Abstract
PURPOSE
We aimed to identify factors that might help differentiate phyllodes tumors from fibroadenomas among cases in which a fibroepithelial breast lesion was diagnosed from core needle biopsy (CNB) under imaging guidance.
MATERIALS AND METHODS
A retrospective review was performed on 213 lesions in 200 patients who had undergone both CNB and excisional biopsy during a four-year period between 2008 and 2011. The final pathology revealed 173 fibroadenomas and 40 phyllodes tumors. The data, including patient characteristics, clinical presentation, and mammography, ultrasonography (US), and pathology findings were analyzed.
RESULTS
Upon univariable analysis, the factors that significantly helped to identify phyllodes tumors consisted of the presenting symptoms (palpable mass or breast pain), increased size on clinical examination, hyperdense mass on mammogram, and the following three US features: heterogeneous echo, presence of round cysts within the mass, and presence of clefts within the mass. The pathologist’s suggestion of a phyllodes tumor was also helpful. The factors that remained statistically significant upon multivariable analysis consisted of symptoms of breast pain, the presence of clefts on US, the presence of round cysts on US and the pathologist’s favoring of phyllodes tumors from a CNB specimen.
CONCLUSION
A multidisciplinary approach was needed to distinguish phyllodes tumors from fibroadenomas in patients who had undergone CNB. US findings (clefts and round cysts), suggestive pathological diagnoses, and clinical symptoms were all useful for the decision to surgically remove the fibroepithelial lesions diagnosed from CNB.
Core needle biopsy (CNB) under imaging guidance is an accepted standard of care for the diagnosis of breast lesions, particularly those that are nonpalpable (1–4). This procedure is safe, cost-effective and minimally invasive compared with surgical excision (1).
In general, CNB allows for appropriate decision-making. Surgery could be obviated by a benign CNB pathology result. At times, however, CNB may provide only an inconclusive histopathology or may yield results associated with a potentially more worrisome pathology. Surgical excision may be needed in such circumstances.
Fibroadenoma is the most common lesion in the breast, occurring in 25% of asymptomatic women (5), and it is usually readily diagnosed via CNB. In the presence of increased stromal cellularity, however, it is less likely to be distinguishable from a phyllodes tumor (1–4). In such cases, the term “fibroepithelial lesion” is used (1–5).
The distinction between fibroadenomas and phyllodes tumors is clinically important. Fibroadenomas may be safely followed without further investigation. Even if an excisional biopsy is needed, the simple enucleation of a fibroadenoma is appropriate (1). In contrast, phyllodes tumors should not be managed by nonoperative means because they commonly and progressively enlarge. Moreover, wide excision of phyllodes tumors with adequate margins is essential to the prevention of local recurrence and to provide an accurate diagnosis as to whether it is benign, borderline or malignant (1–5). A CNB-diagnosed fibroepithelial lesion, therefore, provides the surgeon with the dilemma of whether to operate. Usually, surgical excision is chosen, and a considerable amount of normal breast tissue is sacrificed.
The purpose of the present study was to determine factors that might help differentiate phyllodes tumors from fibroadenomas among cases in which a fibroepithelial lesion was diagnosed from CNB under imaging guidance.
Materials and methods
The study was conducted with institutional review board approval. The authors performed a review of the medical and radiologic records from January 1st, 2008 to December 31st, 2011 of more than 3000 patients who had undergone image-guided CNB of their breast lesions at the breast diagnostic center of Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. There were 518 patients with CNB-diagnosed fibroepithelial lesions. Among them, 318 patients were excluded because no further excisional biopsy was performed for definite histopathologic diagnosis.
A total of 213 lesions from 200 patients who underwent subsequent excisional biopsy were examined in this study. The indications for surgical excision after CNB included the surgeon’s or patient’s preference, the suggestion of the pathologist, and any discordance between the imaging and pathologic findings. Patient characteristics, clinical presentation, mammography and ultrasonography (US) findings, CNB data, details of subsequent surgery, and pathology reports were reviewed.
Mammography was performed in the craniocaudal and mediolateral oblique views using a digital mammography machine (Selenia, Danbury, Connecticut, USA). Additional US was performed for all lesions by two US machines (iU22 Philips Ultrasound, Bothell, Washington, USA). The lesions were described and categorized using the relevant Breast Imaging Reporting and Data System (BIRADS) criteria of the American College of Radiology (6) based on the combination of mammography and US findings.
