Abstract
PURPOSE
We aimed to study side effects, complications, and patient acceptance of magnetic resonance imaging-guided real-time biopsy (MRI-GB) of the prostate.
METHODS
Fifty-four men (49–78 years) with elevated prostate-specific antigen after at least one negative systematic transrectal ultrasound-guided biopsy (TRUS-GB) were included in a prospective clinical study. Suspicious areas on images were selectively sampled by obtaining a median of four specimens (range, 1–9 specimens) using MRI-GB. In TRUS-GB, a median of 10 specimens (range, 6–14 specimens) were obtained. Telephone interviews were conducted one week after outpatient MRI-GB, asking patients about pain and side effects (hematuria, hemospermia, rectal bleeding, fever, and chills) of the two biopsy procedures and which of the two procedures they preferred. Multinomial regression analysis and Fisher’s exact test was used to test for differences.
RESULTS
MRI-GB was preferred by 65% (35/54), and 82% (44/54) would undergo MRI-GB again. Pain intensity (P = 0.005) and bleeding duration (P = 0.004) were significantly lower for MRI-GB compared with TRUS-GB. Hematuria was less common after MRI-GB compared with TRUS-GB (P = 0.006). A high correlation was given between bleeding intensity and bleeding duration for TRUS-GB (r=0.77) and pain intensity and pain duration for MRI-GB (r=0.65). Although hemospermia, rectal hemorrhage, fever, and chills were less common in MRI, they showed no statistically significant difference.
CONCLUSION
MRI-GB of the prostate seems to have fewer side effects and less pain intensity than TRUS-GB and was preferred by the majority of patients.
Several new recommendations about routine prostate-specific antigen (PSA) screening were recently published (1). Nevertheless, guidelines still recommend that patients with PSA levels suspicious for prostate cancer should undergo transrectal ultrasound-guided biopsy (TRUS-GB). The likelihood of detecting cancer by the first TRUS-GB is below 60% and only 16%–29% for repeat biopsy (2, 3). However, a negative systematic TRUS-GB does not definitely rule out prostate cancer in patients with a persistent clinical suspicion (4, 5). In this situation, multiparametric magnetic resonance imaging (MRI) including diffusion-weighted imaging, dynamic contrast-enhanced MRI, and magnetic resonance spectroscopy is being increasingly used to detect prostate cancer and confirm the diagnosis by MRI-guided biopsy (MRI-GB) (6).
While several studies are available on the side effects, complications, and pain experienced by patients who undergo TRUS-GB, no study has been published on the acceptance and side effects of MRI-GB of the prostate (7–9). We therefore conducted a survey among patients who underwent MRI-GB of suspicious areas detected by MRI after a history of at least one negative TRUS-GB. The aim of this survey was to evaluate the acceptance of MRI-GB of the prostate in terms of side effects and complications in comparison with TRUS-GB.
Methods
Patients
A total of 54 patients, aged between 49 and 78 years (median, 68 years), were participants of a prospective clinical study conducted from October 2008 to December 2009 to investigate the role of multiparametric MRI in detecting prostate cancer (10). These patients underwent MRI with subsequent MRI-GB. The survey was approved by the ethics committee, and all patients gave written informed consent. The patients were referred by their treating urologist to the Department of Urology of our hospital and recruited by the urologist cooperating with our department. Inclusion criteria were a suspicious PSA level (median, 12.1 ng/mL; range, 3.3–65.2 ng/mL) and/or rectal digital examination result and at least one TRUS-GB that was negative for prostate cancer. For MRI-GB, patients had to have normal clotting parameters (partial thromboplastin time, international normalized ratio) and had to discontinue acetylsalicylic acid medication 10 days prior to the planned biopsy. Exclusion criteria were the usual contraindications to MRI and known allergy to gadolinium-based contrast medium. No patient was excluded from the study. All 54 study patients underwent TRUS-GB as well as MRI-GB. The median time between the last TRUS and MRI was 13 months.
