Abstract
Objectives:
1) To explore the utility of tuberculosis (TB) symptom screening for symptoms of ≥2 weeks’ duration in a routine setting, and 2) to compare differences in TB diagnosis between human immunodeficiency virus (HIV) infected and non-HIV-infected pregnant women in western Kenya.
Design:
Comparative cross-sectional study among pregnant women with known HIV status screened for TB from 2010 to 2012, in Eldoret, western Kenya.
Results:
Of 2983 participants, respectively 34 (1%), 1488 (50.5%) and 1461 (49.5%) had unknown, positive and negative HIV status. The median age was respectively 30 years (interquartile range [IQR] 26–35) and 26 years (IQR 24–31) in HIV-infected and non-infected participants. A positive symptom screen was found in respectively 8% (119/1488) and 5% (67/1461) of the HIV-infected and non-infected women. The median CD4 count at enrolment was 377 cells/μl (IQR 244–530) for HIV-infected women. One non-HIV-infected patient was sputum-positive. For HIV-infected women, TB was presumptively treated in 1% (16/1488) based on clinical symptoms and chest X-ray. Cumulatively, anti-tuberculosis treatment was offered to 0.6% (17/2949) of the participants.
Conclusion:
This study does not seem to demonstrate the utility of TB symptom screening questionnaires in a routine setting among pregnant women, either HIV-infected or non-infected, in western Kenya.
Keywords: TB symptom screening questionnaire, HIV, TB diagnosis
Abstract
Objective:
1) Explorer l’utilité du dépistage des symptômes de tuberculose (TB) en utilisant les symptômes d’une durée de ≥2 semaines dans un contexte de routine, et 2) comparer les différences en matière de diagnostic de TB entre les femmes enceintes infectées ou non par le virus de l’immunodéficience humaine (VIH) dans l’Ouest du Kenya.
Schéma:
Etude transversale comparative chez les femmes enceintes dont le statut VIH est connu et dépistées pour TB de 2010 à 2012 à Eldoret, Ouest du Kenya.
Résultats:
Sur 2983 participantes, respectivement 34 (1%), 1488 (50,5%) et 1461 (49,5%) avaient un statut VIH inconnu, positif et négatif. L’âge médian en années est de 30 (IQR 26–35) et de 26 (IQR 24–31), respectivement, chez les infectées et les non-infectées par le VIH. On a trouvé un dépistage positif sur symptômes respectivement chez 8% (119/1488) et 5% (67/1461) chez les infectées et non infectées. Le décompte médian de CD4 lors du recrutement a été de 377 cellules/µl (IQR 244–530) pour les femmes infectées par le VIH. Chez une patiente non-infectée par le VIH, l’examen microscopique des crachats à la recherche de la TB a été positif. Pour les femmes infectées par le VIH, la TB a été traitée sur présomption dans 1% (16/ 1488) en se basant sur les symptômes cliniques et le cliché thoracique. De manière cumulative, un traitement pour la TB a été offert à 0,6% (17/2949) des participantes.
Conclusion:
Cette étude ne semble pas démontrer l’utilité d’un questionnaire sur symptômes pour le dépistage de la TB dans un contexte de routine parmi les femmes enceintes, qu’elles soient ou non infectées par le VIH dans l’Ouest du Kenya.
Abstract
Objetivos:
1) Explorar la utilidad del cribado sistemático de los síntomas de tuberculosis (TB) en la práctica ordinaria, con base en los síntomas de ≥2 semanas de duración; y 2) comparar las diferencias en el diagnóstico de la TB en las embarazadas que presentan infección por el virus de la inmunodeficiencia humana (VIH) y las embarazadas exentas de esta infección en Kenia occidental.
Métodos:
Fue este un estudio transversal comparativo de las embarazadas con situación conocida frente al VIH, a quienes se administró un cuestionario de cribado de síntomas de TB entre el 2010 y el 2012, en Eldoret en Kenia occidental.