The biopsy guidance modality was chosen based on the type of lesion observed. If the lesion was clearly visible on US, then US guidance was used. However, if the lesion was invisible on US or observed as a microcalcification on the mammogram, stereotactic guidance was used. Of the 213 breast lesions, 208 (98%) underwent US-guided CNB, and five (2%) underwent stereotactic-guided CNB. The number of core specimens retrieved ranged from four to twenty-four cores.
Ultrasound-guided biopsy was performed with a 12–5 MHz linear array transducer (iU22 Phillips Ultrasound), a 13-gauge coaxial introducer needle, and a 14-gauge cutting needle (MDTech, Gainesville, Florida, USA) with a long-throw (22 mm). All needle biopsies were performed using an automated biopsy gun (Magnum, Bard Peripheral Technologies, Covington, Georgia, USA) with a freehand technique. Six core specimens were typically retrieved from each lesion. Fewer cores would be obtained if the lesions were small, if the patient reported pain, or if significant bleeding was observed.
Stereotactic-guided biopsies were performed with a dedicated core biopsy unit using an 11-gauge directional vacuum-assisted CNB instrument (Mammotome, Biopsys/Ethicon Endo-Surgery, Cincinnati, Ohio, USA), with the patient on a prone breast biopsy table (LORAD MultiCare Platinum, Danbury, Connecticut, USA). Twelve core specimens were routinely retrieved with this technique. All biopsies were performed by three dedicated breast-imaging radiologists with 10 to 15 years of experience.
The pathological reports of each CNB examination were retrieved for the present study. Associated personal and clinical history, age, menopausal status, date at first visit, symptoms and documented suspicions of phyllodes tumor made by clinicians and pathologists were recorded.
Lesions observed from the mammography were characterized as mass, calcification (macro- or microcalcification), combined mass with calcification or focal asymmetrical density. Breast composition, size, shape, margin and density of the lesion, as well as the visibility of the lesion on the mammogram, were also recorded. US images were reviewed for the number of lesions, bilateral or unilateral involvement, number of previous US studies, and evidence of any increasing sizes of lesions. If the lesion was a mass, the features recorded included the following: orientation, shape, margin, echo pattern and posterior acoustic features. Three specific US features were evaluated, as follows: heterogeneous internal echoes without cysts or clefts, internal round cystic spaces, and internal clefts.
Continuous and count variables (for example age, duration of symptoms, size, and number of lesions) were summarized as the mean, standard deviation, median, and range as appropriate. Categorical variables (such as breast density, mammographic results, and US characteristics) were summarized as counts and percentages. Continuous variables were tested for significant differences using an unpaired t test and Mann-Whitney test whenever appropriate. Categorical variables were tested using the chi-square test or Fisher’s exact test. The unit of analysis was the individual lesion, which was assumed to be statistically independent. Multiple logistic regression analysis was used to identify the independent factors associated with the presence of phyllodes tumors. All statistical analyses were performed using Stata version 12 (Stata Corp., College Station, Texas, USA) statistical software. Statistical significance is defined as a two-sided P value of 0.05 or less.
Results
Of the 213 lesions in the present study, 173 were histopathologically diagnosed as fibroadenomas (this group was composed of pure fibroadenoma, fibroadenoma with other proliferative lesions and a few lesions reported as fibrocystic change), and 40 were histopathologically diagnosed as phyllodes tumors.
The mean age of patients with fibroadenomas was 43.6±10.6 years; for patients with phyllodes tumors, 43.4±6.6 years. There was no significant age difference between the two groups (P = 0.903). Palpable masses were found in 95 of 173 fibroadenomas (55%) and in 32 of 40 phyllodes tumors (80%). Associated breast pain was found in 11 of 173 fibroadenomas (6%) and in four of 40 phyllodes tumors (10%). The presenting symptoms were significantly different between the two groups (P = 0.002).
The duration of the clinical symptoms (palpable mass or breast pain) was not significantly different between patients with fibroadenomas (median duration, 5 months; range, 1 day to 120 months) and those with phyllodes tumors (median duration, 6 months; range, 3 days to 72 months; P = 0.742). However, 10 of 31 phyllodes tumors (32%) had a symptomatic increase in the size of the tumors (no information in one patient with a palpable phyllodes tumor), while only 13 of 92 fibroadenomas (14%) had such an increase (no information in three patients with palpable fibroadenomas). This difference was statistically significant (P = 0.025).
The details of the mammography findings are shown in Table 1. Mammographic features which seemed to differ between fibroadenomas and phyllodes tumors included the presence of the lesions on mammograms (P = 0.021) and the density of the mass (P < 0.001).
Table 1.