Transrectal ultrasound-guided biopsy
All collected medical data were gathered directly from the treating urologist and TRUS-GB were also performed by the patients’ urologists. The biopsies were performed in left lateral position (n=46), supine position (n=7), and right lateral position (n=1). A median of 10 biopsies (range, 6–14 biopsies) were taken. The biopsies were obtained with rectally administered anesthesia of lidocaine or xylocaine in 83.3% of the cases (45/54), while no anesthesia was given in 16.7% (9/54). The patients took oral antibiotics (ciprofloxacin or levofloxacin) starting one day before the biopsy procedure and continuing intake for at least three days thereafter. The average duration of biopsy procedures was 15 minutes (range, 10–20 min). The study patients had a median of two negative TRUS-GB (range, 1–6 negative TRUS-GB).
Magnetic resonance imaging-guided biopsy
A multiparametric prostate MRI, consisting of conventional T1-weighted and T2-weighted sequences, 1H-magnetic resonance spectroscopy, diffusion-weighted imaging and dynamic-contrast-enhanced MRI, was performed to detect cancer suspicious areas. These areas were biopsied by MRI-GB on a second examination with a closed 1.5 T whole-body MRI scanner (Avanto, Siemens Healtcare). All biopsies were performed by a radiologist. As with TRUS-GB, the patients took prophylactic oral antibiotics (ciprofloxacin or levofloxacin) starting one day before biopsy and continued for at least three days thereafter. Tissue specimens were removed transrectally using an MRI-compatible biopsy needle (Semi-automatic biopsy gun, Invivo) with the patient in prone position. For anesthesia, all patients received lidocaine gel (Instillagel®, Farco-Pharma) (14, 15). The number of specimens removed was kept to a minimum; a median of four specimens (range, 1–9 specimens) were obtained per patient. The median duration of the biopsy session (room time) was 55 minutes (range, 34–80 min).
Telephone interview
A research fellow conducted a telephone interview based on the questionnaire described below. Patients were called one week after MRI-GB. The interviewer was blinded to biopsy results and was not involved in examination procedures. Fifty-three patients were interviewed by telephone, while one patient was interviewed personally during a visit for a repeat biopsy.
Questionnaire
The questionnaire consisted of three parts. The first part concerned pain related to MRI-GB and the last performed TRUS-GB. Patients were asked to classify the pain intensity on a numerical rating scale (NRS) of 0–10. Zero was defined as no pain and 10 as the most severe pain imaginable. Patients were also asked to specify the site of pain experienced after biopsy by assigning it to one of the following three areas: prostate, area around the prostate, and all other sites. In addition, patients were asked whether there was bleeding after the biopsy or not. If yes, they were asked to give the intensity and duration. The second part of the questionnaire concerned the side effects of both types of prostate biopsy. Specifically, patients were asked whether they experienced hematuria, rectal bleeding, fever, and chills in relation with TRUS-GB and/or MRI-GB. When a patient reported one of these side effects, he was additionally asked to give the duration (in days) and evaluate the intensity by assigning it to one of three predefined categories (mild, moderate, severe). The final question concerned the occurrence of complications and how they were treated.
In the third part, the patient was asked which of the two biopsy modes he preferred and why. The reasons given were retrospectively assigned to one of four categories with the option of assigning the answers to multiple categories (Table 1). Finally, the patient was asked whether he would undergo MRI-GB again.
Table 1.
Categorization of reasons patients gave for their preference of either procedure
| Category | Content of statements |
|---|---|
| Category I | Better result |
| Category II | Procedure-related factors
|
| Category III | Fewer side effects
|
| Category IV | Answers not fitting into categories I–III |
Statistical analysis
To compare the side effects, only those patients who underwent biopsy under anesthesia (45/54 patients) were included in the statistics. The remaining patients (9/54) were excluded from the side effects comparison. In terms of side effects of TRUS-GB, pain-related questions could be answered by 42 patients, duration of bleeding by 44 patients, hematuria by 44 patients, hemospermia by 21 patients and rectal hemorrhage by 25 patients. No data corrections were performed. The location of the pain could not be located by the study patients for TRUS-GB because of the systematic removal of punching cylinders. Categorical data are presented as absolute and relative frequencies, continuous values as median and range. Comparisons of TRUS-GB and MRI-GB were performed using multinomial regression analysis based on generalized estimation equations to account for the intrapatient comparison design and the correlation between observations within the same patient. Intensities of pain and bleeding as well as the duration of pain were analyzed as categorical data. Spearman’s correlation coefficient was calculated to assess the correlation of different parameters. No sample size estimation was performed for this exploratory study, as no basic data were available as basis for any power consideration. All results are therefore suggested as hypothesis-generating and provide a basis for the planning of further clinical studies. Calculations were performed using SAS 9.2 (SAS Institute Inc.).