Resultados:
De 2983 participantes, 34 mujeres desconocían su situación frente al VIH (1%), 1488 eran positivas al VIH (50,5%) y 1461 eran negativas (49,5%). La mediana de la edad fue 30 años (IQR 26–35) en las mujeres positivas al VIH y 26 años (IQR 24–31) en las mujeres sin infección por el virus. Se obtuvo un cribado positivo de síntomas en 8% de las mujeres infectadas por el VIH (IQR 119–1488) y en 5% de las mujeres sin infección (IQR 67–1461). La mediana del recuento de células CD4 en el momento del ingreso al estudio fue 377 células/µl en las mujeres infectadas por el VIH (IQR 244–530). Una mujer negativa para el VIH presentó una baciloscopia de esputo positiva. En el grupo de mujeres infectadas por el VIH se practicó el tratamiento por presunción de TB en 1% de los casos (16/1488) con base en los síntomas clínicos y la radiografía de tórax. Durante el estudio se ofreció tratamiento antituberculoso a 0,6% de las participantes (17/2949).
Conclusión:
Los resultados del presente estudio no parecen demostrar la utilidad del cuestionario de cribado de síntomas de TB en la práctica corriente de atención a las embarazadas, que presenten infección por el VIH o que estén exentas de la infección, en Kenia occidental.
Reduction of tuberculosis (TB) transmission, morbidity and mortality relies largely on intensified case finding, with consequent early initiation of adequate treatment.1,2 This is particularly important among pregnant women in resource-limited settings where TB is a cause of non-obstetrical (indirect) maternal deaths.3,4 This burden is higher in settings with a high prevalence of human immunodeficiency virus (HIV) infection.5,6 Kenya has an adult HIV prevalence of 6.2%,7 with an unacceptably high maternal mortality ratio of 488 per 100 000 live births; 25% of these deaths are attributed to indirect causes such as TB, anaemia, HIV and malaria.8
TB case notification data are not stratified for pregnancy, but women of reproductive age bear a higher burden of TB in sub-Saharan Africa than their male counterparts.1,9 Data from Western Cape, South Africa, indicate that there is a 24.2-fold higher incidence of TB disease among infants born to HIV-infected mothers, diagnosed at a mean age of 6 months, than among those of HIV-negative mothers.10 This suggests probable underdiagnosis of active TB disease during the antenatal and postnatal periods. HIV and TB co-infection during pregnancy have a multiplier effect on maternal morbidity and mortality, and result in poorer pregnancy outcomes.1,11 In Pune, India, TB increased the probability of death by 2.2-fold among HIV-infected women who developed TB and by 3.4-fold for their infants compared to women who did not develop TB.11 In Johannesburg, South Africa, 70% of obstetric deaths in HIV-infected women were mainly attributed to TB.12 These figures suggest that routine screening of pregnant women for TB in endemic settings would be helpful, particularly those who are HIV-infected.
The World Health Organization (WHO) recommends ruling out active TB and identifying those in need of further testing among HIV-infected adults using specific symptoms (current cough of any duration, fever, weight loss or night sweats).13 Although these recommendations were not specific for pregnancy, Gupta et al. used this recommendation and found a 1.4% (11/799) prevalence of active TB among HIV-infected pregnant women who were part of a clinical trial in India.14 Another study of cough of >2 weeks, performed in Kenya by the same clinical team and by the same first author in a routine setting similar to the target population for this study, failed to identify those with TB disease (n = 187).15 The current study differs from the earlier one in its larger sample size and because it compares HIV-infected and non-infected pregnant women.
Data on the utilization of symptom screening among pregnant women in routine settings are scarce. This has been attributed to significant financial and logistical challenges in the implementation of screening in this group of patients.1 The objectives of the present study were 1) to explore the utility of TB symptom screening using symptoms of ≥2 weeks’ duration in a routine setting, and 2) to compare differences in diagnosis of TB among HIV-infected and non-infected pregnant women in western Kenya.
