Abstract
A three-step method for the purification of Enterocytozoon bieneusi spores from stool specimens was developed. The primary process of purification of the spores from bacterial contaminants involved Percoll gradient centrifugation followed by additional separation using cesium chloride density gradient centrifugation. The cesium chloride-isolated spores were further purified using a flow cytometer with cell sorting capabilities. Sorting was performed without the use of antibodies, fluorochromes, or dyes, leaving the sorted spores in their native state, which appears to be less destructive for spores. When quantified by flow cytometry using tubes with known numbers of highly fluorescent polystyrene beads, the sorted material showed a slight decrease in light scatter characteristics compared with the slightly larger Encephalitozoon species spores. Although the overall recovery of the E. bieneusi spores was low, calcofluor and Gram chromotrope staining, indirect immunofluorescence assay, and transmission electron microscopy revealed that the sorted material was highly purified and contained large numbers of E. bieneusi spores and relatively few bacteria and other debris. The sorted material appeared to be sufficiently pure and could be used for in vitro culture and for the development of a variety of diagnostic reagents as well as in studying the genome of E. bieneusi and host-parasite interactions.
Microsporidia are opportunistic intracellular parasites of the phylum Microspora. Of the more than 1,000 species and as many as 100 genera of microsporidia, only 12 well-defined species (Brachiola vesicularum, Brachiola algerae, Brachiola conori, Encephalitozoon cuniculi, Encephalitozoon hellem, Encephalitozoon intestinalis, Enterocytozoon bieneusi, Nosema ocularum, Trachipleistophora hominis, Trachipleistophora anthropophthera, Pleistophora ronneafiei, and Vittaforma corneae) are included in seven genera that are known to cause infections in humans. Microsporidiosis, a disease resulting from infection with these opportunistic protozoa, has been mostly detected in patients with human immunodeficiency virus (HIV)-AIDS, as well as in other immunocompromised persons, including transplant recipients (11, 12, 16). According to some surveys, E. bieneusi is consistently associated with gastrointestinal illness and is the most frequently identified microsporidian in fecal specimens of AIDS patients, ranging from 7 to 50% infectivity in patients with CD4 cells below 100/μl (26). However, it was found that only 3 to 4% of HIV-AIDS patients in Peru were positive for microsporidia (V. Kawai, C. Bern, L. Xiao, E. Ticona, A. Vivar, M. Navincopa, G. S. Visvesvara, and R. H. Gilman, 50th Annu. Meet. Am. Soc. Trop. Med. Hyg., abstr. 84, p. 164, 2001).
Recently, E. bieneusi was identified in fecal specimens of immunocompetent travelers from tropical areas (13, 21). Spores have been identified in water (6, 9), domestic animals (5; C. Aguila, F. Izquierdo, R. Navajas, N. J. Pieniazek, G. Miro, A. T. Alonso, A. da Silva, S. Fenoy, abstract from the 6th Int. Workshop on Opportunistic Protists, J. Eukaryot. Microbiol. 46:8S-9S, 1999; P. Deplazes, A. Mathis, C. Muller, and R. Weber, abstract from the 4th Int. Workshop on Opportunistic Protists, J. Eukaryot. Microbiol. 43:93S, 1996), and HIV-negative patients (14, 15). A number of microsporidian species have been established in vitro; however, the establishment of E. bieneusi is yet to be successful. Infections have been reported in simian immunodeficiency virus-infected primates (22) and in a gnotobiotic pig model (12), but these models do not produce large quantities of spores.
