Abstract
A patient presented with loss of consciousness and conversion. During an exercise test, catecholaminergic polymorphic ventricular tachycardia (CPVT) resulted in cardiac arrest. He started taking medication (a beta-blocker and flecainide) and an implantable cardioverter defibrillator (ICD) was inserted, but the ventricular tachycardia did not resolve. Left cardiac sympathetic denervation (LCSD) was then performed under general anesthesia, and the patient was discharged on the second postoperative day without complications. One month after the operation, no shock had been administered by the ICD, and an exercise stress test did not induce ventricular tachycardia. Although beta-blockers are the gold standard of therapy in patients with CPVT, thoracoscopic LCSD is safe and can be an effective alternative treatment option for patients with intractable CPVT.
Keywords: Catecholaminergic polymorphic ventricular tachycardia, Left cardiac sympathetic denervation
CASE REPORT
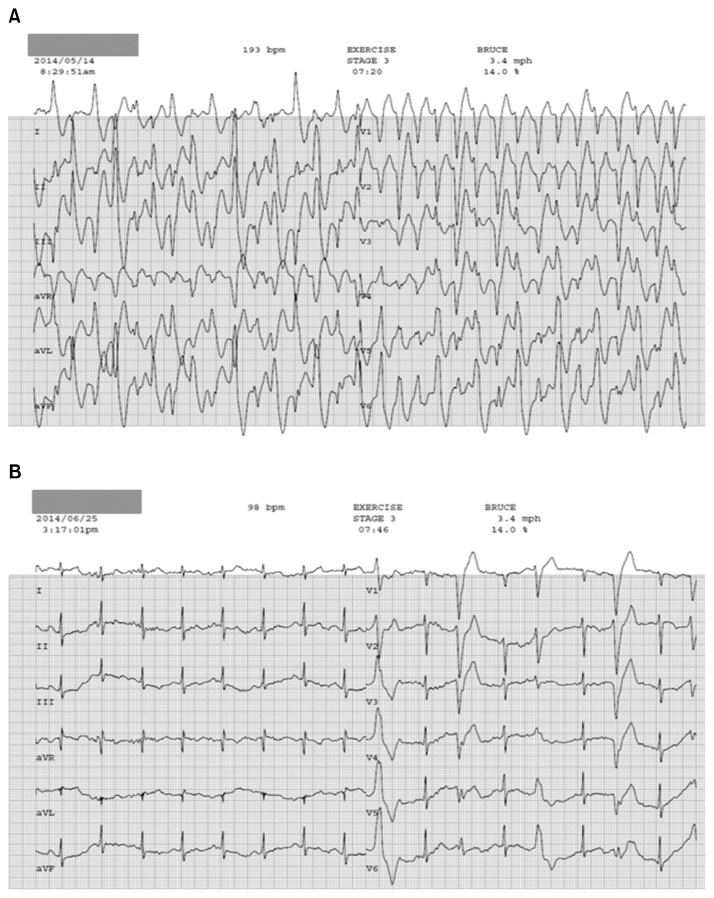
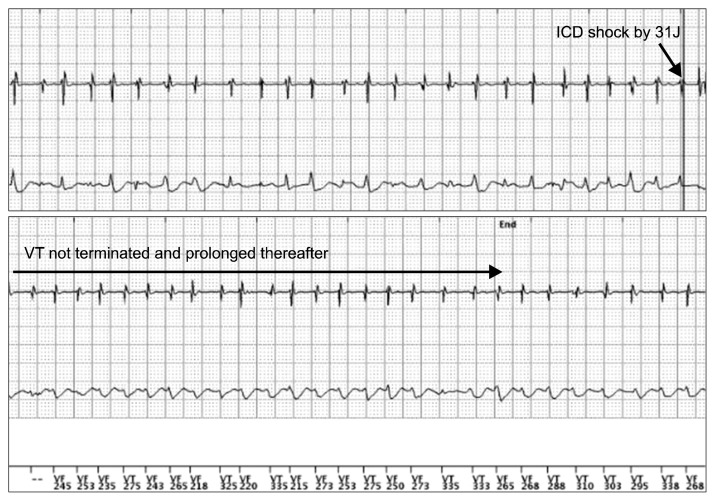
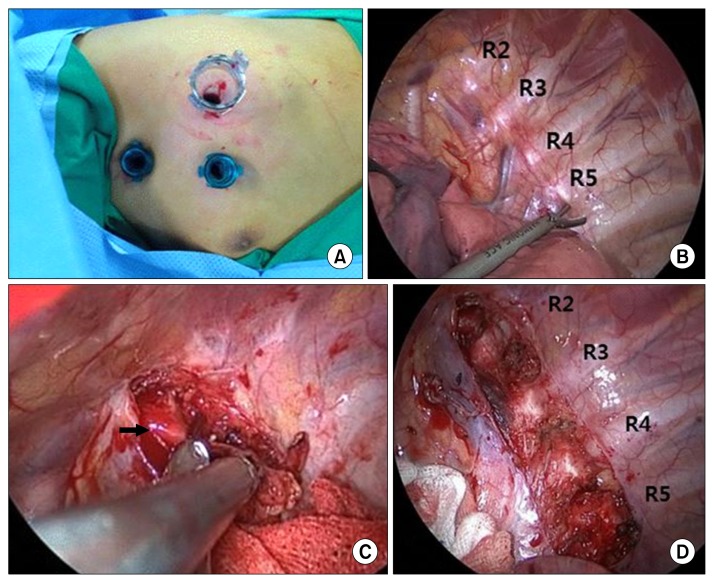
An 8-year-old patient presented with loss of consciousness and conversion. He had an unremarkable medical history and no family history of epilepsy or sudden cardiac death. Brain magnetic resonance imaging, echocardiography (ECG), and 24-hour Holter ECG monitoring revealed no abnormal findings. An electroencephalogram led us to suspect a partial seizure disorder originating from the frontal area. Subsequently, the patient experienced recurrent syncopal episodes despite taking antiepileptic medication for four years and the absence of epileptic discharges on follow-up electroencephalograms. At 12 years of age, his cardiac function was re-evaluated because he reported chest discomfort before syncopal episodes. Echocardiography, 24-hour Holter ECG monitoring, and head-up tilt testing revealed normal findings. However, polymorphic ventricular tachycardia was induced during an exercise stress test (Fig. 1). These findings were consistent with catecholaminergic polymorphic ventricular tachycardia (CPVT), and the patient was started on a beta-blocker. At the age of 15 years, while on the beta-blocker therapy (metoprolol, 200 mg/day), the patient went into cardiac arrest with initial ventricular fibrillation and underwent defibrillation with an automated external defibrillator. An implantable cardioverter defibrillator (ICD) was inserted, and flecainide was added to his medication regimen. However, after ICD insertion, the patient developed recurrent hemodynamically stable ventricular tachycardia (VT), and six shocks were delivered over the course of six months. These episodes of VT were not terminated by shocks from the ICD, but instead persisted for some