Results
MRI-GB was preferred by 65% of patients (35/54) and TRUS-GB by 28% (15/54); 7% (4/54) were undecided. Overall, 82% of patients (44/54) would undergo MRI-GB again, 9% (5/54) said they would not, and 9% (5/54) had no answer to this question.
The most common reasons given for the preference fell into two categories: 33% of patients said they expected a better biopsy result and 35% gave procedure-related factors as reasons (Table 2). Of 15 patients who preferred TRUS-GB, 14 gave reasons that fell into the procedure-related factors category. The most common reasons from this category were discomfort related to lying in a small space for a long period of time in the MRI scanner, followed by noise in the MRI machine, and the strenuous prone position. None of the patients reported feeling confined in the narrow bore of the MRI scanner. Patients who said that MRI-GB was their preferred biopsy procedure, most often gave a reason of better biopsy result for this preference (45%), followed by fewer side-effects (40%) (Table 2).
Table 2.
Reasons that patients gave for their preference of the biopsy procedure*
| Category I Better result |
Category II Procedure-related factors |
Category III Fewer side effects |
Category IV Other reasons |
|
|---|---|---|---|---|
| Total | 33.3 (22/66) | 34.8 (23/66) | 27.3 (18/66) | 4.6 (3/66) |
| Preferred MRI-GB | 45 (20/45) | 13 (6/45) | 40 (18/45) | 2 (1/45) |
| Preferred TRUS-GB | 6 (1/16) | 88 (14/16) | 0 (0/16) | 6 (1/16) |
| Period of lying: 86 (12/14) | ||||
| Noise: 14.3 (2/14) | ||||
| Prone position: 14.3 (2/14) | ||||
| Narrow space: 0 (0/14) |
Data are presented as % (n/N).
MRI-GB, magnetic resonance imaging-guided biopsy; TRUS-GB, transrectal ultrasound-guided biopsy.
Patients could choose as many reasons as applied to their preference.
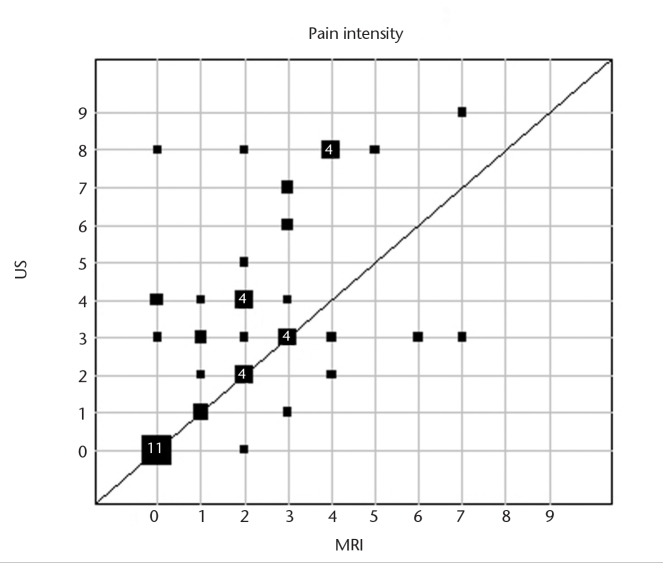