Currently, only fecal specimens from persons infected with E. bieneusi are convenient sources for obtaining large quantities of spores. The difficulty is the purification of the spores from the fecal material. Because immunospecific reagents are not commercially available, positive selection of the spores from the numerous heterogeneous bacteria, possessing size and density characteristics similar to those of the spores, makes purification almost impossible using density gradient separation techniques alone. Thus, additional methods are required to further purify E. bieneusi spores from the bacteria. In this study, we used Percoll and cesium chloride density gradients for the initial purification of E. bieneusi spores from fecal bacteria. Further purification was accomplished using flow cytometry with cell sorting capabilities, and the sorted material was quantified using flow cytometry and tubes with known numbers of highly fluorescent polystyrene beads. Purification of E. bieneusi spores is necessary not only for the development of a variety of diagnostic reagents such as polyclonal and monoclonal antibodies but also for analysis of its genome and studies of host-parasite interactions.
MATERIALS AND METHODS
Stool specimens.
Stool specimens were obtained from three HIV-AIDS patients in hospitals in Lima, Peru. Thin smears made from these specimens were stained by Weber's chromotrope technique (25) and were examined microscopically for E. bieneusi. Specimens that were positive for microsporidian spores were frozen on dry ice and shipped to the Centers for Disease Control and Prevention. After arrival, spore density in the specimens was determined using calcofluor (23) and Gram chromotrope (18) staining. Specimens containing large numbers of spores measuring 0.8 to 1.3 μm and confirmed as E. bieneusi by PCR (Kawai et al., 50th Annu. Meet. Am. Soc. Trop. Med. Hyg., abstr. 84, p. 164, 2001) were selected and processed as described below. Patient specimens were collected using protocols approved by the Institutional Review Board at the Centers for Disease Control and Prevention; Johns Hopkins University, Baltimore, Md.; Hospital Dos de Mayo, Lima, Peru; and Hospital Arzobispo Loayza, Lima, Peru.
Stool specimen processing.
Approximately one part stool specimen was suspended in nine parts 0.01 M phosphate-buffered saline (PBS), pH 7.2 to 7.4, and filtered through a series of nylon sieves (pore sizes, 210, 100, 70, 50, and 20 μm; Small Parts, Inc., Miami Lakes, Fla.). The filtrate was mixed with an antibiotic solution to yield a final concentration of 150 μg of gentamicin, 3 μg of amphotericin B, and 300 μg of imipenem per ml. The spore concentration was estimated by diluting (1:102 to 1:106) a portion of antibiotic-treated spore filtrate, adding to multiwell slides, staining with calcofluor white reagent, and counting the calcofluor-stained spores.
Percoll gradient.
A slight modification of a Percoll gradient method (10) was used in the spore isolation process. Briefly, a Percoll solution was prepared by mixing nine parts Percoll (Sigma Chemical Co., St. Louis, Mo.) to one part 10-fold-concentrated PBS. About 45 ml of the antibiotic-treated stool filtrate was layered over 180 ml of the Percoll and centrifuged at 20,000 × g for 30 min at 4°C. The opaque fraction (about 70 ml), containing spores and bacteria located above the sediment, was collected and washed three times with PBS by centrifugation at 20,000 × g for 30 min at 4°C. The washed sediment was resuspended in 10 ml of PBS. Spore concentration was estimated by diluting a portion of the Percoll gradient spore suspension (1:102 to 1:106), adding to multiwell slides, and counting the calcofluor stained spores.
Cesium gradient.
The Percoll-gradient spore suspension was subjected to additional clarification using cesium chloride gradient centrifugation. Thirty milliliters of cesium chloride (Sigma Chemical Co., St. Louis, Mo.), prepared at 0.46 g/ml, was overlaid by 10 ml of the spore suspension from the Percoll gradient and centrifuged at 20,000 × g for 30 min at 4°C. The opaque fraction at the interface containing the spores and some bacteria was collected and washed three times with PBS by centrifugation at 20,000 × g for 30 min at 4°C. The sediment was resuspended in 5 ml of PBS. The cesium chloride spore suspension was diluted (1:102 to 1:106) and counted as described above.
Sorting spores.