time, eventually terminating spontaneously (Fig. 2). He was referred to the general thoracic department for left cardiac sympathetic denervation (LCSD). The operation was performed under general anesthesia. Intubation was carried out using a dual-lumen endotracheal tube for one-lung ventilation. The patient was placed in the right lateral decubitus position. A 10-mm 30° thoracoscope was used. A 12-mm trocar was inserted on the sixth midaxillary line for the camera, and two 5-mm trocars were inserted on the third and the fifth anterior axillary lines for the endoscopic instruments. The sympathetic chain was identified from T1 to T5 and resected, including the lateral nerve branches, using a harmonic scalpel with sparing of the stellate ganglion (Fig. 3). A 20-Fr chest tube was placed in the left pleural cavity at the end of the operation and was removed on the first postoperative day. The patient was discharged on the second postoperative day without complications and maintained on metoprolol (300 mg/day) and flecainide (100 mg/day). As of one month after the operation, no shocks had been administered by the ICD, and an exercise stress test did not induce VT (Fig. 1).
Fig. 1.
The results of exercise stress tests differed significantly (A) before and (B) after left cardiac sympathetic denervation. (A) Bidirectional polymorphic ventricular tachycardia consistent with typical catecholaminergic polymorphic ventricular tachycardia was induced during the preoperative exercise stress test. (B) Occasional premature ventricular beats without ventricular tachycardia were recorded during the postoperative exercise stress test.
Fig. 2.
The recurrent hemodynamically stable VT that was observed preoperatively could not be terminated by ICD shocks, and instead eventually terminated spontaneously. VT, ventricular tachycardia; ICD, implantable cardioverter defibrillator.
Fig. 3.
Intraoperative findings during left cardiac sympathetic denervation in a patient with catecholaminergic polymorphic ventricular tachycardia. (A) Thoracoscopic port placement. (B–D) Intraoperative findings. The sympathetic chain was identified from T1 to T5 and resected (including the lateral nerve branches) using a harmonic scalpel, while sparing the stellate ganglion (arrow).
DISCUSSION
CPVT was first described in 1975 [1], and is characterized by bidirectional or polymorphic ventricular tachycardia induced by increased sympathetic activity secondary to physical exercise or emotional stress [2]. Genetic studies in the past decade have revealed mutations in two genes (RYR2, CASQ2) [3] that are known to be associated with abnormal intracellular calcium handling and cause adrenergically mediated polymorphic ventricular tachyarrhythmias by delayed after-depolarizations and triggered activity [4].