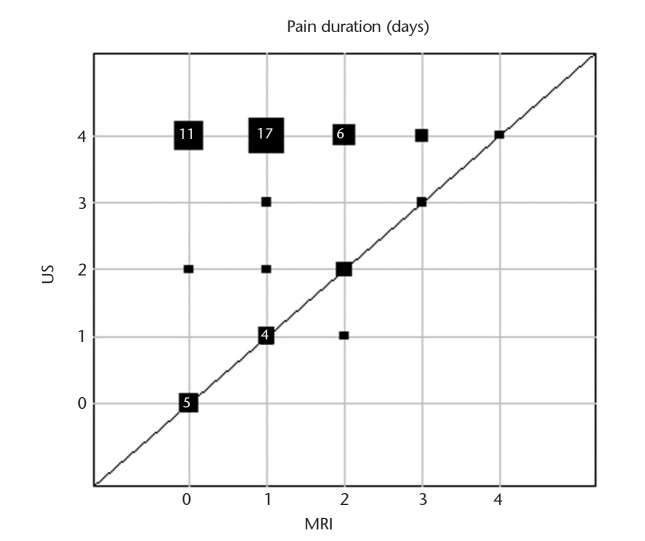
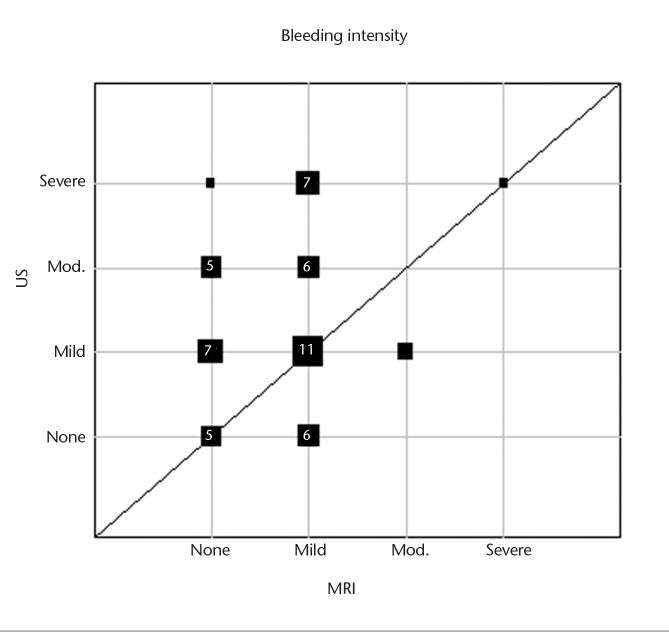
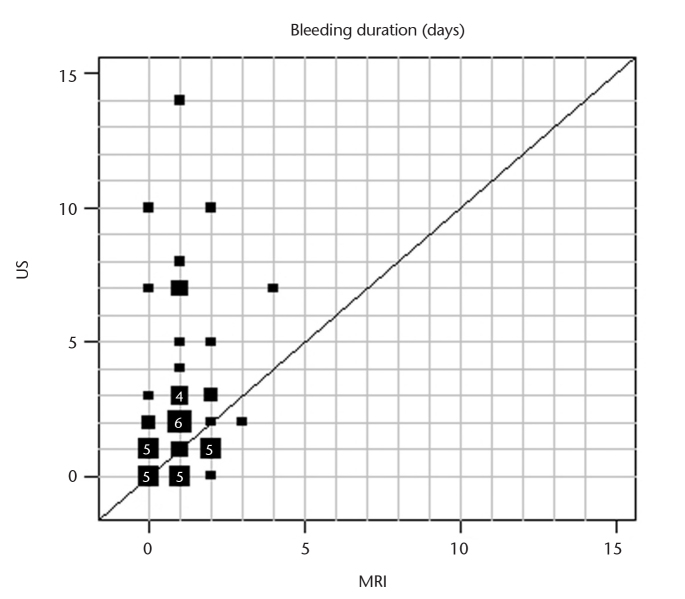
Frequencies of side effects are presented in Table 3. Pain intensity was significantly lower for MRI-GB compared with TRUS-GB (MRI, median 2, range 0–7; TRUS, median 3, range 0–9; P = 0.005) (Fig. 1). Pain duration was shorter after MRI-GB compared with TRUS-GB (Fig. 2). The prostate was the most common site of pain reported after MRI-GB. In total 31% (14/45) reported no pain after MRI-GB and 29% (12/42) reported no pain after TRUS-GB. More patients experienced no bleeding after MRI-GB (49%) than after TRUS-GB (21%). Bleeding intensity was rated as mild after both biopsy procedures (Fig. 3). The bleeding duration was longer with TRUS-GB compared with MRI-GB (P = 0.004) (Fig. 4). The most common side effect of both biopsy techniques was hematuria, followed by hemospermia and rectal hemorrhage. The frequency of hematuria was significantly higher after TRUS-GB compared with MRI-GB (79% vs. 51%, P = 0.006). The incidence of fever and chills amounted to <5% after both procedures. The frequency of hemospermia was not significantly different between MRI-GB and TRUS-GB (36% vs. 33%, respectively, P = 0.82). The other side effects, namely rectal hemorrhage, fever, and chills were also not significantly different between MRI-GB and TRUS-GB (Table 3). The correlation analyses showed a high correlation between bleeding intensity and bleeding duration for TRUS-GB (r=0.77) and pain intensity and pain duration for MRI (r=0.65). All other investigated correlations between bleeding intensity and bleeding or pain duration and between pain intensity and pain or bleeding duration were found to be positive but at a moderate level at most (r<0.50).
Table 3.
Frequency of side effects
| MRI-GB % (n) | TRUS-GB % (n) | P * | ||
