Abstract
Venoarterial extracorporeal membrane oxygenation (VA ECMO) is widely used in patients with cardiogenic shock. Insufficient decompression of the left ventricle (LV) is considered a major factor preventing adequate LV recovery. A 40-year-old male was diagnosed with acute myocardial infarction, and revascularization was performed using percutaneous stenting. However, cardiogenic shock occurred, and VA ECMO was initiated. Severe LV failure developed, and percutaneous transaortic catheter venting (TACV) was incorporated into the venous circuit of VA ECMO under transthoracic echocardiography guidance. The patient was successfully weaned from VA ECMO. Percutaneous TACV is an effective, relatively noninvasive, and rapid method of LV decompression in patients undergoing VA ECMO.
Keywords: Extracorporeal membrane oxygenation, Cardiogenic shock
CASE REPORT
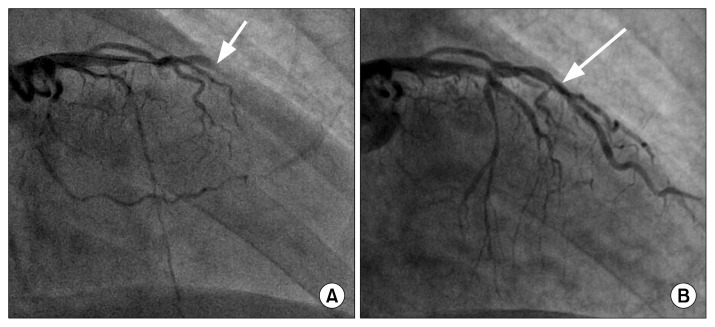
A previously healthy 40-year-old male patient visited Emergency Department of Samsung Changwon Hospital due to dyspnea and sudden-onset chest pain. He was a current smoker, with a 20-pack-year history and no history of diabetes, hypertension, or dyslipidemia. Despite being alert, he was hypotensive, with a systolic blood pressure of approximately 60 mmHg. A chest X-ray was normal. A 12-lead electrocardiogram (EKG) study showed diffuse ST segment elevation in the limb and precordial leads (III, aVF, V4–V5), suggesting anterolateral and inferior wall myocardial infarction. An emergency coronary angiogram revealed multi-vessel disease, including total occlusion of the middle segment of the left anterior descending coronary artery (Fig. 1A). Although percutaneous stenting to the middle segment of the left anterior descending coronary artery was successful (Fig. 1B), the patient’s dyspnea was aggravated, with decreased O2 saturation (66%) that was not improved by immediate intubation and mechanical ventilation.
Fig. 1.
(A) Coronary angiography showing total occlusion of the middle segment of the left anterior descending coronary artery (white arrow). (B) Coronary angiography showing successful coronary stent insertion to the middle segment of the left anterior descending coronary artery (white arrow).
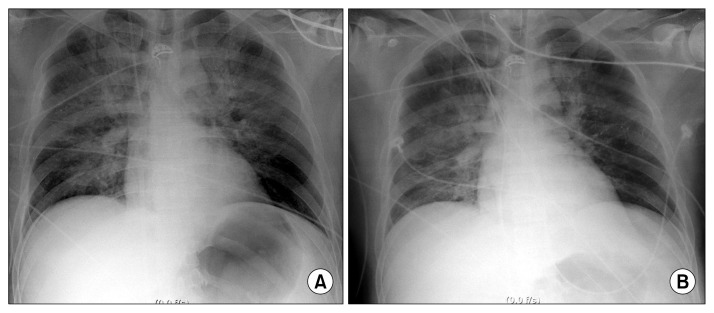
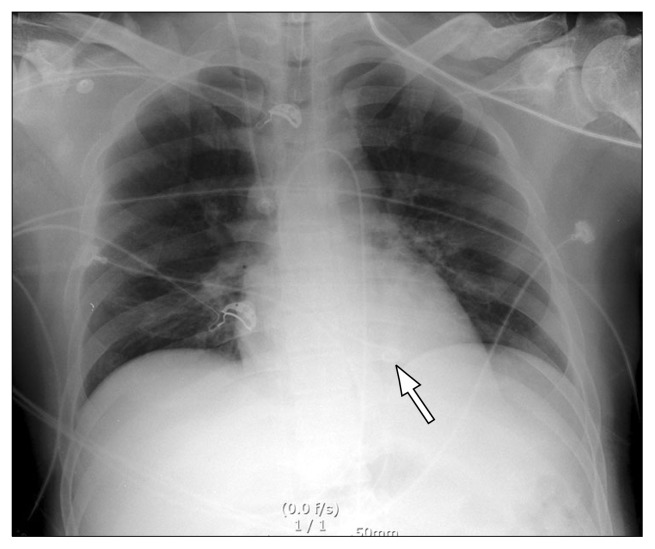
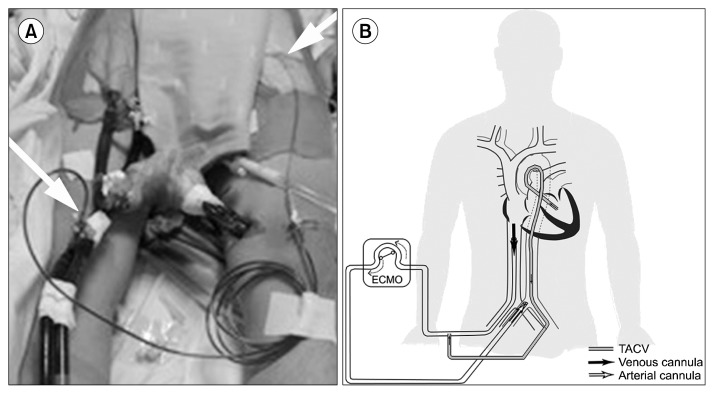