In a previous study we used flow cytometry to sort culture grown Encephalitozoon spores (17). In the present study we have used autofluorescent properties of E. bieneusi spores to sort them by light scatter from fecal material, after they were purified initially by Percoll and cesium chloride density gradient centrifugation. Sorting was done using a FACStarPLUS cell sorter (Becton Dickinson, San Jose, Calif.) equipped with a 70-μm-diameter nozzle, a water-cooled 5-W argon laser, and CellQuest acquisition and analysis software (Becton Dickinson). The laser was tuned to 488 nm, and power was used at 200 mW. Both forward scatter (FSC) and side scatter (SSC) light detectors were set to linear scale, the former at an amplifier gain of 16 and the latter at 4. Voltage for the SSC detector was adjusted so that the spore region was near the origin of the FSC-versus-SSC dot plot.
Two fluorescence detectors were used in log scale with emission detection of 515 to 545 nm (FL1) and 663 to 677 nm (FL31 [FL3 on laser 1]). Voltages to these detectors were adjusted so that the spore region was at the origin of the bi-parameter fluorescence dot plot.
Spores purified using Percoll and cesium chloride gradient centrifugations were suspended in 0.01 M PBS, pH 7.4, containing 0.27 mM KCl and 1.54 mM KH2PO4 (PBS2), to a concentration of 20 × 106 to 23 × 106 spores/bacteria per ml. This yielded about 3,000 events per s using FSC triggering with the sheath pressure set at 11 and the sample differential at 1.2 to 1.4. At these settings and conditions with a drop packet of 3.0, the abort frequency did not exceed 10% of the FSC threshold frequency. The sheath fluid was PBS2. Even though the spores were small and near the limits of detection on the sorter, the FSC threshold was set below 30, which eliminated some electronic noise. To further eliminate electronic noise, low energy was applied to the drop drive, which yielded high drop delays of 14 to 17.
Sorting was accomplished using logical gating of the spores in the FSC-versus-SSC and the FL1-versus-FL31 dot plots. Only events that entered a gate consisting of both the spore region in the FSC-versus-SSC dot plot and the spore region in the FL1-versus-FL31 dot plot were sorted. Sorted material was placed on wet ice and stored at 4°C. After flushing the sample line with filtered PBS2, the same instrument settings were used to acquire data on sorted material to determine the quality of the sort using CellQuest acquisition and analysis software.
Counting sorted material.
To determine the number of sorted events, a fluorescence-activated cell scanner (FACScan; Becton Dickinson) equipped with CellQuest acquisition and analysis software (Becton Dickinson), a 488-nm laser, and tubes with known numbers of highly fluorescent polystyrene beads (TruCount; Becton Dickinson) were used. This was the same instrument and instrument settings used to identify and characterize Encephalitozoon species in a previous study (17). All sorted material was pooled and centrifuged at 1,200 × g for 30 min at 6°C. Most of the supernatant was discarded, and the pellets were resuspended in PBS2. A known volume of sorted material was added to the TruCount tubes. On the flow cytometer, regions were made around the bead and spore regions in both FSC-versus-SSC and FL1-versus-FL3 dot plots, all in log mode. Logical gating was used to accept only those events that entered the bead regions (FSC-versus-SSC and FL1-versus-FL3 dot plots) and those events that entered the spore regions (FSC-versus-SSC and FL1-versus-FL3 dot plots). Calculations on the number of events placed in the TruCount tubes were performed as instructed by the manufacturer, calculated as the inverse of the number of bead events acquired times the number of events acquired in the spore region times the total number of beads in the TruCount tubes (supplied by the manufacturer).
Rabbit polyclonal antiserum and IIF.
An adult New Zealand rabbit was injected seven times intravenously with about 107 cesium chloride-clarified spores (counts determined by dilution technique) per injection over 12 months. Immune serum was tested and compared with preimmune serum by indirect immunofluorescence (IIF) as described below. Ten days before the final bleed the rabbits were injected with 107 sorted spores.