Mammography findings in fibroadenoma and phylloides tumor
| Fibroadenomaa (n=134, unless stated otherwise) | Phyllodes tumor (n=35, unless stated otherwise) | P | |
|---|---|---|---|
| Lesions observed | 109 (81) | 34 (97) | 0.021 |
| Previous mammogram | 35 (26) | 12 (34) | 0.337 |
| Positive previous mammogramb | 25/35 (71) | 10/12 (83) | 0.414 |
| Enlarging mass | 11/25 (44) | 7/10 (70) | 0.164 |
| Breast density | 0.361 | ||
| Low density (fatty and scattered) | 8 (6) | 0 | |
| High density (heterogenous and extreme) | 126 (94) | 35 (100) | |
| Type of lesion | 0.049 | ||
| Mass | 79/109 (72) | 30/34 (88) | |
| Mass with calcification | 10/109 (9) | 4/34 (12) | |
| Pure calcification | 7/109 (6) | 0 | |
| Focal asymmetry | 13/109 (12) | 0 | |
| Calcification | 0.854 | ||
| No calcification | 89/109 (82) | 29/34 (85) | |
| Microcalcification | 12/109 (11) | 4/34 (12) | |
| Macrocalcification | 8/109 (7) | 1/34 (3) | |
| Shape of mass lesion | 0.129 | ||
| Round | 16/88 (18) | 7/34 (21) | |
| Oval | 36/88 (41) | 7/34 (21) | |
| Lobular | 35/88 (40) | 19/34 (56) | |
| Irregular | 1/88 (1) | 1/34 (3) | |
| Margin of mass lesion | 0.153 | ||
| Circumscribed | 30/88 (34) | 18/34 (53) | |
| Obscured | 55/88 (63) | 15/34 (44) | |
| Indistinct | 3/88 (3) | 1/34 (3) | |
| Density of mass lesion | < 0.001 | ||
| Hyperdense | 37/89 (42) | 29/34 (85) | |
| Isodense | 47/89 (53) | 5/34 (15) | |
| Hypodense and mixed | 5/89 (6) | 0 | |
| Maximum size (cm), median (range)c | 1.5 (0.6–3.8) | 1.2 (0.8–2.5) | 0.486 |
Fibroadenoma group consisted of pure fibroadenomas, fibroadenomas mixed with the proliferative change other than phyllodes tumors, and a few cases of fibrocystic change.
Previous mammography was performed at least six months prior to the index mammogram in most cases.
Maximum size was measured in 25 fibroadenomas and 10 phyllodes tumors.
Data are given as n (%), unless stated otherwise.
Details of the US findings are shown in Table 2. Four US features were significantly different between fibroadenomas and phyllodes tumors. These features included the internal heterogeneous echo pattern (Fig. 1), presence of internal round cystic spaces (Fig. 2), presence of internal cleft (Figs. 2, 3), and US size. All differences were associated with P < 0.001. The vast majority of images were categorized as BIRADS 4; very few were categorized as BIRADS 3.
Table 2.
US findings in fibroadenoma and phylloides tumor
| Fibroadenomaa (n=172, unless stated otherwise) | Phyllodes tumor (n=40, unless stated otherwise) | P | |
|---|---|---|---|
| Number of lesions | 0.114 | ||
| 1 | 31/168 (18) | 4 (10) | |
| 2 | 17/168 (10) | 6 (15) | |
| 3 | 20/168 (12) | 4 (10) | |
| 4 | 24/168 (14) | 3 (8) | |
| 5 | 12/168 (7) | 0 | |
| ≥6 | 64/168 (38) | 23 (58) | |
| Bilateral lesions | 114/168 (68) | 29 (73) | 0.569 |
| Previous USb | 39 (23) | 11 (28) | 0.517 |
| Enlarging mass | 18/31 (58) | 6/8 (75) | 0.380 |
| Orientation | 0.705 | ||
| Long axis parallel to skin | 158/167 (95) | 37 (93) | |
| Long axis perpendicular | 9/167 (5) | 3 (8) | |
| Shape of mass | 0.671 | ||
| Round | 7/167 (4) | 2 (5) | |
| Oval | 70/167 (42) | 14 (35) | |
| Irregular | 90/167 (54) | 24 (60) | |
| Margin of mass | 0.386 | ||
| Circumscribed | 59/167 (35) | 11 (28) | |
| Gentle lobulation | 92/167 (55) | 28 (70) | |
| Microlobulation | 4/167 (2) | 1 (3) | |
| Indistinct | 7/167 (4) | 0 | |
| Angular | 5/167 (3) | 0 | |
| Echo pattern | 0.049 | ||
| Markedly hypoechoic | 45/167 (27) | 4 (10) | |
| Mildly hypoechoic | 104/167 (62) | 33 (83) | |
| Isoechoic | 12/167 (7) | 1 (3) | |
| Hyperechoic | 6/167 (4) | 2 (5) | |
| Heterogeneous internal echo | 86/167 (51) | 34 (85) | < 0.001 |
| Internal round cystic space | 24/167 (14) | 24 (60) | < 0.001 |
| Internal cleft | 1/167 (1) | 16 (40) | < 0.001 |
| Posterior acoustic feature | |||
| Enhancement | 95/167 (57) | 32 (80) | 0.046 |
| Shadowing | 11/167 (7) | 1 (3) | |
| Combined | 60/167 (36) | 7 (18) | |
| None | 1/167 (1) | 0 | |
| Maximum size (cm), median (range)c | 1.5 (0.5–10.1) | 2.4 (0.8–11.7) | < 0.001 |
Fibroadenoma group consisted of the fibroadenomas, fibroadenomas mixed with the proliferative change other than phyllodes tumors, and a few cases of fibrocystic change.