METHODS
Study design
This was a descriptive cohort study among HIV-infected and non-infected pregnant women.
Study site and setting
The study was conducted at the Moi Teaching and Referral Hospital (MTRH) antenatal care clinic and the AMPATH Prevention of Mother-To-Child Transmission (PMTCT) clinic in Eldoret, Western Kenya. The AMPATH programme, described elsewhere, is housed by the MTRH, which is the teaching hospital for the Moi University School of Medicine and the second largest referral hospital in Kenya, with the largest PMTCT programme in western Kenya. HIV-infected pregnant women are thus generally referred to this facility for care, increasing the proportion of HIV-infected pregnant women at the MTRH.16–19
HIV-infected pregnant women were offered antenatal and HIV care in the AMPATH clinic, while their non-HIV-infected counterparts were offered antenatal care in the MTRH clinic. Both clinics are located on the MTRH grounds in separate buildings, about 200 m apart, and serve a similar patient population.
TB screening and management
Recommendations by the Kenya National AIDS and STI Control Programme were to screen for TB in all pregnant mothers at every visit. However, as no programme guidelines on how screening should be carried out were available, screening for TB in pregnancy was opportunistic.20 The AMPATH programme screened both HIV-infected and non-infected patients for TB using a routine six-question symptom screening questionnaire in its cough monitor programme (Table 1). Five years of experience among non-pregnant adult population demonstrated a 13% sputum smear-positive rate, with 8% of smear-negative adults positive on either culture or GeneXpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA; unpublished programme data). The Cough Monitor Programme for pregnant women was set up within the AMPATH programme with funding from the International Union Against Tuberculosis and Lung Disease, Paris, France, between October 2010 and July 2012, as part of standard routine care in the AMPATH and MTRH antenatal care clinics.
TABLE 1.
AMPATH symptom screening questionnaire
| Cough of ≥2 weeks Bloody cough in the past year Fever of ≥3 weeks Past history of TB diagnosis History of TB contact in the household Weight loss/failure to gain weight if pregnant in the past year |
TB = tuberculosis.
The symptom screen questionnaire was administered by cough monitors, who were lay staff trained for this purpose. A positive response to any of the questions was regarded as a positive symptom screen, and prompted sputum collection from those with productive cough for TB smear microscopy. The symptom screen questionnaire was administered to each patient only once. Patients whose microscopy and/or culture were positive for Mycobacterium tuberculosis were offered standard anti-tuberculosis treatment per national guidelines. The data from the symptom screen questionnaire were entered into an Excel database for programmatic use (Microsoft, Redmond, WA, USA).
When the attending clinician had a high clinical index of suspicion for TB disease, despite unproductive cough or sputum negative for M. tuberculosis, a shielded chest X-ray (CXR) was performed based on clinical opinion. Based on positive CXR results or suggestive clinical symptoms, presumptive anti-tuberculosis treatment was offered for these patients.
HIV screening and management
About 90% of pregnant women in Kenya were screened for HIV infection as standard of care, unless a woman opted out at the time of the study.21 Women at the MTRH were screened according to the national HIV screening protocol.
Population
The population included all pregnant women cared for at the AMPATH and MTRH antenatal care clinics from October 2010 to July 2012, who were screened for TB using the AMPATH symptom screen questionnaire and who had clinical data captured in the cough monitor database. Patients with unknown HIV status and those admitted to hospital were excluded from the analysis.
Sample size
During the study period, a total of 2983 pregnant women received antenatal services at the AMPATH and MTRH antenatal clinics; these women formed the population of this study.
Data collection and data variables
Routine clinical data were collected retrospectively. There were three sources of data in this study: the symptom screening questionnaire database, the TB laboratory register and patient files. The following variables were collected from pregnant women attending the PMTCT and antenatal care clinics who were administered the symptom screening questionnaire: age, HIV status, CD4 counts if HIV-infected, TB symptoms per the symptom screening questionnaire, CXR patterns, sputum obtained (yes/no) and sputum microscopy results.
