Abstract
For prevention and control of gonorrhea, an objective, highly discriminating, and reproducible molecular epidemiological characterization of Neisseria gonorrhoeae is essential. In the present study, in pursuance of providing such qualities, pyrosequencing technology, a fast real-time DNA sequence analysis, was applied to six short, highly polymorphic porB gene segments, with subsequent genetic variant (genovar) determination of the bacterial isolates. The sequencing templates were obtained by real-time PCR amplification, which also included fluorescence melting curve analysis of the entire porB gene in order to determine the genogroup (porB1a or porB1b allele) prior to pyrosequencing analysis. The PSQ 96 MA system used allowed rapid (in approximately 1.5 h) determination of 96 sequences of 20 to 65 correct nucleotides each. The results were reproducible and mostly in concordance with the results of conventional Sanger dideoxy sequencing, with the exception of shorter read lengths and some uncertainty in determining the correct number of identical nucleotides in homopolymeric segments. The number of sequence variants identified in each of the six highly polymorphic segments of the porB1a and porB1b alleles (encoding surface-exposed amino acid loops of the mature PorB protein) ranged from 5 to 11 and from 8 to 39, respectively. Among porB1a isolates (n = 22) and porB1b isolates (n = 65), 22 and 64 unique genovars, respectively, were identified. All isolates were typeable. The present results provide evidence of a high discriminatory ability, practically the same as that for sequencing of the entire porB gene. In conclusion, the fast and high-throughput pyrosequencing technology can be used for molecular epidemiological characterization of N. gonorrhoeae.
Neisseria gonorrhoeae is the causative agent of gonorrhea, which remains an important sexually transmitted infection in developing nations as well as in developed countries (8). For prevention and control of infection, thorough knowledge about the strain populations circulating in different communities, temporal and geographic changes among the strains, and the emergence and transmission patterns of individual strains is crucial. Thus, for epidemiological and clinical purposes a highly discriminating, objective, and reproducible characterization of N. gonorrhoeae strains is essential. The widely used phenotypic characterizations of the bacterium have several limitations (10, 12, 13, 17, 24, 27, 28), and different molecular genetic methods have thus been developed (12, 15, 18, 23, 25, 26, 27, 28, 30, 32).
The present study investigated whether DNA sequence analysis by pyrosequencing technology, strictly limited to highly polymorphic segments of the porB gene, could be used as a rapid and high-throughput method for molecular epidemiological characterization of N. gonorrhoeae isolates. Pyrosequencing is a recently described, fast real-time DNA sequence analysis by means of synthesis of short DNA stretches (21). It is a primer-directed polymerase extension assay, where the release of pyrophosphate upon nucleotide incorporation is measured in real time.
The rationale of the present study was that N. gonorrhoeae outer membrane protein PorB is universally present and does not undergo high-frequency variation during the course of infection in smaller groups of sexual contacts (33). Individual strains express only one of the two PorB protein groups, either PorB1a or PorB1b (4, 6, 11, 29), which are encoded by the mutually exclusive porB gene alleles, porB1a and porB1b, respectively. Naturally but rarely occurring strains that express PorB1a-PorB1b hybrids have been identified (4, 9). Antigenic diversities of PorB between strains form the basis for the widely used serogroup and serovar determination with monoclonal antibodies (MAbs) (14, 22), and attempts at identifying the antigenic epitopes of PorB of the serovar-specific MAbs have previously been published (1, 3, 4, 5, 16, 27). However, the exact amino acid sequences and structures of many of the epitopes are still unknown.
Previous genetic studies have concluded that porB gene sequencing can be used to trace the transmission of individual N. gonorrhoeae strains within the community, discriminate suspected clusters of gonorrhea cases, and confirm epidemiological connections of patients (5, 12, 28, 31). The segments of the porB gene encoding the eight surface-exposed amino acid loops of PorB (29) exhibit the highest level of polymorphism (1, 4, 5, 7, 12, 27, 28, 29, 31). Sequence analysis of four to six of these short, highly polymorphic segments of the porB gene generates discrimination between isolates that is almost identical to that obtained by sequencing of the entire porB allele (27). Therefore, pyrosequencing technology could be ideal for rapid, large scale sequencing of such carefully selected short, highly polymorphic segments of the porB gene.
The results of the present study show that pyrosequencing technology can be used for genetic variant (genovar) determination of N. gonorrhoeae isolates, a concept that may complement or even replace current internationally established major phenotypic antigen (serovar) determinations in routine use for epidemiological typing.
MATERIALS AND METHODS
Bacterial isolates and culture conditions.
N. gonorrhoeae reference strains (n = 29) and clinical isolates (n = 58) were examined in the present study. The selection of the isolates was based on the results of conventional Sanger dideoxy sequencing obtained in previous studies (27, 28), from which all isolates comprising a unique porB gene sequence (n = 87) were included. The reference strains originated from different geographic localities worldwide between 1973 and 1997, and the clinical isolates were isolated in Sweden between 1998 and 2001. The isolates comprised 28 different serovars and one nonserotypeable serovar according to the serovar determination done with the Genetic Systems panel of MAbs routinely performed on N. gonorrhoeae isolates at the Swedish Reference Laboratory for Pathogenic Neisseria (see Table 2) (27). All isolates were preserved at −70°C and cultured as previously described (28).
TABLE 2.