|---|---|---|---|---|
| NRS (numeric rating scale) | 0 | 31.1 (14/45) | 28.6 (12/42) | 0.005 |
| 1 | 11.1 (5/45) | 9.5 (4/42) | ||
| 2 | 20.0 (9/45) | 9.5 (4/42) | ||
| 3 | 17.8 (8/45) | 14.3 (6/42) | ||
| 4 | 11.1 (5/45) | 9.5 (4/42) | ||
| 5 | 2.2 (1/45) | 0 (0/42) | ||
| 6 | 2.2 (1/45) | 4.8 (2/42) | ||
| 7 | 4.5 (2/45) | 4.8 (2/42) | ||
| 8 | 0 (0/45) | 16.7 (7/42) | ||
| 9 | 0 (0/45) | 2.3 (1/42) | ||
| 10 | 0 (0/45) | 0 (0/42) | ||
| Pain duration (days) | 1–3 | 40 (18/45) | 62 (26/42) | <0.01 |
| ≥4 | 11 (5/45) | 21 (9/42) | ||
| Site of pain | None | 31 (17/54) | 29 (12/42) | |
| Prostate | 50 (27/54) | NA | ||
| Area around the prostate | 17 (9/54) | NA | ||
| Other regions | 2 (2/54) | NA | ||
| Bleeding intensity | None | 37.8 (17/45) | 20.0 (9/45) | <0.01 |
| Mild | 51.1 (23/45) | 44.4 (20/45) | ||
| Moderate | 6.7 (3/45) | 15.6 (7/45) | ||
| Severe | 2.2 (1/45) | 17.8 (8/45) | ||
| Bleeding duration (days) | 0 (no bleeding) | 48.9 (22/45) | 20.5 (9/44) | 0.004 |
| 1–3 | 40.0 (18/45) | 59.1 (26/44) | ||
| >4 | 11.1 (5/45) | 18.1 (8/44) | ||
| >10 | 0 | 2.3 (1/44) | ||
| Hematuria intensity | None | 48.9 (22/45) | 20.9 (9/44) | 0.006 |
| Mild | 51.1(23/45) | 79.1 (34/44) | ||
| Moderate | 0 (0/45) | 0 (0/44) | ||
| Severe | 0 (0/45) | 0 (0/44) | ||
| Hemospermia intensity | None | 64.4 (29/45) | 66.7 (14/21) | 0.82 |
| Mild | 35.6 (16/45) | 33.3 (7/21) | ||
| Moderate | 0 (0/45) | 0 (0/21) | ||
| Severe | 0 (0/45) | 0 (0/21) | ||
| Rectal hemorrhage intensity | None | 84.4 (38/45) | 76.0 (19/25) | 0.29 |
| Mild | 15.6 (7/45) | 24.0 (6/25) | ||
| Moderate | 0 (0/45) | 0 (0/25) | ||
| Severe | 0 (0/45) | 0 (0/25) | ||
| Fever | 2.2 (1/45) | 4.4 (2/45) | 0.57 | |
| Chills | 4.4 (2/45) | 4.4 (2/45) | >0.99 |
MRI-GB, magnetic resonance imaging-guided biopsy; TRUS-GB, transrectal ultrasound-guided biopsy.