Data analysis
Data were retrieved from the cough monitor database and evaluated for inconsistencies and missing data. Where possible, data were corrected from source data. Descriptive data were reported, numbers and proportions were stratified by HIV status, and continuous data were given as median and interquartile range (IQR) for the various subcategories related to TB symptom screening in each group.
Ethics
This study was approved by both the Institutional and Research Ethics Committee of the MTRH, Moi University School of Medicine, Eldoret, Kenya, as well as the Institutional Review Board of Lifespan, Providence, RI, USA. Only de-identified data were analysed. No patient names were recorded, only patient numbers, to enable linkage with the laboratory and patient files. No extra data were collected outside of routine care, and confidentiality was observed at all levels of data collection. The study was classified as an audit, and hence of minimum risk to participants.
RESULTS
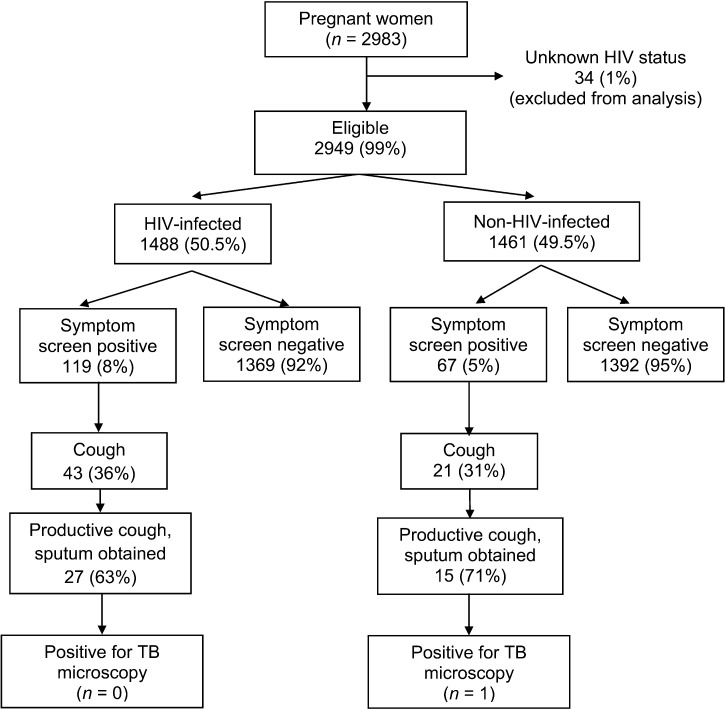
Of 2983 pregnant women enrolled in the study, 34 (1%) had unknown HIV status and were excluded from the analysis. Of the remaining 2949 pregnant women, 1488 (50.5%) were HIV-positive and 1461 (49.5%) were HIV-negative (Figure). The median age of the HIV-infected and non-HIV-infected women was respectively 30 (IQR 26–35) and 26 years (IQR 24–31). Of the HIV-infected women, 1101 had a CD4 count available; the median count at enrolment in the PMTCT programme was 377 cells/μl (IQR 244–530).
FIGURE.
Results of TB symptom screening in pregnant women in western Kenya, 2010–2012.
Of the 1488 HIV-infected women, 119 (8%) had a positive TB symptom screen, which was significantly higher than among the non-HIV-infected women (5%, 67/1461; P < 0.001). As regards specific symptoms, 3% (43/1488) had a cough of ≥2 weeks among the HIV-infected group vs. 1% (21/1461) among the non-HIV-infected group (P = 0.014; Table 2).
TABLE 2.