Previous US was performed at least six months prior to index mammogram.
Maximum size was measured in 167 fibroadenomas and 38 phyllodes tumors. US, ultrasonography.
Data are given as n (%), unless stated otherwise.
Figure 1.

An example of a heterogeneous echo pattern in a 1.8 cm mass. A 39-year-old woman presented with a palpable mass in her right breast. The histopathologic diagnosis from the excision is fibroadenoma.
Figure 2.
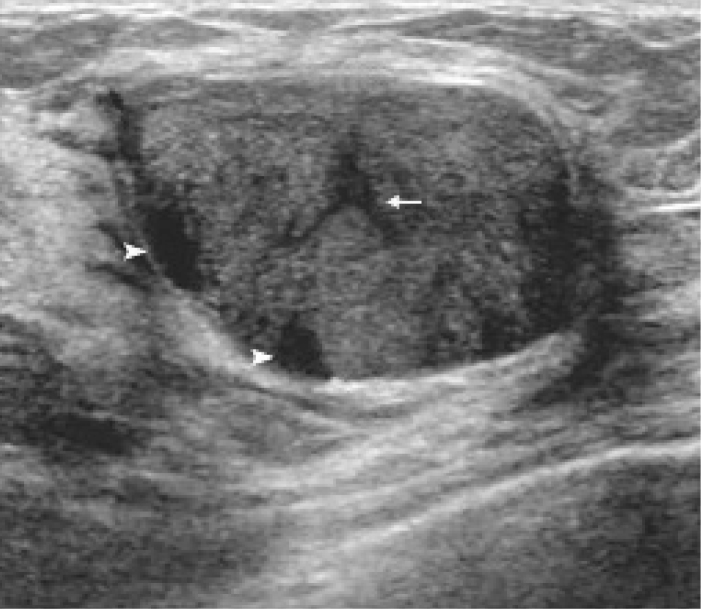
Benign phyllodes tumor in a 42-year-old woman. Arrowheads indicate internal cystic spaces. A cleft is marked by an arrow.
Figure 3. a–f.
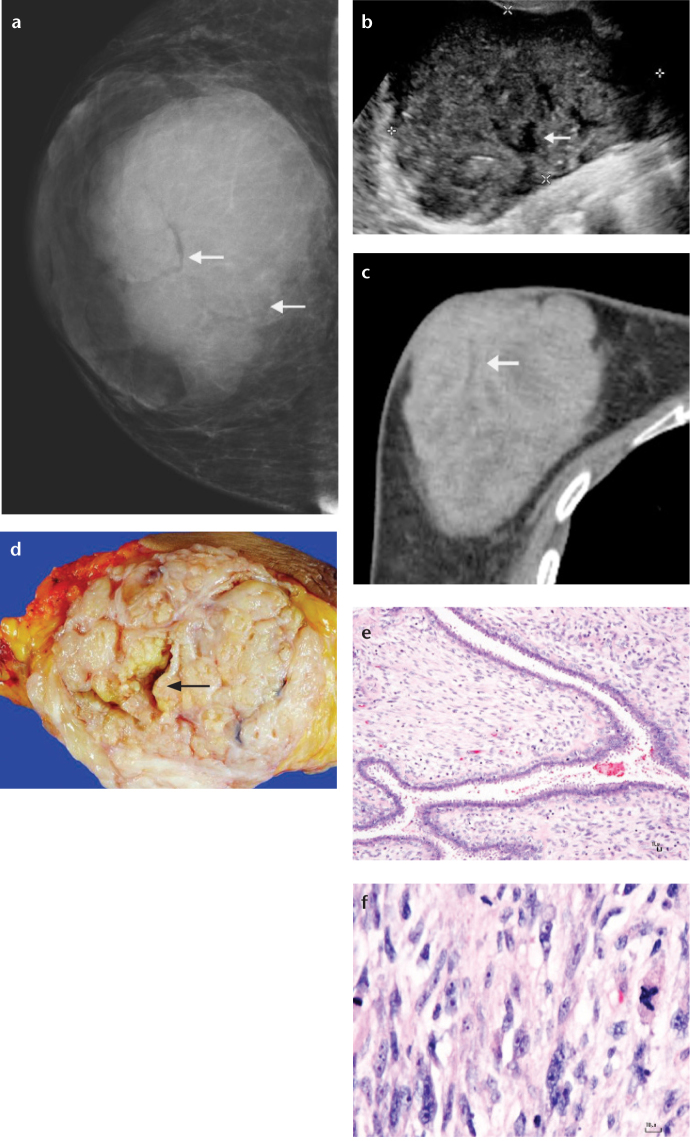
Borderline phyllodes tumor in a 50-year-old woman who presented with a mass in her right breast. This mass progressively enlarged over two years. Mammogram on mediolateral oblique view (a), US (b), and CT scan (c) show a 12 cm lobular mass with circumscribed border. Clefts are indicated by arrows. The cut surface of the gross specimen from a simple mastectomy (d) shows a variegate appearance with clefts (arrow) and small cysts. The border appears to be well circumscribed. The photomicrographs (e, f) show epithelium-lined clefts with moderately cellular stroma revealing hyperchromatic, pleomorphic nuclei with active mitotic activity. Focal ductal hyperplasia with apocrine metaplasia is observed (hematoxylin and eosin, ×100 and ×400, respectively).
The modality of CNB guidance, whether US or stereotactic, could not differentiate between fibroadenomas and phyllodes tumors. This was because almost all lesions were biopsied using a US guide (168 of 173 lesions, 97%, for fibroadenomas; 40 of 40 of lesions, 100%, for phyllodes tumors, P = 0.277).
The pathological report for each lesion usually contained suggestions and recommendations from the pathologist. Whether the pathologists suggested a diagnosis of fibroadenoma or phyllodes tumor from the CNB specimen was significantly different between the lesions histologically diagnosed as fibroadenomas and those diagnosed as phyllodes tumors (P < 0.001; Table 3).
Table 3.