Multinomial regression analysis.
Figure 1.
Graphic representation of subjective pain intensity estimated on a numerical rating scale for intrapatient comparison of MRI-GB and TRUS-GB for those patients who provided the information for both biopsy procedures. Square sizes represent the number of patients with the respective pair of values in a given position. For squares representing four patients or more, the number of patients with that pair of values is given inside the square (MRI, magnetic resonance imaging; US, ultrasound).
Figure 2.
Graphic representation of pain duration for intrapatient comparison of MRI-GB and TRUS-GB for those patients who provided information for both biopsy procedures. Square sizes represent the number of patients with the respective pair of values in a given position. For squares representing four patients or more, the number of patients with that pair of values is given inside the square (US, ultrasound; MRI, magnetic resonance imaging).
Figure 3.
Graphic representation of bleeding intensity for intrapatient comparison of MRI-GB and TRUS-GB for those patients who provided information for both biopsy procedures. Square sizes represent the number of patients with the respective pair of values in a given position. For squares representing four patients or more, the number of patients with that pair of values is given inside the square (US, ultrasound; Mod., moderate; MRI, magnetic resonance imaging).
Figure 4.
Graphic representation of bleeding duration for intrapatient comparison of MRI-GB and TRUS-GB for those patients who provided information for both biopsy procedures. Square sizes represent the number of patients with the respective pair of values in a given position. For squares representing four patients or more, the number of patients with that pair of values is given inside the square (US, ultrasound; MRI, magnetic resonance imaging).
The complication rate was low for both biopsy procedures (<6% [3/54], for both). There were three adverse effects after MRI-GB: temporary dysuria, subcapsular arterial bleeding, and infection with fever of a diabetic patient. The latter two patients were hospitalized for treatment. Following TRUS-GB, there were two cases of infection with fever and one case of epididymitis.
Discussion
Our survey shows that 65% of patients, who underwent MRI-GB after at least one prior negative systematic TRUS-GB and persistent suspicion of prostate cancer, prefer the MRI-guided procedure for prostate biopsy. With its higher patient acceptance and a detection rate of 39%–59%, MRI-GB appears to be a very good alternative biopsy procedure for men with a persistent suspicion of prostate cancer (4–6, 10).
Fewer side effects and lower pain intensity were common reasons given for preferring MRI-GB. In addition, the duration of side effects was shorter compared with TRUS-GB. The lower rate of side effects and less severe pain may be attributable to the removal of fewer biopsy specimens with MRI-GB compared with TRUS-GB, for which current guidelines recommend sampling of 10–12 specimens (1). In MRI-GB, specimens are sampled nonrandomly from areas that appear suspicious on prior MRI. This usually results in the removal of fewer specimens compared with TRUS-GB, which typically involves systematic sampling of one specimen from each region of the prostate. The smaller number of specimens removed by MRI-GB reduces the risk of injury. MRI-GB is less invasive since specimens are removed in a more directed manner based on prior MRI findings, which reduces injury to adjacent structures compared with systematic TRUS-GB (6, 11). It is presumable that lower invasiveness and removal of fewer specimens with MRI-GB resulted in less intense pain in our patients. As expected, a positive correlation between bleeding intensity and the duration of bleeding and pain as well as between the intensity of pain and duration of bleeding and pain was shown. A high correlation was, however, only noted between bleeding intensity and bleeding duration for TRUS-GB and between pain intensity and pain duration for MRI-GB. Since for MRI-GB the bleeding intensity was mostly rated as either none or mild and the bleeding duration lied almost exclusively between 0 and 2 days, a correlation of <0.5 was not surprising. It can therefore be assumed that a clearer correlation can be observed with a larger number of patients.
