Abstract
PURPOSE
We aimed to evaluate iatrogenic renal arterial lesions, including pseudoaneurysm, arteriovenous fistula, and arteriocaliceal fistula, their management by endovascular embolization, and the clinical results.
METHODS
Fifty-five patients (forty males, fifteen females) with a median age of 40 years (range, 8–85 years), who underwent endovascular embolization of iatrogenic renal arterial lesions between March 2003 and December 2013 were included in this retrospective study. Types of iatrogenic lesions and details of embolization procedures were reported. Estimated glomerular filtration rate (eGFR), renal function tests, hemoglobin, and hematocrit levels before and after embolization were recorded and compared.
RESULTS
Median follow-up was 24 months. We identified 53 pseudoaneurysms, 30 arteriovenous fistulas, and 11 arteriocaliceal fistulas in 55 patients, after percutaneous nephrolithotomy (n=26), renal biopsy (n=21), nephrostomy (n=3), renal surgery (n=3), and extracorporeal shock wave lithotripsy (n=2). Median number of pseudoaneurysms was 1 (range, 1–4) with a median size of 7 mm (range, 1.5–35 mm). Fifty-one patients underwent coil embolization. Median number of coils was 5 (range, 2–21) and median renal parenchymal loss was 5% (range, 1%–50%). There were no significant differences between pre- and postoperative eGFR and serum parameters.
CONCLUSION
Iatrogenic renal arterial lesion can be a life threatening condition. Superselective coil embolization is a safe, minimally invasive treatment option with minimal renal parenchymal loss and without significant change in renal function.
Iatrogenic renal arterial lesions including pseudoaneurysm (PA), arteriovenous fistula (AVF), and arteriocaliceal fistula (ACF) are rare, but life-threatening conditions (1). The chief symptom usually includes macroscopic hematuria (2, 3). Catheter angiography is the gold standard for both diagnosis and treatment (1).
Previous studies have evaluated the iatrogenic renal arterial lesions following partial nephrectomy (1, 3–7), but there were only a few studies on iatrogenic renal arterial lesions following any iatrogenic renal interventions (2). In the present study, to the best of our knowledge, we report the largest series of iatrogenic renal arterial lesions following various renal interventions such as biopsy, percutaneous nephrolithotomy (PCNL), percutaneous nephrostomy, and partial nephrectomy. We focused on clinical presentations, imaging findings, management, and outcomes.
Methods
Patients
Data regarding iatrogenic renal arterial lesions treated by endovascular embolization performed at our institution between March 2003 and December 2013 were evaluated retrospectively. A total of 55 patients with iatrogenic renal arterial lesions, who underwent endovascular embolization were included in the study. There were 40 males and 15 females with a median age of 40 years (range, 8–85 years). Patients with traumatic renal arterial lesions treated with endovascular embolization were excluded from the study. Informed consent was obtained from all patients.
Data collection
Patients’ demographics, symptoms, lesions, and concomitant diseases were noted. Arterial lesions on angiograms were classified under three groups: PA, AVF, and ACF. We tried to determine whether these lesions were isolated or associated with other lesions. Additionally, the etiology of the iatrogenic lesions was determined. Other variables were as follows: the number and size of PA, the number of AVF, the number of ACF, the location of the lesion, presence of perirenal hematoma, volume of used contrast media, the number and type of embolic material, the rate of parenchymal loss after embolization, blood transfusion, 30-day mortality, and length of follow-up. Parenchymal loss was evaluated by comparing angiography images obtained before and after the embolization. Angiography images were independently evaluated by two interventional radiologists. Final angiography findings were reached in consensus. The parenchymal loss following the endovascular procedures was categorized in four groups; <5%, 5%–10%, 10%–25%, and 25%–50%. Early and late complications were determined and computed tomography (CT) angiography was performed for follow-up. Preoperative international normalized ratio (INR) and the number of platelets were determined. Preoperative and postoperative serum urea, creatinine, hemoglobin, and hematocrit values were noted. These measurements were performed using patient sera and the Modular System (Roche Cobas 8000 System, Roche Diagnostics). Preoperative and postoperative parameters were assessed one day before embolization and two days after embolization, respectively. Additionally, to evaluate contrast media-induced nephropathy (CIN), we recorded serum creatinine levels during postoperative day 2 to day 5 and considered the highest levels of serum creatinine in this period. CIN, in which renal function indices begin to deteriorate within 24 h, peaking at ∼3–5 days after contrast media administration, is defined as a ≥ 44 μmol/L (0.5 mg/dL) or ≥ 25% increase in serum creatinine levels in the absence of any other cause that might explain deterioration of the renal function (8–10). Preoperative and postoperative estimated glomerular filtration rates (eGFRs) were also noted. The eGFR was calculated with the Modification of Diet in Renal Disease equation (11).