Ten microliters of spore suspension from each isolation step was applied to each well of 12-well slides and air dried. The rabbit anti-E. bieneusi serum (1:600 dilution) was incubated with the spores for 30 min and washed with PBS. Bound rabbit immunoglobulin G antibodies were reacted with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (1:102 dilution; Cappel Laboratories, Cochranville, Pa.) for 30 min. Evans Blue was used to counterstain the spores. Slides were washed with PBS, mounted with glycerol mounting medium, and examined using an Olympus BX60 fluorescence microscope as previously described (24).
Transmission electron microscopy (TEM).
Spores from stool preparations and from sorted material were fixed in 2.5% glutaraldehyde in 0.1 M Na cacodylate buffer, pH 7.4, for 2 h at 4°C. After fixation in 1% OsO4, the spores were dehydrated in ethanol, concentrated in microcentrifuge tubes, and embedded in Epon 812. Ultra-thin sections were cut and were stained with uranyl acetate and lead citrate. Sections were examined using a JEOL 1200 EX transmission electron microscope.
Test for bacterial and fungal contamination.
Portions of the cesium chloride-isolated spore suspensions and the sorted material were inoculated onto blood agar, MacConkey agar, and Sabouraud agar plates. The plates were checked for bacterial and fungal growth after 24 and 48 h of incubation.
RESULTS
Antifungal and antibacterial treatment.
No fungal or bacterial growths were detected on the various media inoculated with Percoll and cesium chloride gradient-purified spores or with sorted material.
E. bieneusi purification process.
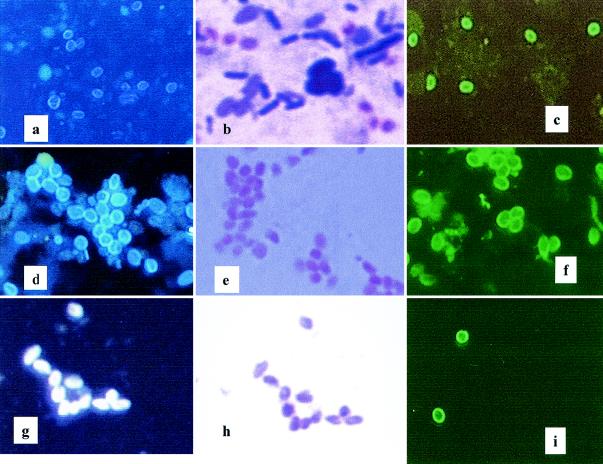
Nine different stool preparations were made from stool specimens obtained from three AIDS patients, patients A, B, and C. All patients had E. bieneusi spores in their stools as confirmed by Weber's chromotrope staining (25). The mean spore counts at each purification step, based on counting stained spores (calcofluor and Gram chromotrope staining), from each of the three patients are displayed in Table 1. In the stool preparations (45 ml of antibiotic-treated filtrate from 1:10 dilution of stool specimen) from the three patients, the mean counts of E. bieneusi spores ranged from 2.10 × 109 to 39.0 × 109 while mean spore counts in the cesium chloride density gradients (5 ml in PBS) ranged from 0.025 × 109 to 0.63 × 109 (Table 1). From stool preparation to cesium chloride density gradients, the mean recovery of spores ranged from 1.03 to 1.57% (Table 1). Various methods (Fig. 1a to c [calcofluor, Gram chromotrope, and IIF, respectively]) were nonspecific, staining many different organisms in addition to E. bieneusi spores. Even though Percoll gradients removed large amounts of debris and non-E. bieneusi organisms, significant debris and unwanted organisms remained with the E. bieneusi spores (data not shown).
TABLE 1.
Mean counts and percent recovery of E. bieneusi spores from stool preparations, Percoll, and cesium chloride gradients
| Patient (no. of stool preparations) | Mean total no. of spores, 109 (SD, 109)
|
Mean % recovery (SD) | ||
|---|---|---|---|---|
| Stool preparations | Percoll | Cesium chloride | ||
| A (4) | 39.0 (9.80) | 3.9 (2.1) | 0.63 (0.25) | 1.57 (0.54) |
| B (3) | 2.10 (1.31) | 0.18 (0.11) | 0.025 (0.01) | 1.24 (0.22) |
| C (2) | 2.50 (0.99) | 0.21 (0.14) | 0.026 (0.01) | 1.03 (0.04) |
FIG. 1.
