Abstract
PURPOSE
Use of oral sorafenib, an antiangiogenic chemotherapeutic agent for hepatocellular carcinoma (HCC), is limited by an unfavorable side effect profile. Transarterial chemoembolization (TACE) employs targeted intravascular drug administration, and has potential as a novel sorafenib delivery method to increase tumoral concentrations and reduce systemic levels. This study aimed to discern the pharmacokinetics of sorafenib TACE in a rabbit VX2 liver tumor model.
METHODS
A 3 mg/kg dose of sorafenib ethiodized oil emulsion was delivered via an arterial catheter to VX2 liver tumors in seven New Zealand white rabbits. Following TACE, serum sorafenib levels were measured at days 0, 1, 2, 3, 7, 10, and 14 until the time of sacrifice, after which rabbit livers were harvested for analysis of sorafenib concentrations within treated tumors and normal liver. Liquid chromatography tandem mass spectrometry was used for drug quantification.
RESULTS
Sorafenib uptake within liver tumor and nontumorous liver tissue peaked at mean 3.53 and 0.75 μg/mL, respectively, immediately post-procedure (5:1 tumor to normal tissue drug uptake ratio), before decreasing with a 10–18 hour half-life. Serum sorafenib levels peaked immediately after TACE at a mean value of 58.58 μg/mL before normalizing with a 5.2-hour half-life, suggesting early drug washout from liver into the systemic circulation. Hepatic lab parameters showed transient increase 24 hours post-TACE with subsequent resolution.
CONCLUSION
While targeted transarterial delivery of sorafenib ethiodized oil emulsion shows preferential tumor uptake compared to normal liver, systemic washout occurs with a short half-life, resulting in high circulating drug levels.
Sorafenib (Nexavar, Bayer Pharmaceuticals) is an antiangiogenic chemotherapeutic multikinase inhibitor that targets vascular endothelial growth factor (VEGF) receptor (1). This drug is approved by the United States Food and Drug Administration (FDA) as an oral agent for treatment of surgically unresectable primary liver cancer (hepatocellular carcinoma, HCC), and is associated with prolongation in tumor time-to-progression and patient overall survival in phase 3 clinical trials (2, 3). The practical utility of sorafenib is tempered, however, by a high incidence of clinically significant side effects related to systemic distribution, including hand-foot syndrome, nausea, diarrhea, and fatigue, which are frequently cited as reasons for patient noncompliance and drug dose limitation (4); serious adverse events occur in 48%–52% of those patients taking sorafenib (2, 3).
Transarterial chemoembolization (TACE) is a widely performed and recognized locoregional drug delivery methodology. During TACE, chemotherapy is administered to solid tumors in a targeted fashion via arterially placed catheters (5). This procedure, which has shown survival benefit in the treatment of HCC (6, 7), exploits the hepatic arterial perfusion of liver cancer to administer targeted therapy with cytotoxic chemotherapeutic agents, and also aims to devascularize neoplastic tissue by occluding feeding arteries. Conventional TACE consists of a mixture of chemotherapeutic agents and embolic ethiodized oil (5, 8), which slows blood flow through the tumor and sequesters chemotherapy medications to achieve high localized intratumoral drug concentration while limiting systemic release. In contrast to standard oral administration, targeted transarterial delivery of sorafenib has the potential to deliver high localized drug concentrations directly to liver tumors while theoretically reducing extrahepatic levels and diminishing adverse systemic effects of the drug.
A previous investigation aimed at translating the high local drug concentrations and low systemic drug levels conferred by targeted TACE toward intrahepatic delivery of sorafenib has demonstrated the feasibility of transcatheter intra-arterial delivery of lipid-emulsified sorafenib in rabbit livers (9). While this method of sorafenib delivery produced high intrahepatic drug concentrations, this study did not elucidate the pharmacokinetics of conventional sorafenib TACE in terms of comparing intra- and extrahepatic drug levels over time. An understanding of the dynamic local and circulating concentrations of sorafenib after transarterial delivery is necessary to provide a foundation on which to base future studies aimed at correlating plasma drug levels with the incidence of side effects and determining the maximum tolerated dose of transarterially delivered sorafenib. The current investigation was thus conducted to determine the pharmacokinetics of conventional sorafenib TACE and to test the hypothesis that sorafenib TACE yields high tissue levels of sorafenib while minimizing systemic release through temporal assessment of liver tissue and circulating drug levels.
