV-1-infected women (7.9 cases/100 py, 95%CI 4–15) was not statistically different from that among non-HIV-1-infected women (6.3 cases/100 py, 95%CI 4–9).
TABLE 2.
Incidence of CIN by HIV status among a cohort of sex workers during a 3-year follow-up period, Nairobi, Kenya, 2009–2012
| HIV status | n | py of observation | Women developing CIN | CIN incidence in new cases/100 py (95%CI) | P value* |
| Total | 296 | 585 | 39 | 6.6 (5–9) | — |
| HIV-1-infected | 59 | 113 | 9 | 7.9 (4–15) | |
| Non-HIV-1-infected | 237 | 472 | 30 | 6.3 (4–9) | 0.54 |
χ2 test.
CIN = cervical intra-epithelial neoplasia; HIV = human immunodeficiency virus; py = person-years; CI = confidence interval.
Regression of CIN
The majority of the incident LSIL events (27/33, 82%) and one third (2/6) of the incident HSIL events regressed to normal or to a lower grade event during follow-up. Median time to regression was 5.9 months for LSIL events (interquartile range [IQR] 5.2–7.6 months) and 5.6 months for HSIL events (IQR 5.3–5.9 months). The rate of regression for LSIL events was 9/100 py (95%CI 10–21). Due to the small sample size, it has not been possible to report on rates of regression for HSIL events or to compare regression rates between HIV-1-infected and non-infected women.
DISCUSSION
In a population of female sex workers in a densely populated settlement area in Nairobi, the prevalence of all cervical neoplasia was 15%, and it was 4% for HSIL. HIV-1-infected women had 2.7 times the risk of CIN compared with non-HIV-1-infected women, with this relative risk being much higher for high-grade lesions compared with low-grade lesions.
The prevalence of pre-cancerous lesions according to HIV status in our study is comparable to that reported from various other parts of Kenya.9,11,12 It is also not dissimilar from reports from other African countries in the region, such as the Democratic Republic of Congo, where the prevalence of CIN among sex workers has been reported to be respectively 27% and 3% for HIV-infected and non-HIV-infected women.13 Elsewhere in Africa, there have been reports that as much as half of HIV-1-infected women in the general population have CIN.14
Our findings are important, as cervical cancer ranks as the second most frequent cancer among women aged 15–44 years in Kenya.1 The target group for screening for cervical cancer by the Kenya Cervical Cancer Prevention Programme is women aged 25–49 years; all HIV-1-infected women with a history of sexual activity aged 18–65 years should be screened at the time of diagnosis or at first contact, repeated every 6 months for the first year, and then annually thereafter.8 These recommendations are based mainly on study findings from resource-rich countries rather than resource-poor settings such as Kenya, where such data are still scarce. In addition, they take little account of the high-risk group of sex workers. Sex workers are at particularly high risk for HIV infection, accounting for 14.2% of all new HIV infections in Kenya.15 In our study, the prevalence of HIV was 24%, higher than that in the general population (9.2%) for women aged 15–49 years in Kenya.16
Although this study had a limited follow-up period (3 years), during that time the overall incidence of CIN was 6.6 cases/100 py compared to 2.5 estimated for women in Kenya.1 This rate was even higher for HIV-1-infected women, at 7.9 cases/100 py, but this was not statistically significantly different from that in non-HIV-1-infected women, at 6.3 cases/100 py.
Study strengths included 1) good follow-up during the 3-year study period, 2) rigorous data collection that was carefully monitored as part of the larger ongoing cohort study, and 3) accurate diagnosis of cervical lesions, whereby two pathologists read the Pap smears independently and made a timely diagnosis of the baseline and follow-up cytology.
The main study limitations were as follows: 1) a relatively small number of patients were followed over a period of only 3 years, and we may thus have underestimated the incidence of CIN. The small sample size also precluded us from being able to assess the effect of HIV status, ART status and CD4 count (for HIV-infected women) on CIN incidence and rates of regression. 2) As the study provided a HIV/STI prevention package, including counseling on safe sex practices, the incidence of CIN among this sub-group of sex workers may have been lower than would be expected among sex workers in general. 3) This study did not provide any data on HPV.
In conclusion, our findings provide good evidence that sex workers in Nairobi have a significantly increased risk of cervical pre-cancerous lesions, particularly when they are HIV-1-infected, and they thus need careful follow-up. This study cannot define an appropriate interval for screening, but supports the Kenyan recommendation8 that these women undergo early cervical screening, with re-screening at least once a year. Technology to determine HPV status would be useful in following these women, allowing for more targeted follow-up of the higher risk women. As women who are HIV-1-infected usually attend ART clinics regularly, this may facilitate more regular check-ups.