Staining of E. bieneusi spores during various stages of the spore purification procedure. Stool preparation: calcofluor (a), Gram chromotrope (b), and IIF (c). Cesium chloride gradient centrifugation: calcofluor (d), Gram chromotrope (e), and IIF (f). Sorted material: calcofluor (g), Gram chromotrope (h), and IIF (i).
The cesium chloride density gradients further removed debris and unwanted material. Various stains of cesium chloride-clarified preparation (Fig. 1d to f [calcofluor, Gram chromotrope, and IIF, respectively]) showed many E. bieneusi spores with some debris and a few non-E. bieneusi organisms. Immune rabbit serum used in the IIF showed a very high titer compared with preimmune serum but the antibody preparation was nonspecific (Fig. 1f). Compared with the stool preparations (Fig. 1a to c), the cesium chloride density gradients provided more purified E. bieneusi spores compared with spores isolated by Percoll.
Sorting.
Figure 2 shows dot plot profiles of unsorted cesium chloride-isolated spore suspensions (Fig. 2 to D) and sorted material (Fig. 2E and F) from cesium chloride-isolated spore suspensions. Different dot plot profiles of the cesium chloride-isolated spore suspensions are shown between patient A (Fig. 2A and B) and patient B (Fig. 2C and D). Only those events that entered a gate consisting of both region 1 (R1) in the FSC-versus-SSC dot plots (Fig. 2A and C) and R2 of the FL1-versus-FL31 fluorescence dot plots (Fig. 2B and D) were sorted. These regions contained the highest concentration of spores in addition to some bacteria, which had autofluorescence and size similar to those of the E. bieneusi spores. Most of the events outside these regions were eliminated because of larger size or higher autofluorescence than the E. bieneusi spores. Sorted material from patient B is shown in a FSC-versus-SSC dot plot (Fig. 2E) and in a FL1-versus-FL31 dot plot (Fig. 2F), showing high uniformity of sorted material (98% and 99%, respectively) assessed by measuring the percentage of events that fell within the gated regions.
FIG. 2.
FSC-versus-SSC and FL1 (515 to 545 nm)-versus-FL31 (633 to 677 nm [FL3 in laser 1]) fluorescence dot plots on unsorted material from cesium chloride density gradients performed on samples from two patients (patient A [A and B, respectively]; and patient B [C and D, respectively]). (E and F) FSC-versus-SSC and F2-versus-F31 dot plots, respectively, on sorted material from patient B. Boxed areas region 1 (R1) and region 2 (R2) enclose concentrations of E. bieneusi spores. Of the sorted events, 98% were contained in R1 of the FSC-versus-SSC dot plot (E) and 99% were contained in R2 of the FL1-versus-FL31 dot plot (Fig. 2F).
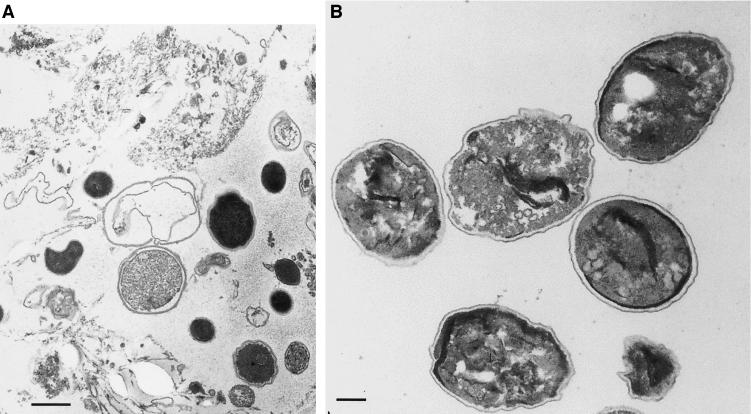
The calcofluor, Gram chromotrope, and IIF staining of sorted samples (Fig. 1g to i) together with the fluorescence-activated cell sorting data (Fig. 2E and F) clearly indicate that the sorting step significantly purified the E. bieneusi spore preparation. The purity of the sorted E. bieneusi preparation was also confirmed by TEM. Figure 3 shows examples of the starting material (Fig. 3A) and the same preparation after sorting (Fig. 3B).