Genetic variants (genovars) of N. gonorrhoeae isolates (n = 87) comprising 28 different serovars and one nonserotypeable serovar according to a previous study (27)a
| Allele (loops) | Strain designation (serovar) | Genovar |
|---|---|---|
| porB1a (1, 2, 3, 4, 6, 8) | 57/01 (NTb) | porB1a.1-1-1-1-4-4 |
| 266/98 (IA-6) | porB1a.1-1-1-1-4-1 | |
| 5/98 (IA-21) | porB1a.1-1-1-2-1-1 | |
| 277/98 (IA-6) | porB1a.1-1-2-2-3-5 | |
| CCUG 36702 (IA-10) | porB1a.1-2-1-2-1-6 | |
| 371/98 (IA-1.2) | porB1a.1-2-6-1-1-1 | |
| 438/98 (IA-8) | porB1a.2-1-1-3-2-1 | |
| 80/99 (IA-8) | porB1a.2-1-4-2-2-1 | |
| 91/98 (IA-21) | porB1a.2-5-1-3-2-1 | |
| CCUG 33978 (IA-1.2) | porB1a.3-2-1-1-1-1 | |
| CCUG 42289 (IA-1.2) | porB1a.3-7-4-1-1-1 | |
| 34/98 (IA-4) | porB1a.4-4-5-4-2-2 | |
| 294/98 (IA-4) | porB1a.4-4-5-5-2-2 | |
| CCUG 13578 (IA-5) | porB1a.5-1-3-1-5-3 | |
| ATCC 43069 (IA-6) | porB1a.5-3-1-1-3-1 | |
| CCUG 13581 (IA-1.2) | porB1a.6-2-1-1-1-1 | |
| CCUG 13576 (IA-1.2) | porB1a.6-6-4-1-1-1 | |
| CCUG 13585 (IA-6) | porB1a.7-1-3-1-2-1 | |
| CCUG 41813 (IA-6) | porB1a.8-3-2-1-3-1 | |
| 163/98 (IA-8) | porB1a.9-3-2-1-3-1 | |
| 72/99 (IA-17) | porB1a.10-2-1-2-4-1 | |
| CCUG 13586 (IA-7) | porB1a.11-1-3-1-5-3 | |
| porB1b (1, 3, 5, 6, 7, 8) | CCUG 33979 (IB-3) | porB1b.1-1-2-7-1-1 |
| CCUG 13574 (IB-3) | porB1b.1-1-3-9-3-4 | |
| 335/98 (IB-3) | porB1b.1-1-9-3-1-1 | |
| CCUG 15821 (IB-9) | porB1b.1-1-10-6-9-1 | |
| CCUG 13579 (IB-3) | porB1b.1-1-10-13-3-1 | |
| 177/98 (IB-31) | porB1b.1-1-12-3-1-1 | |
| 88/98 (IB-3) | porB1b.1-1-15-3-1-1 | |
| 138/99 (IB-3) | porB1b.1-1-19-7-1-1 | |
| 156/98 (IB-3) | porB1b.1-1-24-7-1-1 | |
| 73/99 (IB-31) | porB1b.1-1-26-3-3-1 | |
| 310/98 (IB-11) | porB1b.1-1-27-6-10-1 | |
| 357/98 (IB-3) | porB1b.1-1-35-3-2-7 | |
| 26/98 (IB-1) | porB1b.1-2-3-2-3-1 | |
| CCUG 41812 (IB-1) | porB1b.1-2-3-2-3-5 | |
| 93/98 (IB-5) | porB1b.1-2-5-1-4-1 | |
| 76/98 (IB-10) | porB1b.1-4-13-12-2-3 | |
| CCUG 15823 (IB-1) | porB1b.1-5-3-2-3-1 | |
| 261/98 (IB-36) | porB1b.1-6-1-8-1-1 | |
| 263/98 (IB-26) | porB1b.1-13-36-10-1-1 | |
| CCUG 36701 (IB-1) | porB1b.1-14-39-4-1-1 | |
| CCUG 42290 (IB-26) | porB1b.1-19-1-4-1-1 | |
| CCUG 42285 (IB-26) | porB1b.1-20-1-4-1-1 | |
| 325/98 (IB-2) | porB1b.2-1-2-2-1-1 | |
| 126/99 (IB-2) | porB1b.2-2-1-4-1-1 | |
| 141/98 (IB-5) | porB1b.2-2-5-1-4-1 | |
| 316/98 (IB-2) | porB1b.2-2-6-1-6-1 | |
| 20/99 (IB-26) | porB1b.2-2-18-1-2-1 | |
| 57/98 (IB-5) | porB1b.2-2-34-1-4-1 | |
| 419/98 (IB-2) | porB1b.2-4-6-1-2-9 | |
| 19/99 (IB-26) | porB1b.2-4-11-1-2-3 | |
| 435/98 (IB-2) | porB1b.2-4-20-1-2-3 | |
| 70/98 (IB-1) | porB1b.2-6-1-4-1-1 | |
| 360/98 (IB-2) | porB1b.2-18-21-1-12-2 | |
| 418/98 (IB-2) | porB1b.2-21-28-1-6-3 | |
| 394/98 (IB-3) | porB1b.2-22-1-4-1-1 | |
| 80/98 (IB-12) | porB1b.3-2-16-10-1-1 | |
| CCUG 34447 (IB-6) | porB1b.3-8-2-9-8-1 | |
| 445/98 (IB-2) | porB1b.3-9-2-2-5-1 | |
| 162/98 (IB-24) | porB1b.3-10-29-8-1-4 | |
| CCUG 34327 (IB-4) | porB1b.3-10-32-8-1-4 | |
| CCUG 13583 (IB-22) | porB1b.4-1-14-2-3-1 | |
| 326/98 (IB-3) | porB1b.4-1-23-2-3-1 | |
| CCUG 42287 (IB-36) | porB1b.4-12-38-6-4-1 | |
| 16/98 (IB-3) | porB1b.4-15-25-3-3-1 | |
| 439/98 (IB-2) | porB1b.5-3-4-1-2-2 | |
| 115/99 (IB-21) | porB1b.5-3-4-5-2-2 | |
| 246/98 (IB-2) | porB1b.5-7-22-1-7-6 | |
| 75/98 (IB-21) | porB1b.5-9-2-2-5-1 | |
| 370/98 (IB-2) | porB1b.6-3-4-5-2-2 | |
| 97/98 (IB-2) | porB1b.6-3-7-5-2-2 | |
| 400/98 (IB-2) | porB1b.6-3-7-5-2-2 | |
| 50/98 (IB-3) | porB1b.7-1-30-2-3-1 | |
| 428/98 (IB-3) | porB1b.7-16-17-11-1-1 | |
| 432/98 (IB-3) | porB1b.7-17-1-11-1-1 | |
| 189/98 (IB-2) | porB1b.8-7-4-1-2-2 | |
| 88/99 (IB-8) | porB1b.9-8-8-16-13-1 | |
| CCUG 13577 (IB-4) | porB1b.10-5-37-6-1-1 | |
| CCUG 13572 (IB-7) | porB1b.11-11-8-1-2-2 | |
| 395/98 (IB-2) | porB1b.12-3-33-5-2-2 | |
| CCUG 41811 (IB-4) | porB1b.13-5-5-6-1-1 | |
| 133/99 (IB-3) | porB1b.14-2-1-17-1-1 | |
| CCUG 41810 (IB-6) | porB1b.15-11-1-7-1-1 | |
| 380/98 (IB-13) | porB1b.16-1-9-3-11-1 | |
| 127/98 (IB-31) | porB1b.17-2-31-15-2-1 | |
| 426/98 (IB-2) | porB1b.18-4-6-14-2-3 |
The genogroup, i.e., allele of the porB gene, and the different sequence variants present in each of the six highly polymorphic segments of the porB1a allele or porB1b allele, encoding amino acid loops of the mature PorB protein (29), establish the designation of the genovar.