Based on a chest X-ray showing pulmonary edema (Fig. 2A) and a left ventricular ejection fraction (LVEF) estimated at 20% by transthoracic echocardiography (TTE), cardiogenic shock was suspected and we decided to initiate venoarterial extracorporeal membrane oxygenation (VA ECMO). A 21-Fr inflow catheter (DLP; Medtronic Inc., Minneapolis, MN, USA) was inserted via the left femoral vein, and a 16-Fr outflow catheter (RMI; Edwards Lifesciences LLC, Irvine, CA, USA) was inserted via the right femoral artery (fraction of inspired oxygen=1.0, sweep gas=6 L/min, flow=3.62 L/min, RPM=3,725) (Fig. 2B). TTE performed six hours after initiating VA ECMO revealed a LVEF of 26%, suggesting persistent severe left ventricular systolic dysfunction. After 12 hours on VA ECMO, the arterial blood pressure wave had nearly disappeared, with a pulse pressure less than 10 mmHg, and the EKG was flat, suggesting asystole of the left ventricle (LV). Cardiac massage was initiated to decompress the LV, and a subsequent TTE showed severe LV failure, necessitating immediate decompression of the LV. Under TTE guidance, we successfully inserted a LV vent catheter (5-Fr pigtail catheter; PIG Perfoma; Merit Medical, South Jordan, UT, USA) directly into the LV cavity across the aortic valve (Fig. 3). Percutaneous transaortic catheter venting (TACV) was incorporated into the venous circuit of ECMO (Fig. 4A, B). The procedure was performed percutaneously, using a transfemoral approach. There were no complications related to the procedure. After the procedure, the patient’s arterial blood pressure was elevated from 57/54 mmHg to 99/67 mmHg and the arterial blood pressure waveform was restored. The heart rhythm on EKG monitoring was also restored. TTE performed after the procedure demonstrated two findings showing improved LV function. First, the left ventricular end-diastolic dimension decreased from 38 to 33 mm, suggesting preload reduction. Second, the LVEF was increased (30%) compared to the previous reading of 26%.
Fig. 2.
(A) Chest radiograph showing severe pulmonary edema after coronary stent insertion to the middle segment of the left anterior descending coronary artery. (B) Chest radiograph showing improved pulmonary edema after the application of venoarterial extracorporeal membrane oxygenation.
Fig. 3.
Chest radiograph illustrating percutaneous transaortic catheter venting (white arrow).
Fig. 4.
(A, B) Pictures showing percutaneous TACV incorporated into the venous circuit of ECMO (white arrow). ECMO, extracorporeal membrane oxygenation; TACV, transaortic catheter venting.