Embolization procedure
Transfemoral arteriography was performed under intravenous sedation. After obtaining a vascular access by placing a 5 F sheath, selective catheterization and angiography of the renal artery (4 F or 5 F Cobra or Simmons 2) were performed. Interlobar arteries causing the bleeding were detected and superselective catheterization was performed using microcatheter systems (Progreat Microcatheter; Terumo Medical). The feeding arteries were embolized using coils in 51 patients. Coils were placed to occlude flow into the arterial lesion with minimal parenchymal loss. Embolization was completed when blood flow into the lesion stopped. We used pushable coils (0.018-inch Tornado coils; Cook or VORTX-18 Diamond Shape coils; Boston Scientific) in 49 patients and detachable coils (GDC coils; Boston Scientific) in two patients. In one patient, coils were used together with glue. Amplatzer vascular plug (AVP) was used as an embolic material in three patients and detachable balloon was used in the remaining patient. These four patients had high-flow fistulae and because of migration risk, we used AVP and detachable balloon that permitted endovascular treatment in a more controlled manner than coils. As contrast media, we used Iopamiro 370 (Iopamidol, Bracco), which is a low-osmolar contrast media.
Statistical analysis
Not normally distributed parameters were presented as median (minimum-maximum). Preoperative and postoperative eGFR and serum parameters were presented as mean±standard deviation. We compared pre- and postoperative values of eGFR, serum urea, creatinine, hemoglobin, and hematocrit. Statistical analyses were performed with paired t test. P < 0.05 was considered to indicate statistical significance.
Results
The most common symptom was macroscopic hematuria (n=37). Fourteen patients had flank pain, and four patients had both hematuria and flank pain. Twenty-seven patients had perirenal hematoma on radiological examinations with a median diameter of 19 mm (range, 5–130 mm). Seventeen patients had hypertension, while no patients had diabetes mellitus. Iatrogenic lesions occurred in the early period following the intervention in all but one patient, who developed an iatrogenic lesion two years later. The etiologies of the renal interventions were as follows: PCNL (n=26), renal biopsy (n=21), percutaneous nephrostomy (n=3), renal surgery (n=3), and extracorporeal shock wave lithotripsy (n=2). Renal biopsy was performed in native kidneys of 14 patients due to nephritic syndrome (n=6), proteinuria (n=6), systemic lupus erythematosus nephropathy (n=1), hypertensive nephropathy (n=1), and in transplanted kidneys of the remaining seven patients due to nephritic syndrome (n=6) and renal insufficiency secondary to posterior urethral valve (n=1). Percutaneous nephrostomy was performed in three patients due to hydronephrosis (n=2) and vesical invasion of rectum cancer (n=1). Renal surgeries were partial nephrectomy due to renal tumor (n=1), open nephrolithotomy due to staghorn kidney calculi in ectopic kidney (n=1), and renal transplantation due to hypertensive nephropathy (n=1).