NT, nonserotypeable.
Isolation of genomic DNA.
Isolation of bacterial DNA was performed by using magnetic silica particles in a robotized system (GenoM-48; GenoVision, Oslo, Norway) according to the instructions of the manufacturer. Briefly, bacterial suspensions (approximately 3 × 108 cells/ml) were prepared in 0.15 M sterile NaCl. A total of 750 μl from each suspension was pelleted and resuspended in 200 μl of sterile distilled water. DNA was isolated from these final suspensions with the GenoPrep Tissue DNA kit (GenoVision) and eluted in 75 μl of sterile distilled water according to the instructions of the manufacturer. The DNA preparations were stored at 4°C prior to PCR.
porB gene real-time PCR.
The entire porB gene was amplified by using the previously documented primers PorBU and PorBL (28). The amplifications were performed in a LightCycler system (Roche Molecular Biochemicals, Mannheim, Germany), using SYBR Green I fluorescence melting curve analysis for specific identification of porB1a or porB1b amplicons. Each PCR mixture (20 μl) contained 2 μl of LightCycler-FastStart DNA Master SYBR Green I (Roche Diagnostics GmbH, Mannheim, Germany), 3 mM MgCl2 (Roche Diagnostics), a 0.5 μM concentration of each primer, and 2 μl of DNA template. In each PCR, one porB1a reference strain (ATCC 43069), one porB1b reference strain (CCUG 15821), and one negative control (distilled water instead of DNA template) were included. The cycling parameters of the amplification were as follows: a FastStart enzyme activation step at 95°C for 10 min, followed by 30 sequential cycles of heating up to 95°C, 60°C for 10 s, and 72°C for 42 s. The cycling parameters of the subsequent melting curve analysis were as follows: heating the PCR products up to 95°C, cooling at 65°C for 45 s, and finally slowly heating (0.1°C/s) up to 95°C. For identification of porB1a or porB1b amplicons, the melting temperature (Tm) of the product, which is related to the size of the amplicons, was determined by fluorescence, which was continuously measured during the finishing slow-heating phase. The mean Tms with standard deviations (SDs) for the porB1a and porB1b alleles of all isolates were calculated. Subsequently, the individual isolates were designated as belonging to one or the other genogroup (porB1a or porB1b allele). The PCR products were stored at 4°C.
Real-time PCR of six short, highly polymorphic porB gene segments.
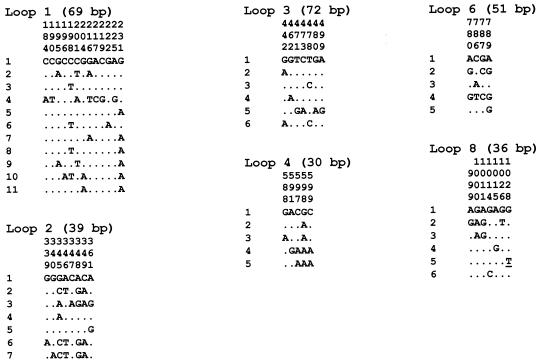
The four and six most polymorphic segments, encoding surface-exposed amino acid loops of the mature PorB (29), of the porB1a and porB1b alleles, respectively, were included (Fig. 1 and 2). This was due to the fact that phylogenetic analysis of these segments and the entire porB alleles shows practically identical discriminatory ability between isolates (27). In the present study, the sequences encoding surface-exposed loop 3 and loop 6 of mature PorB1a protein were also included, since these contain suggested epitopes for the MAbs used in the serovar determination (16) (Fig. 1). The segments of the porB gene were amplified by using primers complementary to conserved regions of the porB gene, according to results in previous studies (27, 28) as well as sequences in GenBank (Table 1). The primers were located some nucleotides before, overlapping the beginning of, or within the segments encoding the loops of PorB (Table 1). All known polymorphic nucleotide sites in these segments were, however, located within the amplicons and clear of the primers used for the pyrosequencing. In each PCR, one of the primers for each segment was labeled with a biotin molecule at the 5′ end for purposes of easy capture. The amplifications were performed in a LightCycler system (Roche Molecular Biochemicals) with SYBR Green I fluorescence melting curve analysis for identification of specific amplicons. In a pilot study, the PCR products were also analyzed by electrophoresis through a 2% agarose gel and ethidium bromide staining in order to ensure the specificity of the amplification. Each PCR mixture (20 μl) contained 2 μl of LightCycler-FastStart DNA Master SYBR Green I (Roche Diagnostics), 3 mM MgCl2 (Roche Diagnostics), a 0.5 μM concentration of each primer, and 2 μl of DNA template. In each PCR, one porB1a reference strain (ATCC 43069) and/or one porB1b reference strain (CCUG 15821) and one negative control (distilled water instead of DNA template) were included. The cycling parameters of the amplification were as follows: a FastStart enzyme activation step at 95°C for 10 min, followed by 40 sequential cycles of heating up to 95°C, 45°C for 10 s, and 72°C for 3 s. The cycling parameters of the subsequent melting curve analysis were as follows: heating the PCR products up to 95°C, cooling at 50°C for 30 s, and finally slowly heating (0.1°C/s) up to 95°C. For identification of a specific amplicon, the Tm of the product was given by the fluorescence that was continuously measured during the finishing slow heating phase. The PCR products were stored at 4°C prior to pyrosequencing.