TB symptoms of pregnant women who were positive on TB symptom screen in western Kenya, 2010–2012
| HIV-infected (n = 1488) n (%) | Non-HIV-infected (n = 1461) n (%) | P value | |
| Symptom screen-positive | 119 (8) | 67 (5) | <0.001 |
| Symptoms | |||
| Cough ≥2 weeks | 43 (3) | 21 (1) | 0.014 |
| Coughed blood in the past year | 2 (0.1) | 1 (0.1) | 0.779 |
| Fever ≥3 weeks | 19 (1) | 31 (2) | 0.128 |
| Weight loss/failure to gain weight in the past year | 19 (1) | 18 (1) | 0.037 |
| Past history of TB contact in the household | 36 (2) | 15 (1) | 0.009 |
| Past history of TB diagnosis | 40 (3) | 0 | <0.001 |
TB = tuberculosis; HIV = human immunodeficiency virus.
Only patients with a productive cough had sputum collected for microscopy. Of the HIV-infected and non-infected women who had cough of ≥2 weeks, respectively 27/43 and 15/21 had productive cough. One non-HIV-infected patient (1/1461) was sputum-positive; none were positive in the HIV-infected group (Figure). The non-HIV-infected TB patient had cough, positive history of contact with an active TB case and weight loss.
During the study period, 1% (16/1488) of the women in the HIV-infected group were treated for TB presumptively; 14 underwent CXR and two did not. Table 3 shows the CXR features of patients treated presumptively for TB. Of the 14 patients with CXR, 10 had features suggestive of TB (pleural effusion, infiltrate and miliary pattern).
TABLE 3.
CXR patterns for HIV-infected pregnant women treated presumptively for tuberculosis in western Kenya, 2010–2013 (n = 14)
| Normal | 3 |
| Pleural effusion | 2 |
| Infiltrate | 6 |
| Milliary pattern | 2 |
| Other abnormalities | 1 |
CXR = chest X-ray; HIV = human immunodeficiency virus.
During the study period, 0.6% (17/2949) of the women were treated for TB: 16 presumptively in the HIV-infected group and one based on a positive smear microscopy result in the non-HIV-infected group. Table 4 shows the clinical characteristics of the patients treated for TB during the study period.
TABLE 4.
Clinical characteristics of pregnant women treated for TB in western Kenya, 2010–2013 (n = 17)
| Age, years, median [IQR] | 30 [27–33] |
| Positive symptom screen | 4 |
| Symptoms | |
| Cough ≥2 weeks | 4 |
| Coughed blood in the past year | 1 |
| Productive cough | 4 |
| Fever ≥3 weeks | 1 |
| Weight loss/failure to gain weight in past year | 2 |
| Past history of TB contact in the household | 2 |
| Past history of TB diagnosis | 0 |
| CD4 counts, cells/µl, median [IQR]* | 256 [106–302] |
1 non-HIV-infected patient.
TB = tuberculosis; IQR = interquartile range; HIV = human immunodeficiency virus.
DISCUSSION
In this study in western Kenya, a symptom screening questionnaire did not produce a high yield of active TB disease among pregnant women, either HIV-infected or non-infected. Although some women were identified using screening, the majority did not have cough with sputum and could not be evaluated using microscopy. CXR in this group with non-productive cough might have revealed more positive TB cases; however, CXR was not performed systematically. Most of the cases treated were thus based on CXR findings, demonstrating a need for further evaluation of this test in pregnant women. Reliance on sputum microscopy did not seem to be useful given the low sputum availability. There seemed to be little difference in results for HIV-positive and -negative women.
These findings are similar to previous findings in the same target population, although the earlier study had a smaller sample size (n = 187).15 The findings are surprising, given the fact that Kenya is a high burden setting for HIV and TB, with an HIV prevalence of 6.2%, and ranks thirteenth among the 22 high TB burden countries globally.7,22 Of the 17 pregnant women treated for TB, only one had a positive smear microscopy result. The large number of patients reporting unproductive cough, particularly in the HIV-infected group, raises concerns that TB diagnosis was perhaps being missed in this population, as sputum microscopy remains the major diagnostic testing strategy.