We identified 53 PAs, 30 AVFs, and 11 ACFs in 55 patients. PA was found in 17 patients, while a combination of PA and AVF (Fig. 1), and a combination PA and ACF were diagnosed in 17 and six patients, respectively. AVF was diagnosed in 10 patients, while ACF (Fig. 2) and a combination of AVF and ACF were found in two and three patients, respectively. None of the patients had all three types of lesions together. Iatrogenic lesions of eight patients were in transplanted kidneys. Of 47 patients, 19 had iatrogenic lesions in the right kidney and 28 in the left kidney. Of 47 patients, 31 had lesions in the lower pole, 10 in the upper pole and six in the middle segments. All lesions were localized in the distal renal artery.
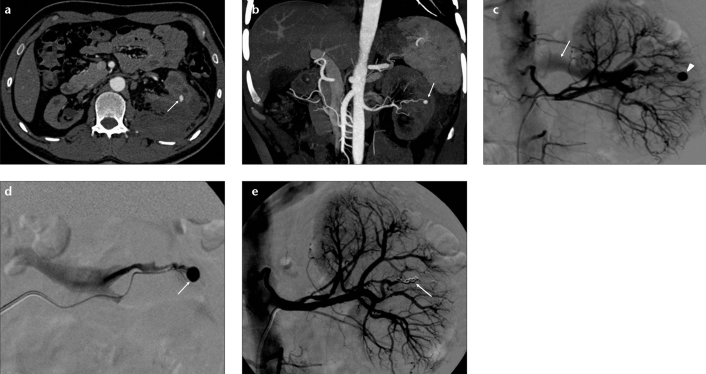
Figure 1. a–e.
Axial CT angiography image (a) of a 31-year-old male patient shows a pseudoaneurysm (arrow) in the left kidney and perirenal hematoma developed following biopsy. Coronal CT angiography reconstruction image (b) shows the pseudoaneurysm (arrow) in the middle segment of the kidney. Selective renal diagnostic angiography image (c) shows the pseudoaneurysm (arrowhead) and filling of renal vein (arrow) indicating an arteriovenous fistula. Superselective renal angiography image (d) shows the pseudoaneurysm (arrow). Control angiography (e) reveals total embolization with coils (arrow).
Figure 2. a–c.
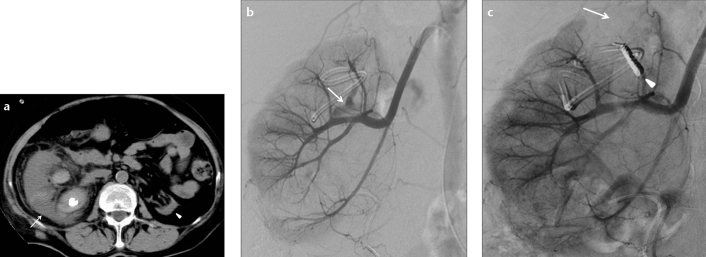
Axial CT image (a) of a 75-year-old female patient who underwent percutaneous nephrolithotomy shows the right kidney (arrow) having hydronephrosis and nephrolithiasis. The left kidney (arrowhead) is atrophic. Selective renal diagnostic angiography image (b) shows caliceal filling (arrow) at the upper pole of right kidney indicating an arteriocaliceal fistula. Control angiography (c) reveals total embolization with coils (arrowhead) and parenchymal area devascularized by the embolization (arrow).
Median number of PAs per patient was 1 (range, 1–4), with a median size of 7 mm (range, 1.5–35 mm) (Fig. 3). Fifty-one patients underwent coil embolization and a median of 5 coils (range, 2–21) were used in each patient. Rate of parenchymal loss was <5% in 16 patients, 5%–10% in 26 patients, 10%–25% in 11 patients, and 25%–50% in two patients. Median rate of renal parenchymal loss was 5% (range, 1%–50%). A median of 0.5 units (range, 0–6 units) of blood was transfused to patients. Mean INR was 1.0±0.09 and mean platelet count was 289 600±128 770 /µL. Median volume of used contrast media was 55 mL (range, 40–80 mL). Pre- and postoperative values of eGFR, serum urea, creatinine, hemoglobin, and hematocrit values were not significantly different (Table). After embolization, CIN occurred in four patients only.