Pathologists’ suggestions and recommendations
| Fibroadenomaa (n=173) | Phyllodes tumor (n=40) | P | |
|---|---|---|---|
| Pathologist suggestion | <0.001 | ||
| FE, no comment | 115 (66) | 19 (48) | |
| FE, favoring fibroadenoma | 28 (16) | 1 (3) | |
| FE, favoring phyllodes | 2 (1) | 13 (33) | |
| FE, inconclusive | 28 (16) | 7 (18) | |
| Excision recommended | 22 (13) | 9 (23) | 0.114 |
Fibroadenoma group consisted of pure fibroadenomas, fibroadenomas mixed with the proliferative change other than phyllodes tumors, and a few cases of fibrocystic change.
FE, fibroepithelial lesion.
Data are given as n (%).
A multiple logistic regression model (Table 4) identified four significant and independent predictors of phyllodes tumors. These predictors consisted of the presence of pain (P = 0.009), pathologists’ suggestion of a diagnosis of phyllodes tumor in their reports (P = 0.001), presence of clefts within the masses on US (P = 0.006), and presence of round cysts within the masses on US (P = 0.032). Based on the likelihood ratio chi-square test, the P value for this model was < 0.001. Palpable mass as the presenting symptom showed marginal significance (P = 0.056). Significant mammographic findings in Table 1 were not used in the multiple logistic regression analysis because a considerable number of lesions (26 of 169 lesions, 15%) could not be observed on mammograms; further, mammography was used in only 169 of 213 lesions (79%).
Table 4.
Multiple logistic regression model for independent predictors of phyllodes tumorsa
| Predictors | Odds ratio (95% confidence interval) | P |
|---|---|---|
| Symptoms | ||
| None | 1 | NA |
| Palpable mass | 3.44 (0.969–12.3) | 0.056 |
| Breast pain | 11.7 (1.95–70.3) | 0.009 |
| Presence of clefts on US | 23.3 (2.46–220) | 0.006 |
| Presence of round cysts on US | 3.32 (1.11–9.92) | 0.032 |
| Pathologist suggestion | ||
| No further comments or equivocal suggestions | 1 | NA |
| Favor fibroadenoma | 0.183 (0.021–1.59) | 0.124 |
| Favor phyllodes tumor | 22.1 (3.73–131) | 0.001 |
Analysis was based on 207 lesions in 194 patients.