In our survey, we used an NRS to objectify the pain experienced by the patients. It is the most widely known pain scale. Patients are asked to express the pain intensity they experienced as a number from 0–10. The NRS can be used in oral and written surveys and is therefore well suited for a telephone survey as in our study. The scale has also been used in visually impaired patients and to evaluate pain intensity after surgery (12, 13). A study assessing pain intensity after TRUS-GB reported a mean NRS score of 3.2 (9). This is consistent with our findings, while pain intensity for MRI-GB was lower.
Hematuria was the most common side-effect of MRI-GB reported by 51% of the patients. In the literature the rate of hematuria after TRUS-GB is in the range of 11%–57%, while a higher rate (79%) of mostly mild hematuria was observed in our patient cohort (8, 9, 14).
A telephone survey is a suitable instrument for obtaining data on side-effects and complications, as it is likely to generate high participation of nearly 100%. Similar studies using written questionnaires have much lower return rates in the range of 69%–90% (15). Patients asked to complete a questionnaire by themselves require much higher motivation, which might not be the case after a painful core biopsy procedure. In a telephone interview, the interviewer and the person questioned are not in a face-to-face communication, which makes it more anonymous and patients are expected to be more at ease and answer more intimate questions. We therefore think that a telephone interview elicits more honest answers. Moreover, the interviewer can provide additional explanations, if a question is not clear, making sure it is understood in the intended sense. This is another advantage over written surveys.
Overall, patients experienced both transrectal MRI-GB and TRUS-GB as procedures of low complication rate. This is primarily attributable to adequate prophylactic oral antibiotic intake. It has been shown that adequate infection prophylaxis lowers the complication rate (16). The low complication rate of both biopsy procedures in our study (<6%, 3/54) confirms the adequacy of the prescribed antibiotic regimens.
A limitation of our survey is that there was a long interval between TRUS-GB and MRI-GB in some patients. Therefore, some patients did not exactly recall the duration and intensity of side effects after TRUS-GB and intrapatient comparison was not possible in all cases. Another limitation is that all patients were questioned only after MRI-GB. This makes the answers obtained regarding MRI-GB more reliable. However, we do not think that this has led to a significant under- or overestimation of side effects and pain intensity experienced after TRUS-GB since a similar proportion of our patients (19%) experienced an unacceptable pain (score of 8–10 on NRS) as compared to 22% of 162 patients in a prospective study about side effects of TRUS-GB (17). Different number of core biopsies in the two biopsy methods can be seen as an additional limitation concerning the comparison of side effects. However, we believe that the side effects and the pain intensity can be compared. A further criticism is the positive bias against MRI-GB, since patients with many negative TRUS-GB and continued fear about prostate cancer are likely to be more positive towards MRI-GB and its ability to detect cancer.
In conclusion, MRI-GB of the prostate seems to be accepted and preferred to TRUS-GB by a majority of patients. Our study shows that 82% of our patients who underwent MRI-GB after at least one prior negative TRUS-GB and persistent suspicion of prostate cancer, would undergo MRI-GB again. The lower rate of side effects that is possibly due to the smaller number of punch biopsies in MRI-GB may explain this preference.