Figure 3. a–c.
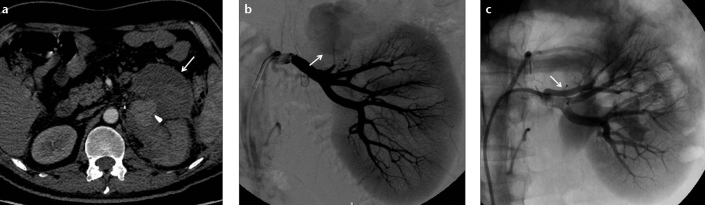
Axial CT angiography image (a) of a 43-year-old male patient shows a pseudoaneurysm (arrowhead) in the left kidney, which occurred following partial nephrectomy and perirenal hematoma (arrow). Selective renal diagnostic angiography image (b) shows the pseudoaneurysm (arrow) at the upper pole of the kidney. Control angiography (c) reveals complete embolization with an Amplatzer vascular plug (arrow).
Median follow-up was 24 months (range, 1–132 months). Six patients had rebleeding in the early follow-up period and underwent reembolization with coils. One of these patients had a massive hematoma in retroperitoneal space three days after embolization; the left renal artery was embolized with polyvinyl alcohol and coils, but the patient expired a few hours after reembolization. One patient had a late complication; she experienced a renal arteriovenous malformation after five years following AVF embolization and the malformation was embolized with Onyx.
Discussion
This study evaluated iatrogenic renal arterial lesions, their management by endovascular embolization, and the clinical results. The initial results were positive, demonstrating high technical and clinical success rates in patients. In the early follow-up period, endovascular embolization did not lead to any deterioration of renal function, and rebleeding, treated by reembolization, was determined in six of 55 patients (10.9%).
Hemorrhage is the most common complication after renal interventions, with a reported incidence of up to 6% (4–6). It can appear clinically as macroscopic hematuria or perirenal hematoma. PA seems to be the most common lesion after renal interventions (4, 7, 12, 13). However, in a series of 200 patients who underwent laparoscopic partial nephrectomy, PA was not seen in any of 12 patients experiencing hemorrhage (6). Rare complications may include urinary fistula and renal artery thrombosis (5). The time period between renal intervention and presence of iatrogenic renal arterial lesions may be varied. Complications usually occur within three weeks following the interventions. However, it should be noted that occasionally complications may occur a long time after the intervention.
Richstone et al. (14) reported that 57 out of 4695 patients (1.2%) required selective endovascular embolization following percutaneous renal interventions (PCNL was the etiology for 44 of 57 patients). Srivastava et al. (15) reported embolization after PCNL in 24 out of 1854 patients and found a combination of PA and AVF in four cases. In their study, serum creatinine values were not significantly different after PCNL or embolization. In our series 26 patients underwent embolization after PCNL, and a combination of PA and AVF was present in five of these 26 patients. Moreover, isolated ACF was seen in two patients after PCNL and a combination of ACF and another arterial lesion was seen in six of these 26 patients. Rastogi et al. (16) reported a case with a combination of AVF and caliceovenous fistula after PCNL and pointed out that serum creatinine values increased in this patient. However, caliceovenous fistula was self-limiting unlike arterial bleeding (14, 15, 17).
Today partial nephrectomy, called nephron-sparing surgery, can be performed in urology clinics, specifically in the case of solitary kidney, renal insufficiency, or even in patients with a normal contralateral kidney (18). PA and AVF are rare complications of partial nephrectomy. Approximately 40 cases of PA after partial nephrectomy have been reported (19–24). In the literature, PA rate was about 0.43% for open partial nephrectomy (4) and 1.7% for laparoscopic partial nephrectomy (3). Ghoneim et al. (25) reported their complication rates as 0.6% for open partial nephrectomy and 2.6% for laparoscopic partial nephrectomy. In a multicenter study of 998 patients who underwent minimally invasive partial nephrectomy, Hyams et al. (1) found the frequency of iatrogenic vascular lesions and PA as 2% and 1.7%, respectively. However, the real frequency of PA might be higher when considering the asymptomatic PAs. There was only one patient who underwent open partial nephrectomy in our study, and a combination of PA and ACF was detected in this patient.