FIG. 1.
Sequence variants and polymorphic nucleotide sites in each of the highly polymorphic segments of the porB1a alleles, encoding amino acid loops of the mature PorB1a protein (29), of N. gonorrhoeae isolates (n = 22). The nucleotide present at each polymorphic site among all of the sequence variants is shown for sequence variant 1 in each loop segment. For the other sequence variants, those sites that differ are shown. Dots indicate identity with sequence variant 1, and underlining indicates synonymous mutations. Nucleotide sites that are conserved in all sequence variants are excluded. The length of each loop segment is included, and the sites are numbered above in vertical format based on the nucleotide numbering of a porB1a gene (GenBank accession no. J03029).
FIG. 2.
Sequence variants and polymorphic nucleotide sites in each of the highly polymorphic segments of the porB1b alleles, encoding amino acid loops of the mature PorB1b protein (29), of N. gonorrhoeae isolates (n = 65). The nucleotide present at each polymorphic site among all of the sequence variants is shown for sequence variant 1 in each loop segment. For the other sequence variants, those sites that differ are shown. Dots indicate identity with sequence variant 1, dashes represent alignment gaps (due to indels in some of the variants), and underlining indicates synonymous mutations. Nucleotide sites that are conserved in all sequence variants are excluded. The length of each loop segment, according to the multiple-sequence alignment, is included, and the sites are numbered above in vertical format based on the nucleotide numbering of a porB1b gene (GenBank accession no. J03017).
TABLE 1.
Primers used in real-time PCR and pyrosequencing of the six highly polymorphic segments of the porB1a and porB1b alleles of N. gonorrhoeae
| Targeta | Primerb | Sequence (5′ → 3′) | Size of PCR amplicons (bp) | Location (bp)c |
|---|---|---|---|---|
| porB1a | ||||
| Loop 1 | PorB1a1F | AGTCGGCGTAGAA | 93 | 167-179 |
| PorB1a1Rd | TTCGAACCCAAATCAGC | 259-243 | ||
| Loop 2 | PorB1a2F | AACAAAAAGCCTACGTC | 61 | 319-335 |
| PorB1a2R | CCGATGAAGGATTGG | 379-365 | ||
| Loop 3 | PorB1a3F | AACATCCTGAAAGACACC | 98 | 423-440 |
| PorB1a3R | GAAACGTGGCGTTCT | 520-506 | ||
| Loop 4 | PorB1a4F | CGTACAATACGTGCCTA | 60 | 560-576 |
| PorB1a4Rd | CCTGCATGGTAAGATTC | 619-603 | ||
| Loop 6 | PorB1a6Fd | CAACAAGACGCGAAA | 72 | 762-776 |
| PorB1a6Rd | CRCGGTAGYGGCAAC | 833-819 | ||
| Loop 8 | PorB1a8F | TTCTGCCGGTTGGTTG | 66 | 980-995 |
| PorB1a8Rd | CCGACACCGCCGNC | 1045-1032 | ||
| porB1b | ||||
| Loop 1 | PorB1b1F | CGTACAAACTTACCGTTC | 74 | 160-177 |
| PorB1b1R | CGGCGATTTCGCT | 233-221 | ||
| Loop 3 | PorB1b3FA | CCCCCTGAAAAACA | 105 | 412-425 |
| PorB1b3FB | GTCAATGCTTGGGAATC | 437-453 | ||
| PorB1b3R | AGGTAGCGGTGTTCC | 516-502 | ||
| Loop 5 | PorB1b5F | GGCGAAGGCACTAAA | 80 | 677-691 |
| PorB1b5R | TGAACTTGCAGTTTTTCA | 756-739 | ||
| Loop 6 | PorB1b6Fd | AACAACAAGATGCCAAA | 61 | 810-826 |
| PorB1b6Rd | TCGGTTTGAGAGTTGTG | 870-854 | ||
| Loop 7 | PorB1b7F | GCTTCAAAGGCACTGTT | 65 | 933-949 |
| PorB1b7Rd | CGCRCCGACAACCAC | 997-983 | ||
| Loop 8 | PorB1b8F | TGCCTTGGTTTCTG | 75 | 1024-1037 |
| PorB1b8Rd | ACGACGGCRCTGGC | 1098-1085 |
Allele of the porB gene and sequences encoding the surface-exposed loops of the mature PorB protein (29).
Synthesized by Scandinavian Gene Synthesis AB, Köping, Sweden. All primers were also produced in a biotinylated version (5′ end) for the pyrosequencing.
Location of the primer according to the previously published nucleotide sequences of a porB1a gene (GenBank accession no. J03029) (2) and a porB1b gene (GenBank accession no. J03017) (11).
Determined the entire target segment when used as sequencing primer in the pyrosequencing.
Pyrosequencing.