CPVT presents clinically with syncope, aborted cardiac arrest, or sudden cardiac death, all of which are triggered by physical or emotional stress. Moreover, symptoms often develop at a young age. In some cases, sudden death is the first manifestation [5]. Therefore, the accurate and early diagnosis of CPVT is critical for achieving the best possible clinical results in these patients. Reproducibility of the typical arrhythmia by provocative testing, such as an exercise stress test or an isoproterenol infusion test, is important in establishing the diagnosis [5]. However, CPVT-related syncopal episodes may include seizures involving urinary or fecal incontinence, and usually present with normal ECG findings. Therefore, the average delay in diagnosis has been reported to be as high as 2.0±0.8 years after the manifestation of the first symptoms [6]. Our patient’s condition was initially mis-diagnosed as epilepsy, and antiepileptic medication was administered for four years, which resulted in a delayed diagnosis of CPVT.
Beta-blockers are the first-line therapy for CPVT, and they are known to provide most patients with sufficient protection. Since fatal ventricular storms can be initiated by shocks from an ICD that trigger subsequent catecholamine release [7], ICD placement should only be considered in patients who have been resuscitated after cardiac arrest and who have experienced recurring syncope or sustained VT despite beta-blocker therapy [8]. Other pharmacologic treatments (sodium channel blockers, amiodarone, verapamil) have been shown to have an additive benefit in combination with beta-blockers [5]. LCSD can be considered when ventricular arrhythmias remain uncontrolled by beta-blockers and other additive drug therapies. After Wilde et al. [2] first reported that LCSD is an effective therapy for CPVT, several other researchers have confirmed that LCSD is highly effective and safe in severely affected CPVT patients [9]. Moreover, Hofferberth et al. [10] presented the results of thoracoscopic LCSD in 24 patients with life-threatening ventricular arrhythmias, including nine patients with CPVT. In that study, 16 of the 22 patients who underwent long-term follow-up experienced a marked reduction in arrhythmic events, and 12 patients were completely free of arrhythmia. Moreover, no major complications and only three minor complications (two cases of pneumothorax and one case of Harlequin syndrome) were reported. The stellate ganglion was saved in all but one patient. Horner’s syndrome was not reported in any patients.
Schwartz [11] recently reviewed the experimental evidence pertaining to LCSD effectiveness. The author had previously reported that left stellectomy in dogs, which is the equivalent of LCSD in humans, raised the ventricular fibrillation threshold, whereas right stellectomy lowered the ventricular fibrillation threshold. Other studies have reported the following findings: (1) left ventricular contractility is not reduced after left stellectomy; (2) no evidence indicates that LCSD induces post-denervation supersensitivity; (3) left stellectomy increases reactive hyperemia via the coronary artery; (4) after LCSD, the heart rate increases more than usual; and (5) LCSD increases the activity of the cardiac vagal efferent nerve. Clinical evidence has indicated that LCSD may have a role in treating patients with channelopathies such as long QT syndrome, previous myocardial infarction, and CPVT [11].
In summary, CPVT is a genetic disorder involving intracellular calcium handling that leads to adrenergically-mediated life threatening ventricular tachyarrhythmias. Although beta-blockers are the gold standard of therapy in patients with CPVT, thoracoscopic LCSD is safe and can be an effective alternative treatment option. LCSD is a particularly important option for patients for whom beta-blockers are contraindicated and in patients who experience recurrent ICD shocks despite optimal treatment with medication.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Reid DS, Tynan M, Braidwood L, Fitzgerald GR. Bidirectional tachycardia in a child: a study using His bundle electrography. Br Heart J. 1975;37:339–44. doi: 10.1136/hrt.37.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilde AA, Bhuiyan ZA, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–9. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 3.Laitinen PJ, Brown KM, Piippo K, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–90. doi: 10.1161/01.CIR.103.4.485. [DOI] [PubMed] [Google Scholar]
- 4.Liu N, Colombi B, Memmi M, et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ Res. 2006;99:292–8. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 5.Napolitano C, Priori SG. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:675–8. doi: 10.1016/j.hrthm.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 6.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.CIR.0000020013.73106.D8. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed U, Gollob MH, Gow RM, Krahn AD. Sudden cardiac death despite an implantable cardioverter-defibrillator in a young female with catecholaminergic ventricular tachycardia. Heart Rhythm. 2006;3:1486–9. doi: 10.1016/j.hrthm.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5:e1–62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Atallah J, Fynn-Thompson F, Cecchin F, DiBardino DJ, Walsh EP, Berul CI. Video-assisted thoracoscopic cardiac denervation: a potential novel therapeutic option for children with intractable ventricular arrhythmias. Ann Thorac Surg. 2008;86:1620–5. doi: 10.1016/j.athoracsur.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Hofferberth SC, Cecchin F, Loberman D, Fynn-Thompson F. Left thoracoscopic sympathectomy for cardiac denervation in patients with life-threatening ventricular arrhythmias. J Thorac Cardiovasc Surg. 2014;147:404–9. doi: 10.1016/j.jtcvs.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz PJ. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat Rev Cardiol. 2014;11:346–53. doi: 10.1038/nrcardio.2014.19. [DOI] [PubMed] [Google Scholar]