PA is a collection of blood leakage completely out of renal artery, but confined next to the vessel by the surrounding tissue. PA was the most frequent iatrogenic renal arterial lesion occurring in 40 of 55 patients in our study. In case of a renal arterial injury, hemostatic mechanisms such as decreased blood flow, coagulation, and pressure of surrounding tissues attempt to control bleeding. When these mechanisms fail and blood flow to the injured artery increases, blood leakage may form a PA. A true aneurysm is distinguished from a PA by the involvement of all three layers of the arterial wall (intima, media, and adventitia). When the PA becomes larger and blood leakage occurs into the collecting system, macroscopic hematuria may occur (26). In the literature, macroscopic hematuria and/or flank pain were reported in almost all cases with PA (25, 27). In line with previously reported studies, all patients were symptomatic in the present series. Albani and Novick (4) reported one asymptomatic patient with PA, and Ghoneim et al. (25) reported two asymptomatic patients with PA.
An AVF is an abnormal communication between the arterial and venous systems without an intervening capillary bed (25). AVF may be asymptomatic, but may cause macroscopic hematuria, hypertension or cardiac failure (in rare cases), and renal transplant dysfunction due to “intrarenal steal phenomenon” (27). The frequency of simultaneous PA and AVF is unknown, yet. PA and AVF were present together in 17 of 55 patients, in our study.
An ACF is an abnormal communication between the arterial and caliceal systems. In our study, two patients had isolated ACF while nine patients had ACF with another arterial lesion. Unlike our study, ACF was not mentioned as an iatrogenic lesion following renal interventions in the previous studies.
CT angiography is often used as one of the first suggested radiological examination for diagnosis. In emergency conditions, CT angiography is advantageous over the other imaging modalities. Arterial and nephrographic phases of CT angiography should be preferably performed to detect iatrogenic renal vascular lesions. In our study, all patients were examined by CT angiography (80–100 mL of Iopamiro 370). Iatrogenic renal arterial lesions can be best visible on the arterial phase of CT angiography. PA appears as a clear hyperdense lesion on early phases of CT angiography, while it looks like a cystic lesion on grey-scale ultrasonography. Thus, grey-scale ultrasonography is not sufficient for differential diagnosis of PA. However, flow within the lesion indicating a PA can be seen on color Doppler ultrasonography, which can be used in stable patients. Magnetic resonance (MR) angiography can also be used in patients with iodine contrast allergy (28).
Angiography is required for definitive diagnosis of the iatrogenic vascular lesions following renal interventions. Before endovascular embolization, surgery was the only treatment choice. Nowadays, endovascular embolization is one of the first minimally invasive treatment options for these lesions (3). Successful renal artery embolization is defined as total and permanent closure of the injured renal artery branch (12). However, it should be considered that closure of proximal vessels or the nearby intact vessels may lead to a remarkable renal parenchymal loss. In our study, coils were used in 51 of 55 patients and we performed successful embolization in 49 of 55 patients. Six patients underwent reembolization because of early recurrence, and one of these six patients expired a few hours after reembolization. Ghoneim et al. (25) demonstrated that 14 of 15 patients (93%) were successfully treated by embolization and the remaining patient with hemophilia underwent surgery. Sildiroglu et al. (29) stated that thrombin and coils can be used for embolization of large and central PAs with minimal parenchymal loss. Difficulties of the embolization procedures include renal insufficiency, tortuous vessels, and renal artery stenosis (12). Carbon dioxide may be used instead of iodinated contrast media to avoid contrast nephropathy in patients with renal insufficiency. In case of a renal artery stenosis, embolization may be performed after the recanalization treatment of renal artery. Complications of endovascular embolization such as renal artery dissection, postembolization syndrome (21) and loss of renal function (30) are rare. Renal function can be negatively affected by nephrotoxic iodinated contrast media or devascularization of a part of the renal parenchyma secondary to embolization. Superselective embolization performed as distal as possible minimizes parenchymal loss of functional renal tissue to 0% to 15% (30). No deterioration of renal function after embolization was detected in previous series (12). In accordance with the literature, we did not find a negative effect of embolization on renal function. Only four patients experienced CIN in our study. CIN may be caused by CT angiography examinations performed shortly before embolization or by embolization procedures.