NA, not applicable; US, ultrasonography.
Discussion
Fibroadenomas and phyllodes tumors share many common features. Clinically, both present as rounded or lobulated, circumscribed, moveable masses. Histologically, both are composed of an epithelial element surrounded by variable amounts of stroma (7), and thus they can also be given the name “fibroepithelial lesion”. However, their natural history is often different. Phyllodes tumors tend to show more rapid growth and tend to recur if incompletely excised. Moreover, borderline and malignant phyllodes tumors may metastasize (8). In contrast, fibroadenomas usually need not be removed, and even when surgery is needed, enucleation is sufficient. For these reasons, an accurate preoperative diagnosis, for which CNB is a popular method of distinguishing between the two entities, is very important to ensure proper management (7). The present study focused on CNB-diagnosed fibroepithelial lesions in which the pathologist was unable to make a definitive distinction between fibroadenoma or phyllodes tumor (2).
Patients with phyllodes tumors are usually 40 to 50 years of age at the time of diagnosis (9), generally older than patients with fibroadenomas (1, 4, 9). Patients older than 50 to 55 years are more likely to have a phyllodes tumor (7). In the present study, age was not a useful discriminant, unlike other studies (2, 4, 9). This finding may be explained by the fact that a surgical excision was usually performed in older patients at our institution if an initial CNB revealed a fibroepithelial lesion, regardless of other features suggestive of a fibroadenoma. This was mainly due to patient’s and the surgeon’s preference.
Phyllodes tumors usually present with a clinically benign breast lump, which may be rapid growing (1, 10). Fibroadenomas, by contrast, tend to be stable or have minimal growth (10). The present study confirmed this observation; 80% of patients with phyllodes tumors presented with a palpable breast mass, compared to 55% of the patients with fibroadenomas. Further, more phyllodes tumors increased in size over time (32% for phyllodes tumors and 14% for fibroadenomas, P = 0.025).
Interestingly, the present study identified a relationship between breast pain and phyllodes tumor (P = 0.009 on multivariable analysis). To our knowledge, this feature has not been previously reported. Although possibly a statistical artifact, this finding may also be explained by the nature of phyllodes tumors, which interfere with the surrounding breast parenchyma, either by stretching or by compressing it. In addition, the rapidly increasing size may cause pain. Nonetheless, pain is a very common and subjective symptom that is usually unreliable or of limited use as a clinical marker for any given disease. More evidence is needed for a definitive conclusion regarding this point.
Phyllodes tumors tend to be larger in size than fibroadenomas (2, 9, 10), as observed in the present study. Nevertheless, size was not a significant discriminant upon multivariable analysis. This finding is easily explained by the fact that larger tumors tended to have clefts and cysts within the mass, and pathologists tended to diagnose phyllodes tumors based on their size. The presence of clefts and cysts was also highly correlated with the presence of phyllodes tumors, as will be seen later. In a multivariable analysis, less important but collinear variables, such as size, were eliminated.
Previous studies reported a substantial overlap in the characteristics of phyllodes tumors and fibroadenomas on the mammograms. Both tumors manifest as well-circumscribed, oval or lobulated masses (1, 4, 9, 11). However, high-density masses on a mammogram have been reported to be more common in phyllodes tumors (10, 11). A high-density mass was also a useful mammographic feature suggestive of phyllodes tumors in the present study (P < 0.001). Because phyllodes tumors tended to be larger and have higher densities in the present study, they were also more likely to be visualized on a mammogram in the present study (P = 0.021).
The presence or absence of intratumoral calcifications have been reported to be a significant discriminator by previous studies (12, 13). Calcifications are more common in long-standing fibroadenomas but are rare in phyllodes tumors (12, 13). However, the frequency of calcified lesions did not differ between phyllodes tumors and fibroadenomas in the study of Yilmaz et al. (11), in agreement with the present study. Most of the fibroepithelial lesions in the present study did not contain calcifications (82% of fibroadenomas and 85% of phyllodes tumors).
Advances in US imaging technology have allowed for more detailed evaluations of lesions. Thus, US may provide accurate discrimination between phyllodes tumors and fibroadenomas. One useful US feature regarding phyllodes tumors is the presence of cysts within a solid mass (Fig. 2) (1, 9, 11–14). These cystic areas represent focal necrosis or degeneration (11, 13, 14). The present study confirmed that the presence of round cystic spaces within the mass was associated with phyllodes tumors on both univariable analysis (P < 0.001) and multivariable analysis (P = 0.032). Other studies (10, 13, 15), however, found very few phyllodes tumors with internal cysts, concluding that these cysts were not pathognomonic for phyllodes tumors. These cysts may also be present in other well-circumscribed tumors, such as fibroadenomas or medullary carcinomas (11, 13, 15).