Acknowledgments
We thank Bettina Herwig, BA and Laura Graziani, BA for translation support and manuscript correction.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. http://dx.doi.org/10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinlan MR, Casey RG, Flynn R, Grainger R, McDermott TE, Thornhill JA. A review of repeat prostate biopsies and the influence of technique on cancer detection: our experience. Ir J Med Sci. 2009;178:287–290. doi: 10.1007/s11845-009-0362-0. http://dx.doi.org/10.1007/s11845-009-0362-0. [DOI] [PubMed] [Google Scholar]
- 3.Roehrborn CG, Pickens GJ, Sanders JS. Diagnostic yield of repeated transrectal ultrasound-guided biopsies stratified by specific histopathologic diagnoses and prostate specific antigen levels. Urology. 1996;47:347–352. doi: 10.1016/s0090-4295(99)80451-8. http://dx.doi.org/10.1016/S0090-4295(99)80451-8. [DOI] [PubMed] [Google Scholar]
- 4.Anastasiadis AG, Lichy MP, Nagele U, et al. MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur Urol. 2006;50:738–748. doi: 10.1016/j.eururo.2006.03.007. http://dx.doi.org/10.1016/j.eururo.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz M. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology. 2005;234:576–581. doi: 10.1148/radiol.2342031887. http://dx.doi.org/10.1148/radiol.2342031887. [DOI] [PubMed] [Google Scholar]
- 6.Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520–527. doi: 10.1016/j.juro.2009.10.022. http://dx.doi.org/10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Irani J, Fournier F, Bon D, Gremmo E, Dore B, Aubert J. Patient tolerance of transrectal ultrasound-guided biopsy of the prostate. Br J Urol. 1997;79:608–610. doi: 10.1046/j.1464-410x.1997.00120.x. http://dx.doi.org/10.1046/j.1464-410X.1997.00120.x. [DOI] [PubMed] [Google Scholar]
- 8.Palisaar J, Eggert T, Graefen M, Haese A, Huland H. Transrectal ultrasound-guided punch biopsies of the prostate. Indication, technique, results, and complications. Der Urologe Ausg A. 2003;42:1188–1195. doi: 10.1007/s00120-003-0422-4. http://dx.doi.org/10.1007/s00120-003-0422-4. [DOI] [PubMed] [Google Scholar]
- 9.Peyromaure M, Ravery V, Messas A, Toublanc M, Boccon-Gibod L, Boccon-Gibod L. Pain and morbidity of an extensive prostate 10-biopsy protocol: a prospective study in 289 patients. J Urol. 2002;167:218–221. http://dx.doi.org/10.1097/00005392-200201000-00049. [PubMed] [Google Scholar]
- 10.Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopy in patients with at least one transrectal US-guided biopsy with a negative finding – multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–172. doi: 10.1148/radiol.10101251. http://dx.doi.org/10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 11.Pondman KM, Futterer JJ, ten Haken B, et al. MR-guided biopsy of the prostate: an overview of techniques and a systematic review. Eur Urol. 2008;54:517–527. doi: 10.1016/j.eururo.2008.06.001. http://dx.doi.org/10.1016/j.eururo.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Breivik EK, Bjornsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16:22–28. doi: 10.1097/00002508-200003000-00005. http://dx.doi.org/10.1097/00002508-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Laubenthal H, Becker M, Neugebauer E. Guideline: Treatment of acute perioperative and posttraumatic pain. Updating from the S2- to the S3-level: a preliminary report. Anasthesiol Intensivmed Notfallmed Schmerzther. 2006;41:470–472. doi: 10.1055/s-2006-949507. http://dx.doi.org/10.1055/s-2006-949507. [DOI] [PubMed] [Google Scholar]
- 14.Krishna NS, Kumar PM, Morrison L. Patients’ tolerance of transrectal ultrasound-guided prostatic biopsy: an audit of 104 cases. BJU Int. 1999;84:890. [PubMed] [Google Scholar]
- 15.Crundwell MC, Cooke PW, Wallace DM. Patients’ tolerance of transrectal ultrasound-guided prostatic biopsy: an audit of 104 cases. BJU Int. 1999;83:792–795. doi: 10.1046/j.1464-410x.1999.00011.x. http://dx.doi.org/10.1046/j.1464-410x.1999.00011.x [DOI] [PubMed] [Google Scholar]
- 16.Sieber PR, Rommel FM, Agusta VE, Breslin JA, Huffnagle HW, Harpster LE. Antibiotic prophylaxis in ultrasound guided transrectal prostate biopsy. J Urol. 1997;157:2199–2200. http://dx.doi.org/10.1016/S0022-5347(01)64716-5. [PubMed] [Google Scholar]
- 17.Chopra S, Rowe EW, Laniado M, Patel A. A prospective study analysing the effect of pain on probe insertion, and the biopsy strategy, on the patients’ perception of pain during TRUS-guided biopsy of the prostate. N Z Med J. 2008;121:39–43. [PubMed] [Google Scholar]