Limitations of our study include its retrospective design and variable patient follow-up. Additionally, our evaluation for renal function impairment is limited to renal function tests and eGFR. Lastly, parenchymal loss can be more correctly evaluated with volumetric CT or MR angiography measurements, but angiography was not performed in all patients after embolization. Because our study was retrospective, we evaluated parenchymal loss on angiography images. However, according to our best knowledge, this is the largest reported series of iatrogenic renal arterial lesions following renal interventions in the literature.
In conclusion, renal iatrogenic arterial lesions should be considered in patients with macroscopic hematuria or flank pain following renal interventions. CT and MR angiography are usually performed for diagnosis of these lesions. Endovascular embolization can be safely and effectively performed in iatrogenic renal arterial lesions, and it has no negative effect on renal function.
Table.
Comparison of pre- and postprocedural eGFR and serum parameters
| Parameters | Before embolization (n=55) | After embolization (n=55) | P |
|---|---|---|---|
| eGFR (mL/min/1.73 m2) | 71.3±32.7 | 65.5±34.1 | 0.194 |
| Serum urea (mg/dL) | 48.2±30.3 | 49±30 | 0.455 |
| Serum creatinine (mg/dL) | 1.6±1.4 | 1.7±1.5 | 0.202 |
| Serum Hb (g/dL) | 10.3±1.6 | 10.2±1.2 | 0.903 |
| Serum Htc (%) | 30.7±4.8 | 30.6±3.8 | 0.893 |
Data are given as mean±standard deviation. Paired t test was used for statistical analyses.
eGFR, estimated glomerular filtration rate; Hb, hemoglobin; Htc, hematocrit.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Hyams ES, Pierorazio P, Proteek O, et al. Iatrogenic vascular lesions after minimally invasive partial nephrectomy: a multi-institutional study of clinical and renal functional outcomes. Urology. 2011;78:820–826. doi: 10.1016/j.urology.2011.04.063. http://dx.doi.org/10.1016/j.urology.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Inci K, Cil B, Yazici S, et al. Renal artery pseudoaneurysm: complication of minimally invasive kidney surgery. J Endourol. 2010;24:149–154. doi: 10.1089/end.2009.0342. http://dx.doi.org/10.1089/end.2009.0342. [DOI] [PubMed] [Google Scholar]
- 3.Singh D, Gill IS. Renal artery pseudoaneurysm following laparoscopic partial nephrectomy. J Urol. 2005;174:2256–2259. doi: 10.1097/01.ju.0000181827.49239.8e. http://dx.doi.org/10.1097/01.ju.0000181827.49239.8e. [DOI] [PubMed] [Google Scholar]
- 4.Albani JM, Novick AC. Renal artery pseudoaneurysm after partial nephrectomy: three case reports and a literature review. Urology. 2003;62:227–231. doi: 10.1016/s0090-4295(03)00364-9. http://dx.doi.org/10.1016/S0090-4295(03)00364-9. [DOI] [PubMed] [Google Scholar]
- 5.Van Poppel H, Bamelis B, Oyen R, Baert L. Partial nephrectomy for renal cell carcinoma can achieve long-term tumor control. J Urol. 1998;160:674–678. doi: 10.1016/S0022-5347(01)62751-4. http://dx.doi.org/10.1097/00005392-199809010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ramani AP, Desai MM, Steinberg AP, et al. Complications of laparoscopic partial nephrectomy in 200 cases. J Urol. 2005;173:42–47. doi: 10.1097/01.ju.0000147177.20458.73. http://dx.doi.org/10.1097/01.ju.0000147177.20458.73. [DOI] [PubMed] [Google Scholar]