Although the US findings of clefts and round cystic spaces in phyllodes tumors might be important if confirmed by future studies, the current BIRADS classification does not explicitly use these findings. Future modifications of the BIRADS system might incorporate these findings with the aim of distinguishing phyllodes tumors from fibroadenomas, perhaps by appropriately upgrading the BIRADS classification whenever clefts and cysts are clearly noted in the radiologic images.
Microscopically, phyllodes tumors are characterized by a double-layered epithelial component arrayed in clefts and surrounded by a hypercellular stromal mesenchymal component. The stroma often protrudes into the epithelial lining spaces, forming a slit-like space or a leaf-like pattern; hence, it was given the name phyllodes, which means “leaf-like” in Greek (8). The slit-like nature of the cystic spaces in phyllodes tumors results in one of its US characteristics: the horizontally oriented linear echoes or clefts (14). This finding is particularly important in our study. The presence of a cleft within the solid mass was strongly associated with phyllodes tumors upon both univariable (P < 0.001) and multivariable analyses (P = 0.006) (Figs. 2, 3).
Heterogeneous echo patterns of the mass have been reported as one of the US features of phyllodes tumors (9, 10, 13–15). The present study also showed that most phyllodes tumors (85%) displayed a heterogeneous echotexture, compared with 51% of fibroadenomas. Although this contrast was significant upon univariable analysis (P < 0.001), it was not significant upon multivariable analysis due to collinearity with other US features.
Other US features, including the orientation of the mass, shape, margin, echo pattern, and posterior acoustic features, were not significantly different between phyllodes tumors and fibroadenomas, in agreement with several studies (1, 2, 4, 9). On the other hand, some studies (13, 15) have suggested that a lobulated-shaped mass on US is often associated with phyllodes tumors, while Yilmaz et al. (11) reported that a marked posterior acoustic enhancement is also an important characteristic of phyllodes tumors.
The present study investigated the significance of the presence of multiple or bilateral lesions, based on the knowledge that multiple fibroadenomas may occur in association with phyllodes tumors (10). Foxcroft et al. (10) reported that 31% of patients with phyllodes tumors had at least one concurrent fibroadenoma. According to the present study, 90% of patients with phyllodes tumors had more than one lesion, and 73% had bilateral lesions. However, a multiplicity of tumors and bilateral involvement were also common in patients with fibroadenomas in the present study, in which 81% of these patients had more than one lesion, and 68% had bilateral involvement. Note that, because cysts and solid nodules could not always be clearly distinguished in reviewed US images, the present findings should be interpreted with caution.
As stated previously, it is difficult to discriminate histologically between phyllodes tumors and fibroadenomas (3, 7), particularly in CNB specimens. Even after the entire lesion has been excised, “gray areas” exist in diagnosis (7). Because CNB uses a sampling technique, it is even more difficult for pathologists to make a clear-cut diagnosis. Previous studies (3, 4, 7, 16) have described histologic features suggestive of phyllodes tumors based on CNB specimens. These features consist of the degree of stromal hypercellularity, stromal overgrowth, nuclear cytologic atypia, number of mitoses, amount of stroma relative to epithelium, and infiltrative tumor borders. Using these morphologic criteria, pathologists are able to make a diagnosis of phyllodes tumor from some CNB specimens. The present study found a high concordance between the suggestion of pathologists for a diagnosis of phyllodes tumor or fibroadenoma from CNB pathology and the surgical pathology after a complete excision (P < 0.001). Dillon et al. (3) also found such a concordance. The importance of suggestions by the pathologist was confirmed by multivariable analysis in the present study.
There were several limitations to the present study. First, its retrospective nature resulted in some missing clinical data and limited our ability to evaluate the US features in some cases. Second, the study attempted to imitate real clinical practices, so pathologic slides and specimens were not reviewed. Thus, there might be some misclassification of CNB specimens. Third, approximately 40% of patients diagnosed as having fibroepithelial lesions from CNB did not undergo an excision. Therefore, many patients were excluded, resulting in substantial selection bias. For example, patients with a higher risk of having malignant lesions, such as those with older age or larger lesions, were selected for surgery. We did not investigate the long-term follow-up of the excluded group of patients. It was possible that a substantial number of patients with phyllodes tumors were in this group.