Acknowledgments
The authors thank the women who agreed to participate in the ongoing prospective cohort study; A Gakure and M Chitwa, the clinic and recruitment/retention staff of SWOP-Korogocho for their efforts in the follow-up of participants; and the Operational Research Unit, Médecins Sans Frontières–Luxembourg for technical support. The first author is supported as an operational research trainee by the Operational Research Unit, MSF–Luxembourg.
Conflict of interest: none declared.
References
- 1.World Health Organization/Institut Català d’Oncologia Information Centre on HPV and Cervical Cancer (HPV Information Centre) Summary report 2010. Geneva, Switzerland: WHO; 2013. Human papillomavirus and related cancers in Kenya. www.who.int/hpvcentre Accessed November 2013. [Google Scholar]
- 2.Jemal A, Bray F, Center M M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Control of cancer of the cervix uteri: review article based on a report of a WHO meeting, November 1985, Geneva. Bull World Health Organ 1986. 64:607–618. [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch F X, Manos M M, Muñoz N, et al. International Biological Study on Cervical Cancer (IBSCC) Study Group. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 5.Feingold A R, Vermund S H, Burk R D, et al. Cervical cytologic abnormalities and papillomavirus in women infected with human immunodeficiency virus. J Acquir Immune Defic Syndr. 1990;3:896–903. [PubMed] [Google Scholar]
- 6.Branca M, Garbuglia A R, Benedetto A, et al. Factors predicting the persistence of genital human papillomavirus infections and PAP smear abnormality in HIV-positive and HIV-negative women during prospective follow up. Int J STD AIDS. 2003;14:417. doi: 10.1258/095646203765371321. [DOI] [PubMed] [Google Scholar]
- 7.Ahdieh L, Klein R S, Burk R, et al. Prevalence, incidence and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184:682–690. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Public Health and Sanitation, Ministry of Medical Services . Nairobi, Kenya: Ministry of Medical Services; 2012. National guidelines for prevention and management of cervical, breast and prostate cancers, Kenya. http://www.iedea-ea.org/joomla/attachments/article/305/National%20RT%20Cancer%20guidelines%20FINAL%20FEB%202012.pdf Accessed November 2013. [Google Scholar]
- 9.Yamada R, Sasagawa T, Kirumbi L, et al. Human papillomavirus infection and cervical abnormalities in Nairobi, Kenya, an area with a high prevalence of human immunodeficiency virus infection. J Med Virol. 2008;80:847–855. doi: 10.1002/jmv.21170. [DOI] [PubMed] [Google Scholar]
- 10.Patel S J, Mugo N R, Cohen C R, et al. Multiple human papillomavirus infections and HIV seropositivity as risk factors for abnormal cervical cytology among female sex workers in Nairobi. Int J STD AIDS. 2013;24:221–225. doi: 10.1177/0956462412472446. [DOI] [PubMed] [Google Scholar]
- 11.Memiah P, Mbuthia W, Kiiru G, et al. Prevalence and risk factors associated with precancerous cervical cancer lesions among HIV-infected women in resource-limited settings. AIDS Res Treat. 2012;2012:953743. doi: 10.1155/2012/953743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luchters S, Vanden Broeck D, Chersich M, et al. Association of HIV infection with distribution and viral load of HPV types in Kenya: a survey with 820 female sex workers. BMC Infect Dis. 2010;10:18. doi: 10.1186/1471-2334-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laga M, Icenogle J P, Marsella R, et al. Genital papillomavirus infection and cervical dysplasia—opportunistic complications of HIV infection. Int J Cancer. 1992;50:45–48. doi: 10.1002/ijc.2910500110. [DOI] [PubMed] [Google Scholar]
- 14.Moodley J R, Hoffman M, Carrara H, et al. HIV and pre-neoplastic and neoplastic lesions of the cervix in South Africa: a case-control study. BMC Cancer. 2006;6:135. doi: 10.1186/1471-2407-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Global AIDS Program, The World Bank . Nairobi, Kenya: Government of Kenya; 2009. Kenya HIV prevention response and modes of transmission analysis. http://siteresources.worldbank.org/INTHIVAIDS/Resources/375798-1103037153392/KenyaMOT22March09Final.pdf Accessed November 2013. [Google Scholar]
- 16.Government of Kenya . Full report 2009. Nairobi, Kenya: Government of Kenya; 2007. Kenya AIDS Indicator Survey 2007. http://www.nascop.or.ke/library/3d/Official_KAIS_Report_20091.pdf Accessed November 2013. [Google Scholar]