These findings could be attributed to the following five possibilities: 1) the TB symptom screening tool is not sensitive enough during pregnancy. In a study in South Africa that documented a high prevalence of TB among HIV-infected women (47/1415, 3.3%), the majority (73%) did not report any TB symptoms.23 2) Symptoms may be attenuated in people living with HIV, often yielding negative results on smear microscopy.24 Participants in this study who were treated for TB had a lower CD4 count than those who were not treated for TB (256 cells/μl, IQR 106–302 vs. 377 cells/μl, IQR 244–530). 3) Physiological changes associated with pregnancy may mask the symptoms of TB.23 4) There may a low prevalence of TB in pregnancy—this is less plausible considering TB is a major cause of non-obstetrical maternal deaths.3 5) A combination of all of the above.
In our setting, the utility of shielded CXR in screening for TB in pregnancy among HIV-infected women needs to be evaluated. Of the 17 women treated for TB in this study, 10 had CXR features suggestive of TB. Of particular note are two cases who had a miliary pattern. This finding is consistent with previous findings in the same population, where 10/187 patients were presumptively treated for TB based on suggestive CXR, three of whom had a miliary pattern.15 However, a study in India demonstrated marginal to no added value of CXR in this group.14
Our study had three major limitations. First, a gold standard for TB diagnosis was lacking, and not all patients underwent culture for TB, CXR or further evaluation other than clinical examination. Second, the WHO TB symptom screening protocol of current cough of any duration, fever, weight loss or night sweats was not used because the routine data analysed was from before implementation of the WHO recommendation. Third, data on antiretroviral use among HIV-positive participants were not collected. However, our large sample size, the fact that MTRH is a referral hospital, which meant that our study had an equal proportion of HIV-negative and -positive participants, and the fact that this was carried out under routine programme conditions, are of value.
CONCLUSION
This study does not seem to demonstrate the utility of TB symptom screening questionnaires in routine settings among pregnant women in Western Kenya. There may, however, be a role of CXR in screening for TB among HIV-infected pregnant women. More analytical hypothesis-driven studies need to be developed to determine the best screening tool for TB in pregnancy in high HIV-TB burden, low-resource settings.
Acknowledgments
This research was supported in part by a grant to the United States Agency for International Development (USAID) AMPATH Partnership from the USAID as part of the President’s Emergency Plan for AIDS Relief (PEPFAR). Support was also provided through an operational research course that was jointly developed and run by the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France, and the Operational Research Unit, Médecins Sans Frontières, Brussels, Belgium.
RJK was partly supported by the Miriam/Brown/Tufts Fogarty AIDS International Training and Research Program (Providence, RI, USA) D43-TW-000237. DS was supported by the National Institutes of Health Office of the Director, Fogarty International Center through the International Clinical Research Scholars and Fellows Program at Vanderbilt University, Nashville, TN, USA (R24 TW007988), and the American Relief and Recovery Act, Washington, DC, USA.
The authors thank the women who participated in this study and Professor A D Harries, Senior Advisor at The Union, for his invaluable input to this manuscript.
Conflict of interest: none declared.