Both DNA strands of the six highly polymorphic segments of the porB1a and porB1b alleles were used as templates in the pyrosequencing. The pyrosequencing was performed by also using the PCR primers as sequencing primers. However, one additional forward sequencing primer, PorB1b3FB, had to be used in order to determine the entire segment that encodes loop 3 of the mature PorB1b protein (29) (Table 1). Twenty microliters of the biotinylated PCR products was immobilized on streptavidin-coated magnetic beads (10 μl of Dynabeads M-280 streptavidin solution [Dynal, Oslo, Norway]) in 25 μl of 2× binding-washing buffer II (10 mM Tris-HCl, 2 M NaCl, 1 mM EDTA, and 0.1% Tween 20 [pH 7.6]) at 65°C for 15 min in a shaking mixer (1,400 rpm). The PCR product-Dynabeads complexes were captured with a PSQ 96 Sample Prep Tool (Pyrosequencing AB, Uppsala, Sweden) and transferred to a 96-well PSQ 96 SQA microtiter plate containing 0.5 M NaOH (50 μl per well), and single-stranded DNA was obtained through incubation for 1 min. This denaturation was followed by washing, by releasing and recapturing the beads into different microtiter plates, of the single-stranded-DNA-Dynabeads complexes in 1× annealing buffer (20 mM Tris-acetate and 5 mM Mg-acetate) (100 μl per well), followed by washing three times in 95% ethanol (100 μl per well) and finally in 1× annealing buffer (100 μl per well) once again. Subsequently, after transfer of the complexes to a new PSQ 96 SQA microtiter plate, 1 μl of sequencing primer (15 pmol of the nonbiotinylated PCR primer) was annealed to the immobilized single-stranded DNA in 1× annealing buffer (44 μl per well) at 80°C for 2 min. After cooling to room temperature, 1 μl (0.55 μg/μl) of single-stranded DNA binding protein (USB Corporation, Cleveland, Ohio) was added to each well. For pyrosequencing, a PSQ 96 SQA reagent kit (dATPαS, dCTP, dGTP, dTTP, enzyme mixture [DNA polymerase, ATP-sulfurylase, luciferase, and apyrase], and substrate mixture [luciferin and adenosine 5′-phosphosulfate]) was used according to the instructions of the manufacturer (Pyrosequencing AB) in a PSQ 96 MA system with PSQ SQA (version 2.0) software. The different types of deoxynucleoside triphosphates were separately dispensed in a sequence-directed and/or cyclic (dispensation order, 20 × CTGA or 25 × CTGA) manner. The sequences were automatically interpreted from the pyrograms by the PSQ SQA (version 2.0) software and manually edited after visual inspection. All sequencing results were compared to previous results obtained by using conventional Sanger sequencing (27, 28).
Sequence comparisons and genovar designation.
Different multiple-sequence alignments of the six highly polymorphic porB gene segments were performed with BioEdit (version 5.0.9) software and by manual adjustment. The nucleotide sequences in each of the segments of all of the isolates were compared, and each unique sequence was assigned a sequence variant number (Fig. 1 and 2). The most prevalent sequence variant in each segment was designated 1, the second most prevalent was designated 2, and so on. Subsequently, the isolates were designated as different genovars due to different sequence variants present in the polymorphic segments (Table 2). The sequence variants as well as the genovars of all of the isolates were entered into an Excel database.
RESULTS
Real-time PCR of the porB genes of 87 N. gonorrhoeae isolates.
Well-defined melting peaks were observed for all the isolates. The mean Tm of the porB1a allele was 88.0°C (SD = 0.37°C), with a range of 87.4 to 88.7°C. For the porB1b allele, the mean Tm was 89.9°C (SD = 0.49°C), with a range of 88.9 to 91.4°C. Consequently, the genogroup (porB1a or porB1b allele) of each individual isolate could be determined by real-time PCR amplification of the porB gene.
Pyrosequencing.
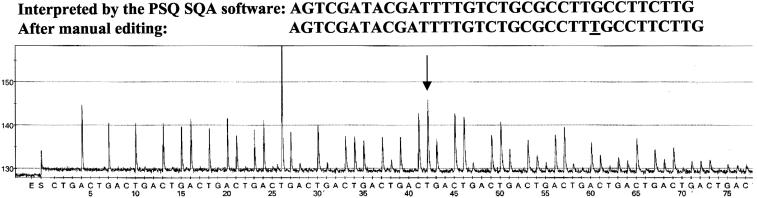
Real-time PCR amplification followed by sequence analysis by using pyrosequencing technology for the six highly polymorphic segments of the porB gene was performed without technical obstacles. Normally, pyrosequencing allowed rapid (approximately 1.5 h) determination of 96 sequences with 20 to 65 correct nucleotides each by means of automatic pyrogram analysis included in the software. Occasionally, manual editing of the obtained sequence was necessary. For porB gene segments encoding loops 1, 4, 6, and 8 and loops 6, 7, and 8 of the PorB1a and PorB1b proteins, respectively, the entire sequences could be determined by using only one of the DNA strands and, consequently, one sequencing primer in the pyrosequencing. The software-interpreted results of the pyrosequencing were in concordance with the conventional Sanger sequencing, with the exception of some uncertainties concerning the correct number of identical nucleotides in homopolymeric segments and some other minor discrepancies presumably due to nonsynchronized extension of some sequences, which limited the length of obtained reliable and correct sequences. The pyrogram of each analysis was reproducible, and a pyrogram of the sequence analysis of the loop 8 segment of the porB1b allele, which also illustrates the need of manual editing for homopolymeric segments in some cases, is shown in Fig. 3.
FIG. 3.
Pyrogram of the sequence analysis of the loop 8 segment of the porB1b allele (sequence variant 1, reverse strand), encoding surface-exposed amino acid loop 8 of the mature PorB1b protein (29), in N. gonorrhoeae. The nucleotide sequences of the entire loop 8 segment, as interpreted by the PSQ SQA (version 2.0) software and after manual editing are shown above the pyrogram. The discrepancy is indicated by underlining in the manually edited sequence and by an arrow in the pyrogram. The cyclic dispensation order of the deoxynucleoside triphosphates is indicated below the pyrogram.