- 7.Chatziioannou A, Mourikis D, Awad M, Konstantinedes P, Panourgias E, Vlachos L. Embolization of a segmental renal artery pseudoaneurysm after partial nephrectomy in a solitary kidney. Urol Int. 2000;64:223–225. doi: 10.1159/000030536. http://dx.doi.org/10.1159/000030536. [DOI] [PubMed] [Google Scholar]
- 8.Lindholt JS. Radiocontrast induced nephropathy. Eur J Vasc Endovasc Surg. 2005;25:296–304. doi: 10.1053/ejvs.2002.1824. http://dx.doi.org/10.1053/ejvs.2002.1824. [DOI] [PubMed] [Google Scholar]
- 9.Stacul F, van der Molen AJ, Reimer P. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. doi: 10.1007/s00330-011-2225-0. http://dx.doi.org/10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 10.Bedolla-Barajas M, Hernández-Colín DD, Morales-Romero J, Serrano-Salinas C. Immediate and nonimmediate reactions induced by contrast media: incidence, severity and risk factors. Asia Pac Allergy. 2013;3:241–248. doi: 10.5415/apallergy.2013.3.4.241. http://dx.doi.org/10.5415/apallergy.2013.3.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. http://dx.doi.org/10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Heye S, Maleux G, Van Poppel H, Oyen R, Wilms G. Hemorrhagic complications after nephron-sparing surgery: angiographic diagnosis and management by transcatheter embolization. AJR Am J Roentgenol. 2005;184:1661–1664. doi: 10.2214/ajr.184.5.01841661. http://dx.doi.org/10.2214/ajr.184.5.01841661. [DOI] [PubMed] [Google Scholar]
- 13.Parsons JK, Schoenberg MP. Renal artery pseudoaneurysm occurring after partial nephrectomy. Urology. 2001;58:105. doi: 10.1016/s0090-4295(01)00967-0. http://dx.doi.org/10.1016/S0090-4295(01)00967-0. [DOI] [PubMed] [Google Scholar]
- 14.Richstone L, Reggio E, Ost MC, et al. Hemorrhage following percutaneous renal surgery: characterization of angiographic findings. J Endourol. 2008;22:1129–1135. doi: 10.1089/end.2008.0061. http://dx.doi.org/10.1089/end.2008.0061. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava A, Singh KJ, Suri A, et al. Vascular complications after percutaneous nephrolithotomy: are there any predictive factors? Urology. 2005;66:38–40. doi: 10.1016/j.urology.2005.02.010. http://dx.doi.org/10.1016/j.urology.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi N, Zawacki W, Alencar H. Coexisting intrarenal arteriovenous and caliceovenous fistulae after percutaneous nephrolithotomy: Case report and literature review. Interv Med Appl Sci. 2013;5:81–84. doi: 10.1556/IMAS.5.2013.2.5. http://dx.doi.org/10.1556/IMAS.5.2013.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Rosette J, Assimos D, Desai M, et al. The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: indications, complications, and outcomes in 5803 patients. J Endourol. 2011;25:11–17. doi: 10.1089/end.2010.0424. http://dx.doi.org/10.1089/end.2010.0424. [DOI] [PubMed] [Google Scholar]
- 18.Van Poppel H, Dilen K, Baert L. Incidental renal cell carcinoma and nephron sparing surgery. Curr Opin Urol. 2001;11:281–286. doi: 10.1097/00042307-200105000-00007. http://dx.doi.org/10.1097/00042307-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Cohenpour M, Strauss S, Gottlieb P, et al. Pseudoaneurysm of the renal artery following partial nephrectomy: imaging findings and coil embolization. Clin Radiol. 2007;62:1104–1109. doi: 10.1016/j.crad.2007.06.004. http://dx.doi.org/10.1016/j.crad.