In conclusion, the present study emphasized a multidisciplinary approach to distinguish phyllodes tumors from fibroadenomas in patients with CNB-diagnosed fibroepithelial lesions. Radiologists should search for the US characteristics of phyllodes tumors, particularly the presence of clefts or round cysts within solid masses. Pathologists should search for histopathologic characteristics of phyllodes tumors and make suggestions as to the most likely diagnosis. Clinical findings associated with the mass, particularly the symptom of breast pain, should be documented. All of these data could help the surgeon to decide whether the lesion should be surgically removed.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Jacklin RK, Ridgway PF, Ziprin P, Healy V, Hadjiminas D, Darzi A. Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol. 2006;59:454–459. doi: 10.1136/jcp.2005.025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komenaka IK, El-Tamer M, Pile-Spellman E, Hibshoosh H. Core needle biopsy as a diagnostic tool to differentiate phyllodes tumor from fibroadenoma. Arch Surg. 2003;138:987–990. doi: 10.1001/archsurg.138.9.987. [DOI] [PubMed] [Google Scholar]
- 3.Dillon MF, Quinn CM, McDermott EW, O’Doherty A, O’Higgins N, Hill AD. Needle core biopsy in the diagnosis of phyllodes neoplasm. Surgery. 2006;140:779–784. doi: 10.1016/j.surg.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Lee AH, Hodi Z, Ellis IO, Elston CW. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology. 2007;51:336–344. doi: 10.1111/j.1365-2559.2007.02786.x. [DOI] [PubMed] [Google Scholar]
- 5.Guray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. Oncologist. 2006;11:435–449. doi: 10.1634/theoncologist.11-5-435. [DOI] [PubMed] [Google Scholar]
- 6.American College of Radiology . Illustrated breast imaging reporting and data system (BI-RADS) 4th ed. Reston: American College of Radiology; 2003. [Google Scholar]
- 7.Morgan JM, Douglas-Jones AG, Gupta SK. Analysis of histological features in needle core biopsy of breast useful in preoperative distinction between fibroadenoma and phyllodes tumour. Histopathology. 2010;56:489–500. doi: 10.1111/j.1365-2559.2010.03514.x. [DOI] [PubMed] [Google Scholar]
- 8.Tse GM, Niu Y, Shi HJ. Phyllodes tumor of the breast: an update. Breast Cancer. 2010;17:29–34. doi: 10.1007/s12282-009-0114-z. [DOI] [PubMed] [Google Scholar]
- 9.Bode MK, Rissanen T, Apaja-Sarkkinen M. Ultrasonography and core needle biopsy in the differential diagnosis of fibroadenoma and tumor phyllodes. Acta Radiol. 2007;48:708–13. doi: 10.1080/02841850701367911. [DOI] [PubMed] [Google Scholar]
- 10.Foxcroft LM, Evans EB, Porter AJ. Difficulties in the preoperative diagnosis of phyllodes tumours of the breast: a study of 84 cases. Breast. 2007;16:27–37. doi: 10.1016/j.breast.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz E, Sal S, Lebe B. Differentiation of phyllodes tumors versus fibroadenomas: mammographic and sonographic features. Acta Radiol. 2002;43:34–39. doi: 10.1080/028418502127347619. [DOI] [PubMed] [Google Scholar]
- 12.Liberman L, Bonaccio E, Hamele-Bena D, Abramson AF, Cohen MA, Dershaw DD. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996;198:121–124. doi: 10.1148/radiology.198.1.8539362. [DOI] [PubMed] [Google Scholar]
- 13.Chao TC, Lo YF, Chen SC, Chen MF. Sonographic features of phyllodes tumors of the breast. Ultrasound Obstet Gynecol. 2002;20:64–71. doi: 10.1046/j.1469-0705.2002.00736.x. [DOI] [PubMed] [Google Scholar]
- 14.Stavros AT. Atypical, high-risk, premalignant, and locally aggressive lesions. In: Stavros AT, editor. Breast ultrasound. 1st ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 689–711. [Google Scholar]
- 15.Chao TC, Lo YF, Chen SC, Chen MF. Phyllodes tumors of the breast. Eur Radiol. 2003;13:88–93. doi: 10.1007/s00330-002-1370-x. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs TW, Chen YY, Guinee DG, Jr, et al. Fibroepithelial lesions with cellular stroma on breast core needle biopsy: Are there predictors of outcome on surgical excision? Am J Clin Pathol. 2005;124:342–354. doi: 10.1309/5N2C-4N5X-CB8X-W8JL. [DOI] [PubMed] [Google Scholar]