Pyrosequencing of six highly polymorphic segments of the porB1a alleles from 22 N. gonorrhoeae strains and subsequent genovar determination.
The 22 PorB1a isolates comprised 22 unique porB1a gene sequences, with 67 polymorphic nucleotide sites (of 924) in the multiple-sequence alignment, in accordance with previous studies using conventional Sanger sequencing (27, 28). The number of polymorphic sites in the six highly polymorphic segments varied from 4 to 13 (ratios of 7.8 to 20.5 per 100 sites), and the locations of the sites for all sequence variants are shown in Fig. 1. The majority of the polymorphic sites were distributed in the sequences encoding the apexes of the amino acid loops of the PorB1a proteins (29). The polymorphism was caused by single-nucleotide substitutions and clustered substitutions.
The numbers of sequence variants identified in the six highly polymorphic segments of the porB1a allele, based on conventional sequencing (27, 28) and mostly confirmed by pyrosequencing, were 11 (segment encoding loop 1), 7 (loop 2), 6 (loop 3), 5 (loop 4), 5 (loop 6), and 6 (loop 8) (Fig. 1). Consequently, the number of sequence variants ranged from 5 to 11, with a mean of 6.7 per segment (Fig. 1). Thus, theoretically, the number of sequence variants in the highly polymorphic segments of the porB1a allele would allow >6.9 × 104 different porB1a genovars to be distinguished.
Among the porB1a isolates (n = 22) comprising nine different serovars and one nonserotypeable serovar, 22 unique genovars were assigned (Table 2).
Pyrosequencing of six highly polymorphic segments of the porB1b alleles from 65 N. gonorrhoeae strains and subsequent genovar determination.
The 65 porB1b isolates comprised 65 unique porB1b gene sequences, with 145 polymorphic nucleotide sites (of 999) in the unambiguous multiple-sequence alignment, in accordance with previous studies using conventional Sanger sequencing (27, 28). The number of polymorphic sites in the six highly polymorphic segments varied from 8 to 31 (ratios of 20.5 to 38.3 per 100 sites), and the locations of the sites for all sequence variants are shown in Fig. 2. Most of the polymorphic sites were distributed in the sequences encoding the apexes of the amino acid loops of PorB1b (29). The polymorphism was due to single-nucleotide substitutions, clustered substitutions, and indels.
The numbers of sequence variants identified in the six highly polymorphic segments of the porB1b allele, based on conventional sequencing (27, 28) and mostly confirmed by pyrosequencing, were 18 (segment encoding loop 1), 22 (loop 3), 39 (loop 5), 17 (loop 6), 13 (loop 7), and 8 (loop 8) (Fig. 2). Accordingly, the number of sequence variants ranged from 8 to 39, with a mean of 19.5 per segment (Fig. 2). Thus, theoretically, the number of sequence variants in the highly polymorphic segments of the porB1b allele would allow >2.7 × 107 different porB1b genovars to be discriminated.
Among the porB1b isolates (n = 65) comprising 19 different serovars, 64 distinguishable genovars were identified (Table 2).
DISCUSSION
In the present study, pyrosequencing technology was successfully applied for rapid, highly discriminating, and high-throughput molecular typing of N. gonorrhoeae isolates, with subsequent genovar determination, based on six highly polymorphic porB gene segments. This strategy included a real-time PCR amplification of the entire porB gene in order to determine the genogroup (porB1a or porB1b allele) of the isolates prior to the DNA sequencing. The porB1a allele was smaller (57 to 69 bp) than the porB1b allele and therefore had a lower Tm (a difference of 1.9°C in the mean Tms of the alleles). Consequently, the genogroup (porB1a or porB1b allele) of the individual isolate could be determined by real-time PCR amplification of the porB gene in all cases. However, an exactly defined range of the Tm for each allele is difficult to predict due to minor intra- and interassay variabilities in the Tm of the same allele, presumably dependent on the amounts of DNA template in the PCRs (data not shown). In addition, intra-allelic variations in the G+C contents of both the porB1a and porB1b alleles, as well as in the length of the porB1b allele, influence the Tms.
The present sequencing results (Fig. 1 and 2) provide evidence of a high discriminatory ability, practically the same as for sequencing the entire porB gene (27), and excellent typeability (all isolates were typeable) (Table 2). By using real-time PCR and pyrosequencing technology for DNA sequencing of polymorphic segments of the porB gene, rapid sequence data that are objective, portable for comparison between laboratories, and reproducible for epidemiological characterization of N. gonorrhoeae isolates are generated. This molecular strategy also continuously identifies new sequence variants in the highly polymorphic segments of the porB gene, reveals new genovars, and can be optimized to amplify the porB gene from clinical samples without culturing of the bacteria. By creating a database that comprises all sequence variants in each polymorphic segment, sequence variant numbers can be derived and isolates can be automatically designated as being different genovars.
Depending on the level of discrimination desired, the number of highly polymorphic segments of the porB gene analyzed can most likely be reduced in the future, and the nomenclature of the genovars can be simplified. Laboratories lacking access to pyrosequencing technology and using conventional Sanger sequencing could also adopt the same nomenclature of sequence variants and genovars. These laboratories have the option of analyzing the entire porB gene, but perhaps the most appealing alternative would be to sequence a fragment of approximately 400 to 600 bp of the porB gene, spanning, for instance, loops 1 to 4 of the porB1a allele and loops 3 to 7 of the porB1b allele, by using only one sequencing primer. Subsequently, sequence variants of some of the different highly polymorphic segments and genovars could be identified. However, pyrosequencing would seem to be the more convenient alternative.