FIG. 3.
TEM of a stool preparation from an AIDS patient with confirmed E. bieneusi infection (A) (bar = 500 nm) and from sorted material from the same patient (B) (bar = 200 nm).
TEM shows also the good preservation of sorted spores indicating that this method is less destructive for spores.
Counts on sorted material.
The sorted material was quantified using flow cytometry and TruCount tubes containing known numbers of highly fluorescent polystyrene beads. The sorted material and beads were clearly separated, and the E. bieneusi spores showed slightly less light scatter characteristics than that of the slightly larger Encephalitozoon species previously studied (17; data not shown). Using the described cell sorting technique, an average of about 13 to 15 million sorted events could be obtained during a 3-h run.
DISCUSSION
Percoll gradient purification is a widely used technique (20). Accoceberry et al. (2) isolated E. bieneusi spores from stool preparations using a discontinuous Percoll gradient consisting of four different concentrations (100, 67.5, 45, and 22.5%). The spores were sufficiently purified for the production of monoclonal antibodies. In a subsequent study, they used expanded-bed adsorption technology using a mouse monoclonal antibody bound directly to recombinant protein A adsorbent for direct recovery of E. bieneusi spores from diluted stool specimen (1). However, monoclonal antibodies to E. bieneusi spores are not available commercially.
In the present study, we sequentially used Percoll and cesium chloride gradients for the purification of E. bieneusi spores from human stool specimens. This technique has been successfully used for the purification of Cryptosporidium parvum oocysts from the feces of infected calves (M. J. Arrowood and K. Donaldson, abstr. 4th Int. Workshop Opportunistic Protists, J. Eukaryot. Microbiol. 43:89S, 1996). Although the Percoll and cesium chloride gradients described here generated relatively pure E. bieneusi spores from human stool specimens, some debris and bacteria remained with the spores.
Flow cytometry has been used in various studies of parasitic protozoa. Single-color flow cytometry has been used to identify microsporidia belonging to the genus Encephalitozoon (21), and two-color flow cytometry has been used to identify C. parvum oocysts in environmental water samples (8) and to quantify C. parvum oocysts in the feces of infected mice using TruCount tubes (16). Specific antibodies conjugated with fluorochrome have been used in flow cytometry to quantify trophozoites of Giardia duodenalis (4). Over a period of 1 year, flow cytometry showed that the light scatter and autofluorescence properties of fish microsporidia remained unchanged (3). Further, density gradients and flow cytometry with sorting capabilities have purified oocyst walls of Toxoplasma gondii that were heavily contaminated with fragments of host cells (7). Sorting was also used to isolate E. bieneusi spores that had been stained with the fluorochrome Uvitex 2B, which labels a spore wall chitin (S. Challier, S. Brown, C. Ombrouck, I. Desportes-Livage, D. De Nay, and M. Gentilini, abstract from the 3rd Int. Workshop on Opportunistic Protists, J. Eukaryot. Microbiol. 41:27S, 1994).