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Negoro H, Kawakita M, Koda Y. Renal artery pseudoaneurysm after laparoscopic partial nephrectomy for renal cell carcinoma in a solitary kidney. Int J Urol. 2005;12:683–685. doi: 10.1111/j.1442-2042.2005.01130.x. http://dx.doi.org/10.1111/j.1442-2042.2005.01130.x. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MJ, Smith EB, Trost DW, Vaughan ED., Jr Renal artery embolization: clinical indications and experience from over 100 cases. BJU Int. 2007;99:881–886. doi: 10.1111/j.1464-410X.2006.06653.x. http://dx.doi.org/10.1111/j.1464-410X.2006.06653.x. [DOI] [PubMed] [Google Scholar]
- 22.Bozgeyik Z, Ozdemir H, Orhan I, Cihangiroglu M, Cetinkaya Z. Pseudoaneurysm and renal arteriovenous fistula after nephrectomy: two cases treated by transcatheter coil embolization. Emerg Radiol. 2008;15:119–122. doi: 10.1007/s10140-007-0646-5. http://dx.doi.org/10.1007/s10140-007-0646-5. [DOI] [PubMed] [Google Scholar]
- 23.Taneja M, Tan KT. Renal vascular injuries following nephron-sparing surgery and their endovascular management. Singapore Med J. 2008;49:63–66. [PubMed] [Google Scholar]
- 24.Zelenák K, Sopilko I, Svihra J, Kliment J. Successful embolization of a renal artery pseudoaneurysm with arteriovenous fistula and extravasations using Onyx after partial nephrectomy for renal cell carcinoma. Cardiovasc Intervent Radiol. 2009;32:163–165. doi: 10.1007/s00270-008-9332-6. http://dx.doi.org/10.1007/s00270-008-9332-6. [DOI] [PubMed] [Google Scholar]
- 25.Ghoneim TP, Thornton RH, Solomon SB, Adamy A, Favaretto RL, Russo P. Selective arterial embolization for pseudoaneurysms and arteriovenous fistula of renal artery branches following partial nephrectomy. J Urol. 2011;185:2061–2065. doi: 10.1016/j.juro.2011.02.049. http://dx.doi.org/10.1016/j.juro.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro EY, Hakimi AA, Hyams ES, Cynamon J, Stifelman M, Ghavamian R. Renal artery pseudoaneurysm following laparoscopic partial nephrectomy. Urology. 2009;74:819–823. doi: 10.1016/j.urology.2009.03.056. http://dx.doi.org/10.1016/j.urology.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi K, Censullo ML, Rossman LL, Kyriakides PN, Kahan BD, Cohen AM. Interventional radiologic management of renal transplant dysfunction: indications, limitations, and technical considerations. Radiographics. 2007;27:1109–1130. doi: 10.1148/rg.274065135. http://dx.doi.org/10.1148/rg.274065135. [DOI] [PubMed] [Google Scholar]
- 28.Uberoi J, Badwan KH, Wang DS. Renal-artery pseudoaneurysm after laparoscopic partial nephrectomy. J Endourol. 2007;21:330–333. doi: 10.1089/end.2006.0260. http://dx.doi.org/10.1089/end.2006.0260. [DOI] [PubMed] [Google Scholar]
- 29.Sildiroglu O, Saad WE, Hagspiel KD, Matsumoto AH, Turba UC. Endovascular management of iatrogenic native renal arterial pseudoaneurysms. Cardiovasc Intervent Radiol. 2012;35:1340–1345. doi: 10.1007/s00270-011-0325-5. http://dx.doi.org/10.1007/s00270-011-0325-5. [DOI] [PubMed] [Google Scholar]
- 30.Poulakis V, Ferakis N, Becht E, Deliveliotis C, Duex M. Treatment of renal-vascular injury by transcatheter embolization: immediate and long-term effects on renal function. J Endourol. 2006;20:405–409. doi: 10.1089/end.2006.20.405. http://dx.doi.org/10.1089/end.2006.20.405. [DOI] [PubMed] [Google Scholar]