Overall, sequence analysis by using high-throughput pyrosequencing technology is accurate and reproducible and can be almost fully automated. The technology is also less expensive, time-consuming, and labor-intensive, as well as easier to perform, than conventional Sanger sequencing. Disadvantages of the pyrosequencing technology are short read lengths (which is partly compensated for by the possibility of reading from the first base), the uncertainty in determining the correct number of incorporated identical nucleotides in homopolymeric segments, and the occasional occurrence of nonsynchronized extension of some sequences due to minus or plus frameshifting, which limits the length of obtained reliable and correct sequences (19, 20, 21). These areas need to be further developed. In the present study, homopolymers of 3 to 7 nucleotides in several of the loop segments were identified for some of the isolates, making it difficult for the software to interpret the correct number of incorporated identical nucleotides. This inherent problem in pyrosequencing technology is due to the nonlinear light response, which is not completely compensated for by the software algorithms, following incorporation of more than three or four identical nucleotides. However, the effect is less pronounced for G and C homopolymeric regions. The homopolymers may also cause nonsynchronized extension of the sequences due to insufficient completion of the homopolymers in some DNA strands, i.e., minus frameshifting, that can subsequently result in uncertain or erroneous interpretation of the pyrograms (19, 20, 21). Nevertheless, a sequence-directed dispensation order applied to conserved nucleotides preceding the polymorphic nucleotide positions, in combination with cyclic dispensation of the deoxynucleoside triphosphates (20 × CTGA) thereafter, use of reference peaks, and manual editing after visual inspection of the pyrograms, enabled the correct number of nucleotides in homopolymers to be revealed in most cases. A pyrogram of the sequence analysis of the loop 8 segment of the porB1b allele before and after manual editing is shown in Fig. 3. The method may also be further optimized by using sequencing primers with 3′ ends that partly overlap the homopolymers of interest.
The widely used serovar determination for epidemiological characterization of N. gonorrhoeae has important limitations (10, 12, 13, 17, 24, 27, 28). In addition, for several of the MAbs used in the serovar determination, the precise amino acid residues of PorB that are critical for single-MAb reactivity have been difficult to identify (1, 3, 4, 5, 16, 27). Consequently, a rapid and stable molecular genetic method for routine use in the characterization of N. gonorrhoeae, and which overcomes the limitations of serovar determination, is definitely needed. However, the prospects of developing a genetic typing system comprising a completely congruent translation of the serovar determination seem to be limited.
In conclusion, the present study illustrates for the first time how pyrosequencing technology, strictly applied to highly polymorphic segments of the porB gene, can be used for molecular typing of N. gonorrhoeae. This paves the way for rapid molecular epidemiological characterization of N. gonorrhoeae isolates based on the porB gene, coding for the PorB protein, which is internationally established and routinely used as a major phenotypic antigen (serovar determination). The genetic variant (genovar) determination of N. gonorrhoeae isolates is a concept which, because of its simplicity, high discriminatory ability, portability, and reproducibility, complements and might eventually replace current internationally established major phenotypic antigen (serovar) determinations in routine use for epidemiological typing.
Acknowledgments
The present study was supported by grants from the Research Committee of Örebro County, the Örebro University Hospital Research Foundation, and the National Institute for Public Health, Sweden.
REFERENCES
- 1.Butt, N. J., M. Virji, F. Vayreda, P. R. Lambden, and J. E. Heckels. 1990. Gonococcal outer-membrane protein PIB: comparative sequence analysis and localization of epitopes which are recognized by type-specific and cross-reacting monoclonal antibodies. J. Gen. Microbiol. 136:2165-2172. [DOI] [PubMed] [Google Scholar]
- 2.Carbonetti, N. H., and P. F. Sparling. 1987. Molecular cloning and characterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 84:9084-9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow, V. T. K., Q. C. Lau, and C. L. Poh. 1994. Mapping of serovar-specific monoclonal antibody epitopes by DNA and amino acid sequence analysis of Neisseria gonorrhoeae outer membrane protein IB strains. Immun. Infect. Dis. 4:202-206. [Google Scholar]
- 4.Cooke, S. J., K. Jolley, C. A. Ison, H. Young, and J. E. Heckels. 1998. Naturally occurring isolates of Neisseria gonorrhoeae which display anomalous serovar properties, express PIA/PIB hybrid porins, deletions in PIB or novel PIA molecules. FEMS Microbiol. Lett. 162:75-82. [DOI] [PubMed] [Google Scholar]
- 5.Cooke, S. J., H. de la Paz, C. L. Poh, C. A. Ison, and J. E. Heckels. 1997. Variation within serovars of Neisseria gonorrhoeae detected by structural analysis of outer-membrane protein PIB and by pulsed-field gel electrophoresis. Microbiology 143:1415-1422. [DOI] [PubMed] [Google Scholar]
- 6.Derrick, J. P., R. Urwin, J. Suker, I. M. Feavers, and M. C. J. Maiden. 1999. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect. Immun. 67:2406-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fudyk, T. C., I. W. Maclean, N. J. Simonsen, E. N. Njagi, J. Kimani, R. C. Brunham, and F. A. Plummer. 1999. Genetic diversity and mosaicism at the por locus of Neisseria gonorrhoeae. J. Bacteriol. 181:5591-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerbase, A. C., J. T. Rowley, D. H. Heymann, S. F. Berkley, and P. Piot. 1998. Global prevalence and incidence estimates of selected curable STDs. Sex. Transm. Infect. 74(Suppl. 1):S12-S16. [PubMed] [Google Scholar]