In the present study, we used flow cytometry with cell sorting capabilities to further purify E. bieneusi spores that were isolated using Percoll and cesium chloride density gradients. As observed by calcofluor and Gram chromotrope staining and IIF, the sorted material appeared to be highly purified (Fig. 1g to i). However, all of these stains were nonspecific and were not suitable for use in sorting. The light scatter and autofluorescence profiles of the sorted material indicated a purity of 98 and 99%, respectively, within the regions used in a gate designated for sorting (Fig. 2E and F, respectively). In this study, quantification of sorted events containing E. bieneusi spores using TruCount tubes and flow cytometry was convenient, and, as expected, the light scatter profiles obtained with the E. bieneusi spores were slightly less in intensity than that obtained in a previous study on the slightly larger Encephalitozoon species (17). Quantification of the spores using TruCount tubes will be useful in other studies, and TruCount tubes and flow cytometry have quantified CD4 cells in the peripheral blood of HIV-AIDS patients for critical decisions regarding their treatment and classification (19). The ability to purify and quantify E. bieneusi spores will facilitate further molecular-biological, biochemical, and immunological studies of this parasite.
Acknowledgments
This study was supported in part by U.S. Public Health Service grant RR03034 and by EPA project R-82804201-0.
REFERENCES
- 1.Accoceberry, I., M. Thellier, A. Datry, I. Desportes-Livage, S. Biligui, M. Danis, and X. Santarelli. 2001. One-step purification of Enterocytozoon bieneusi spores from human stools by immunoaffinity expanded-bed adsorption. J. Clin. Microbiol. 39:1947-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accoceberry, I., M. Thellier, I. Desportes-Livage, A. Achbarou, S. Biligui, M. Danis, and A. Datry. 1999. Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi. J. Clin. Microbiol. 37:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amigo, J. M., M. P. Gracia, J. Comas, H. Salvado, and C. P. Vivares. 1994. Comparative study of microsporidian spores by flow cytometric analysis. J. Eukaryot. Microbiol. 41:210-214. [DOI] [PubMed] [Google Scholar]
- 4.Bruderer, T., E. Niederer, and P. Kohler. 1994. Separation of a cysteine-rich surface antigen-expressing variant from a cloned Giardia isolate by fluorescence-activated cell sorting. Parasitol. Res. 80:303-306. [DOI] [PubMed] [Google Scholar]
- 5.Dengjel, B., M. Zahler, W. Hermanns, K. Heinritzi, T. Spillmann, A. Thomschke, T. Loscher, R. Gothe, and H. Rinder. 2001. Zoonotic potential of Enterocytozoon bieneusi. J. Clin. Microbiol. 39:4495-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowd, S. E., C. P. Gerba, and I. L. Pepper. 1998. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl. Environ. Microbiol. 64:3332-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everson, W. V., M. W. Ware, J. P. Dubey, and H. D. Lindquist. 2002. Isolation of purified oocyst walls and sporocysts from Toxoplasma gondii. J. Eukaryot. Microbiol. 49:344-349. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari, B. C., G. Vesey, K. A. Davis, M. Gauci, and D. Veal. 2000. A novel two-color flow cytometric assay for the detection of Cryptosporidium in environmental water samples. Cytometry 41:216-222. [PubMed] [Google Scholar]
- 9.Fournier, S., O. Liguory, M. Santillana-Hayat, E. Guillot, C. Sarfati, N. Dumoutier, J. Molina, and F. Derouin. 2000. Detection of microsporidia in surface water: a one-year follow-up study. FEMS Immunol. Med. Microbiol. 29:95-100. [DOI] [PubMed] [Google Scholar]
- 10.Green, L. C., P. J. Didier, and E. S. Didier. 1999. Fractionation of sporogonial stages of the microsporidian Encephalitozoon cuniculi by Percoll gradients. J. Eukaryot. Microbiol. 46:434-438. [DOI] [PubMed] [Google Scholar]