On hospital day 4, we decided to remove the LV vent catheter and considered weaning the patient from VA ECMO based on the presence of stable vital signs and the TTE data (left ventricular end-diastolic dimension and LVEF) presented above. An intra-aortic balloon pump (IABP) was inserted as a bridge therapy. In the Angioroom, ECMO was removed. On hospital day 6, the LVEF was measured as 58%, with IABP support. On hospital day 7, the patient’s hemodynamic values had further stabilized and the IABP was removed. On the same day, it was possible to wean the patient from the ventilator and extubate him. The patient was weaned from all vasoactive/inotropic agents on hospital day 9. On hospital day 10, the patient was transferred to the general ward, because his general condition had undergone significant improvement. On hospital day 15, he was discharged without complications.
DISCUSSION
VA ECMO is widely used in pediatric and adult patients with cardiogenic shock. However, some patients cannot be successfully weaned from VA ECMO support. Insufficient LV unloading is a main cause of unsuccessful LV recovery during VA ECMO [1]. Kurihara et al. [1] have suggested that TACV may be an adjunctive treatment to VA ECMO in patients with LV failure.
Dilatation of the LV may occur due to several factors: (1) the afterload induced by VA ECMO in cases of LV failure, (2) suboptimal venous return accompanying right heart recovery, (3) heavy collateral bronchial flow, and (4) aortic insufficiency [2]. The importance of left heart decompression during VA ECMO is generally recognized, but percutaneous drainage of the left heart is rarely reported and no consensus exists regarding the timing of LV decompression during VA ECMO.
In our hospital, we consider applying percutaneous TACV for LV decompression in patients who show either severe LV dysfunction (LVEF<25%) with persistent pulmonary edema or asystole of the LV with or without mitral insufficiency.
Fumagalli et al. [3] were the first to report draining blood from the LV in a patient on VA ECMO as a bridge to heart transplantation, using a percutaneously placed transaortic cannula that pumped directly into the femoral artery, normalizing the left heart filling pressure and resolving pulmonary edema [3]. Kang et al. [4] reported a case of successful percutaneous trans-septal left atrial drainage using a 28-Fr cannula. Nonetheless, left atrial drainage via septal puncture entails a risk of septal injury and/or a resultant left-to-right shunt. Furthermore, it is debatable whether atrial decompression or ventricular decompression is more effective, especially in case of severe distension with asystole of the LV. We are of the opinion that left atrial drainage can avoid pulmonary edema, but has no effect on LV decompression in the absence of mitral insufficiency in cases of LV asystole. The other method of LV decompression is the use of the Impella LP 2.5 device, as reported by Koeckert et al. [5]. The use of the Impella LP 2.5 device for LV decompression is an expensive technique. Although an IABP can decrease LV afterload, doing so has no direct effect on LV decompression. Despite the use of a relatively small catheter (5 Fr), immediate decompression was possible in our case within a few hours, suggesting that direct ventricular decompression may be a more effective and faster method.
In summary, our patient was successfully treated with percutaneous TACV, which is an effective, relatively non-invasive, and rapid method of left heart decompression in patients undergoing VA ECMO.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kurihara H, Kitamura M, Shibuya M, Tsuda Y, Endo M, Koyangi H. Effect of transaortic catheter venting on left ventricular function during venoarterial bypass. ASAIO J. 1997;43:M838–41. doi: 10.1097/00002480-199703000-00122. [DOI] [PubMed] [Google Scholar]
- 2.Chocron S, Perrotti A, Durst C, Aupecle B. Left ventricular venting through the right subclavian artery access during peripheral extracorporeal life support. Interact Cardiovasc Thorac Surg. 2013;17:187–9. doi: 10.1093/icvts/ivt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fumagalli R, Bombino M, Borelli M, et al. Percutaneous bridge to heart transplantation by venoarterial ECMO and transaortic left ventricular venting. Int J Artif Organs. 2004;27:410–3. doi: 10.1177/039139880402700510. [DOI] [PubMed] [Google Scholar]
- 4.Kang MH, Hahn JY, Gwon HC, et al. Percutaneous trans-septal left atrial drainage for decompression of the left heart in an adult patient during percutaneous cardiopulmonary support. Korean Circ J. 2011;41:402–4. doi: 10.4070/kcj.2011.41.7.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koeckert MS, Jorde UP, Naka Y, Moses JW, Takayama H. Impella LP 2.5 for left ventricular unloading during venoarterial extracorporeal membrane oxygenation support. J Card Surg. 2011;26:666–8. doi: 10.1111/j.1540-8191.2011.01338.x. [DOI] [PubMed] [Google Scholar]