- 9.Gill, M. J., J. Jayamohan, M. P. A. Lessing, and C. A. Ison. 1994. Naturally occurring PIA/PIB hybrids of Neisseria gonorrhoeae. FEMS Microbiol. Lett. 119:161-166. [DOI] [PubMed] [Google Scholar]
- 10.Gill, M. J. 1991. Serotyping Neisseria gonorrhoeae: a report of the Fourth International Workshop. Genitourin. Med. 67:53-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotschlich, E. C., M. E. Seiff, M. S. Blake, and M. Koomey. 1987. Porin protein of Neisseria gonorrhoeae: cloning and gene structure. Proc. Natl. Acad. Sci. USA 84:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs, M. M., T. M. Alcorn, R. H. Davis, W. Fischer, J. C. Thomas, I. Martin, C. Ison, P. F. Sparling, and M. S. Cohen. 1999. Molecular typing of Neisseria gonorrhoeae causing repeated infections: evolution of porin during passage within a community. J. Infect. Dis. 179:371-381. [DOI] [PubMed] [Google Scholar]
- 13.Ison, C. A., L. Whitaker, and A. Renton. 1992. Concordance of auxotype/serovar classes of Neisseria gonorrhoeae between sexual contacts. Epidemiol. Infect. 109:265-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapp, J. S., M. R. Tam, R. C. Nowinski, K. K. Holmes, and E. G. Sandström. 1984. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J. Infect. Dis. 150:44-48. [DOI] [PubMed] [Google Scholar]
- 15.McKnew, D. L., F. Lynn, J. M. Zenilman, and M. C. Bash. 2003. Porin variation among clinical isolates of Neisseria gonorrhoeae over a 10-year period, as determined by por variable region typing. J. Infect. Dis. 187:1213-1222. [DOI] [PubMed] [Google Scholar]
- 16.Mee, B. J., H. Thomas, S. J. Cooke, P. R. Lambden, and J. E. Heckels. 1993. Structural comparison and epitope analysis of outer-membrane protein PIA from strains of Neisseria gonorrhoeae with differing serovars specificities. J. Gen. Microbiol. 139:2613-2620. [DOI] [PubMed] [Google Scholar]
- 17.Ng, L.-K., M. Carballo, and J.-A. R. Dillon. 1995. Differentiation of Neisseria gonorrhoeae isolates requiring proline, citrulline, and uracil by plasmid content, serotyping, and pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:1039-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Rourke, M., C. A. Ison, A. M. Renton, and B. G. Spratt. 1995. Opa-typing: a high-resolution tool for studying the epidemiology of gonorrhoea. Mol. Microbiol. 17:865-875. [DOI] [PubMed] [Google Scholar]
- 19.Ronaghi, M. 2001. Pyrosequencing sheds light on DNA sequencing. Genome Res. 11:3-11. [DOI] [PubMed] [Google Scholar]
- 20.Ronaghi, M., M. Nygren, J. Lundeberg, and P. Nyrén. 1999. Analyses of secondary structures in DNA by sequencing. Anal. Biochem. 267:65-71. [DOI] [PubMed] [Google Scholar]
- 21.Ronaghi, M., M. Uhlén, and P. Nyrén. 1998. A sequencing method based on real-time pyrophosphate. Science 281:363-365. [DOI] [PubMed] [Google Scholar]
- 22.Sandström, E., and D. Danielsson. 1980. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Pathol. Microbiol. Scand. Sect. B 88:27-38. [DOI] [PubMed] [Google Scholar]
- 23.Spaargaren, J., J. Stoof, H. Fennema, R. Coutinho, and P. Savelkoul. 2001. Amplified fragment length polymorphism fingerprinting for identification of a core group of Neisseria gonorrhoeae transmitters in the population attending a clinic for treatment of sexually transmitted diseases in Amsterdam, The Netherlands. J. Clin. Microbiol. 39:2335-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tam, M. R., T. M. Buchanan, E. G. Sandström, K. K. Holmes, J. S. Knapp, A. W. Siadak, and R. C. Nowinski. 1982. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect. Immun. 36:1042-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trees, D. L., A. J. Schultz, and J. S. Knapp. 2000. Use of the neisserial lipoprotein (Lip) for subtyping Neisseria gonorrhoeae. J. Clin. Microbiol. 38:2914-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unemo, M., T. Berglund, P. Olcén, and H. Fredlund. 2002. Pulsed-field gel electrophoresis as an epidemiologic tool for Neisseria gonorrhoeae; identification of clusters within serovars. Sex. Transm. Dis. 29:25-31. [DOI] [PubMed] [Google Scholar]
- 27.Unemo, M., P. Olcén, J. Albert, and H. Fredlund. 2003. Comparison of serologic and genetic porB-based typing of Neisseria gonorrhoeae: consequences for future characterization. J. Clin. Microbiol. 41:4141-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unemo, M., P. Olcén, T. Berglund, J. Albert, and H. Fredlund. 2002. Molecular epidemiology of Neisseria gonorrhoeae: sequence analysis of the porB gene confirms presence of two circulating strains. J. Clin. Microbiol. 40:3741-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Ley, P., J. E. Heckels, M. Virji, P. Hoogerhout, and J. T. Poolman. 1991. Topology of outer membrane porins in pathogenic Neisseria spp. Infect. Immun. 59:2963-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Looveren, M., C. A. Ison, M. Ieven, P. Vandamme, I. M. Martin, K. Vermeulen, A. Renton, and H. Goossens. 1999. Evaluation of the discriminatory power of typing methods for Neisseria gonorrhoeae. J. Clin. Microbiol. 37:2183-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viscidi, R. P., J. C. Demma, J. Gu, and J. Zenilman. 2000. Comparison of sequencing of the por gene and typing of the opa gene for discrimination of Neisseria gonorrhoeae strains from sexual contacts. J. Clin. Microbiol. 38:4430-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viscidi, R. P., and J. C. Demma. 2003. Genetic diversity of Neisseria gonorrhoeae housekeeping genes. J. Clin. Microbiol. 41:197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zak, K., J.-L. Diaz, D. Jackson, and J. E. Heckels. 1984. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J. Infect. Dis. 149:166-174. [DOI] [PubMed] [Google Scholar]