- 11.Kelkar, R., P. S. Sastry, S. S. Kulkarni, T. K. Saikia, P. M. Parikh, and S. H. Advani. 1997. Pulmonary microsporidial infection in a patient with CML undergoing allogeneic marrow transplant. Bone Marrow Transplant. 19:179-182. [DOI] [PubMed] [Google Scholar]
- 12.Kondova, I., K. Mansfield, M. A. Buckholt, B. Stein, G. Widmer, A. Carville, A. Lackner, and S. Tzipori. 1998. Transmission and serial propagation of Enterocytozoon bieneusi from humans and rhesus macaques in gnotobiotic piglets. Infect. Immun. 66:5515-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Velez, R., M. C. Turrientes, C. Garron, P. Montilla, R. Navajas, S. Fenoy, and C. del Aguila. 1999. Microsporidiosis in travelers with diarrhea from the tropics. J. Travel Med. 6:223-227. [DOI] [PubMed] [Google Scholar]
- 14.Lores, B., I. Lopez-Miragaya, C. Arias, S. Fenoy, J. Torres, and C. del Aguila. 2002. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus-negative patients from Vigo, Spain. Clin. Infect. Dis. 34:918-921. [DOI] [PubMed] [Google Scholar]
- 15.Metge, S., J. T. Van Nhieu, D. Dahmane, P. Grimbert, F. Foulet, C. Sarfati, and S. Bretagne. 2000. A case of Enterocytozoon bieneusi infection in an HIV-negative renal transplant recipient. Eur. J. Clin. Microbiol. Infect. Dis. 19:221-223. [DOI] [PubMed] [Google Scholar]
- 16.Moss, D. M., and M. J. Arrowood. 2001. Quantification of Cryptosporidium parvum oocysts in mouse fecal specimens using immunomagnetic particles and two-color flow cytometry. J. Parasitol. 87:406-412. [DOI] [PubMed] [Google Scholar]
- 17.Moss, D. M., G. P. Croppo, S. Wallace, and G. S. Visvesvara. 1999. Flow cytometric analysis of microsporidia belonging to the genus Encephalitozoon. J. Clin. Microbiol. 37:371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moura, H., D. A. Schwartz, F. Bornay-Llinares, F. C. Sodre, S. Wallace, and G. S. Visvesvara. 1997. A new and improved “quick-hot Gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch. Pathol. Lab. Med. 121:888-893. [PubMed] [Google Scholar]
- 19.Nicholson, J. K., D. Stein, T. Mui, R. Mack, M. Hubbard, and T. Denny. 1997. Evaluation of a method for counting absolute numbers of cells with a flow cytometer. Clin. Diagn. Lab. Immunol. 4:309-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pertoft, H. 2000. Fractionation of cells and subcellular particles with Percoll. J. Biochem. Biophys. Methods 44:1-30. [DOI] [PubMed] [Google Scholar]
- 21.Sandfort, J., A. Hannemann, H. Gelderblom, K. Stark, R. L. Owen, and B. Ruf. 1994. Enterocytozoon bieneusi infection in an immunocompetent patient who had acute diarrhea and who was not infected with the human immunodeficiency virus. Clin. Infect. Dis. 19:514-516. [DOI] [PubMed] [Google Scholar]
- 22.Tzipori, S., A. Carville, G. Widmer, D. Kotler, K. Mansfield, and A. Lackner. 1997. Transmission and establishment of a persistent infection of Enterocytozoon bieneusi, derived from a human with AIDS, in simian immunodeficiency virus-infected rhesus monkeys. J. Infect. Dis. 175:1016-1020. [DOI] [PubMed] [Google Scholar]
- 23.Vavra, J., R. Dahbiova, W. S. Hollister, and E. U. Canning. 1993. Staining of microsporidian spores by optical brighteners with remarks on the use of brighteners for the diagnosis of AIDS associated human microsporidioses. Fol. Parasitol. 40:267-272. [PubMed] [Google Scholar]
- 24.Visvesvara, G. S., G. J. Leitch, A. J. da Silva, G. P. Croppo, H. Moura, S. Wallace, S. B. Slemenda, D. A. Schwartz, D. Moss, and R. T. Bryan. 1994. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J. Clin. Microbiol. 32:2760-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber, R., R. T. Bryan, R. L. Owen, C. M. Wilcox, L. Gorelkin, G. S. Visvesvara, et al. 1992. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N. Engl. J. Med. 326:161-166. [DOI] [PubMed] [Google Scholar]
- 26.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]