References
- 1.Deluca A, Chaisson R E, Martinson N A. Intensified case finding for tuberculosis in prevention of mother-to-child transmission programs: a simple and potentially vital addition for maternal and child health. J Acquir Immune Defic Syndr. 2009;50:196–199. doi: 10.1097/QAI.0b013e3181900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golub J E, Mohan C I, Comstock G W, Chaisson R E. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9:1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull E R, Kancheya N G, Harris J B, Topp S M, Henostroza G, Reid S E. A model of tuberculosis screening for pregnant women in resource-limited settings using Xpert MTB/RIF. J Pregnancy. 2012;2012:565049. doi: 10.1155/2012/565049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grange J, Adhikari M, Ahmed Y, et al. Tuberculosis in association with HIV/AIDS emerges as a major nonobstetric cause of maternal mortality in sub-Saharan Africa. Int J Gynaecol Obstet. 2010;108:181–183. doi: 10.1016/j.ijgo.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Lönnroth K, Castro K G, Chakaya J M, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 6.Swindells S, Komarow L, Tripathy S, et al. Screening for pulmonary tuberculosis in HIV-infected individuals: AIDS Clinical Trials Group Protocol A5253. Int J Tuberc Lung Dis. 2013;17:532–539. doi: 10.5588/ijtld.12.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National AIDS Control Council, National AIDS and STD Control Programme. Kenya AIDS epidemic: update 2011. Nairobi, Kenya: NACC, NASCOP; 2012. http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_KE_Narrative_Report.pdf. Accessed November 2013. [Google Scholar]
- 8.Kenya National Bureau of Statistics. Kenya demographic and health survey 2008–09. Nairobi, Kenya: KNBS; 2010. [Google Scholar]
- 9.Mnyani C N, McIntyre J A. Tuberculosis in pregnancy. BJOG. 2011;118:226–231. doi: 10.1111/j.1471-0528.2010.02771.x. [DOI] [PubMed] [Google Scholar]
- 10.Hesseling A C, Cotton M F, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48:108–114. doi: 10.1086/595012. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis. 2007;45:241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 12.Black V, Brooke S, Chersich M F. Effect of human immunodeficiency virus treatment on maternal mortality at a tertiary center in South Africa: a 5-year audit. Obstet Gynecol. 2009;114(2 Pt 1):292–299. doi: 10.1097/AOG.0b013e3181af33e6. [DOI] [PubMed] [Google Scholar]
- 13.Getahun H, Kittikraisak W, Heilig C M, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLOS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Chandrasekhar A, Gupte N, et al. Symptom screening among HIV-infected pregnant women is acceptable and has high negative predictive value for active tuberculosis. Clin Infect Dis. 2011;53:1015–1018. doi: 10.1093/cid/cir605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosgei R J, Ndavi P M, Ong’ech J O, et al. Symptom screen: diagnostic usefulness in detecting pulmonary tuberculosis in HIV-infected pregnant women in Kenya. Public Health Action. 2011;1:30–33. doi: 10.5588/pha.11.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einterz R M, Kimaiyo S, Mengech H N, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med. 2007;82:812–818. doi: 10.1097/ACM.0b013e3180cc29f1. [DOI] [PubMed] [Google Scholar]
- 17.Inui T S, Nyandiko W M, Kimaiyo S N, et al. AMPATH: living proof that no one has to die from HIV. J Gen Intern Med. 2007;22:1745–1750. doi: 10.1007/s11606-007-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamlin J K, Kimaiyo S, Nyandiko W, Tierney W. Academic institutions linking access to treatment and prevention: case study. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 19.Voelker R. Conquering HIV and stigma in Kenya. JAMA. 2004;292:157–159. doi: 10.1001/jama.292.2.157. [DOI] [PubMed] [Google Scholar]
- 20.National AIDS and STD Control Programme. Guidelines for prevention of mother to child transmission (PMTCT) of HIV/AIDS in Kenya. Nairobi, Kenya: NASCOP; 2012. http://nascop.or.ke/pmtctpubs.php Accessed November 2013. [Google Scholar]
- 21.National AIDS and STD Control Programme. Prevention of mother to child transmission, 2013. Nairobi, Kenya: NASCOP; 2013. http://nascop.or.ke/prevention_of_mother_to_child.php Accessed November 2013. [Google Scholar]
- 22.World Health Organization. Global tuberculois report, 2012. WHO/HTM/TB/2012.6. Geneva, Switzerland: WHO; 2012. www.who.int/tb/publications/global_report/gtbr12_main.pdf Accessed November 2013. [Google Scholar]
- 23.Hoffmann C J, Variava E, Rakgokong M, et al. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLOS ONE. 2013;8:e62211. doi: 10.1371/journal.pone.0062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqi K, Lambert M L, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis. 2003;3:288–296. doi: 10.1016/s1473-3099(03)00609-1. [DOI] [PubMed] [Google Scholar]