Methods
Institutional Animal Care and Use Committee approval was obtained for this prospective study. The experimental protocol consisted of: (a) development and propagation of a rabbit VX2 hepatic tumor line, a leporine anaplastic squamous cell carcinoma widely accepted and commonly employed by interventional radiologists in preclinical investigations of HCC (10); (b) production of a lipid-emulsified sorafenib preparation; (c) in vivo TACE intravascular delivery of sorafenib emulsion into tumor-laden New Zealand white rabbit livers; (d) sequential assessment of circulating serum sorafenib levels; (e) liver explantation and direct tissue chemotherapeutic analysis to determine intratumoral and intraparenchymal drug concentrations; and (f) assessment of liver function parameters for possible hepatotoxic effects of sorafenib delivery. Of note, the pharmacokinetics of transcatheter arterial chemoinfusion was not investigated in the current study because its relatively reduced clinical use compared to TACE in the transcatheter therapy of HCC.
Rabbit VX2 tumor development and propagation
Development and propagation of the VX2 cell line was performed according to previously described methodology (11). Briefly, flash-frozen rabbit VX2 tumor samples (0.75–1 mL, approximately 107 cells) were defrosted, mixed in methylcellulose media (Stemcell Technologies) in a 1:1 ratio, and injected into the hind limb muscle of donor New Zealand white rabbits for propagation. Approximately 2–3 weeks after implantation, the donor rabbits were sacrificed and the hind limb tumors were excised and transected. Several 1–3 mm pieces of tumor were selected for surgical liver implantation and the remaining viable tumor was harvested from the specimen and strained in order to create a cell suspension using Roswell Park Memorial Institute (RPMI) media (Cellgro). The collected cells were centrifuged at 1600 rotations per minute for eight minutes, after which the supernatant was disposed of and the remaining cell pellet resuspended and mixed in methylcellulose media (Stemcell Technologies) in a 1:1 ratio. The new VX2 cell samples were then immediately injected into the hind limb of another rabbit in order to propagate the cell line.
VX2 liver tumor implantation
All surgical VX2 liver tumor implantations were performed under aseptic conditions with rabbits intubated and maintained under general anesthesia (induction with ketamine 15–30 mg/kg IM and dexmeditomidine 50–250 µg/kg IM, followed by maintenance with 1%–3% isoflurane). Intravenous antibiotic prophylaxis was provided. A mini-laparotomy was created in the subxiphoid area, exposing the liver. Then, two 1–3 mm freshly harvested tumor fragments were implanted in the left hepatic lobe of each animal (n=7). Two small stab incisions were made 2–3 cm apart and approximately 0.5 cm deep in the liver parenchyma, and the tumor pieces placed deep into both. The wounds were then closed with a 0.25 cm2 piece of hemostatic gauze (BloodSTOP iX, PRN Pharmacal) placed over the liver puncture sites. The abdomen was closed in two layers using PDS suture (Ethicon) in the facial layer and absorbable Vicryl suture (Ethicon) for subcuticular closure. After the procedure, the animals were aroused and recovered, returned to cages, and monitored daily for wound healing and appetite until TACE. Liver tumors were incubated for three weeks prior to TACE based on previous experience of suitable 2–3 cm diameter tumor growth within 14–28 days (11).
Preparation of sorafenib solution
Preparation of sorafenib emulsion has been previously described (9). Briefly, sorafenib solution was prepared using a solvent mixture consisting of 12.5% Cremophor EL (Sigma-Aldrich), 12.5% ethyl alcohol (Sigma-Aldrich), and 75% distilled water (Sigma-Aldrich). Sorafenib powder was dissolved in 50% Cremophor EL and 50% ethyl alcohol mixture at 24 mg/mL, facilitated by heating of the mixture to 60°C, after which distilled water was added gradually with mixing to generate the 12 mg/mL dosing solution. The sorafenib solution was then allowed to cool to room temperature prior to use in TACE procedures.
Sorafenib TACE
For sorafenib TACE, New Zealand white rabbits (n=7) were intubated and maintained under general anesthesia. Angiography was performed with a C-arm unit (OEC Medical Systems series 9600, GE Healthcare). The femoral artery was accessed through a surgical cut-down and catheterized with a 3 F vascular sheath (Cook Medical), after which a 2 F catheter (JB1, Cook Medical) was advanced over a guidewire and the celiac artery was selectively catheterized. Angiography of the common and proper hepatic arteries was then performed via injections of iohexol (Omnipaque-300, Amersham Health). After obtaining angiographic confirmation of catheter placement within the proper hepatic artery, sorafenib solution was mixed in a 1:1 ratio with ethiodized oil (Lipiodol, Guerbet) and injected by hand under fluoroscopic visualization until an endpoint of vascular stasis was achieved. The utilized ratio of sorafenib solution to ethiodized oil was selected in order to ensure that the ensuing emulsion consisted of more oil than water phase, as it is known that water-in-oil emulsions result in lower systemic chemotherapy agent concentrations during TACE than oil-in-water emulsions (12); in this manner, the ratio of chemotherapy solution to embolic ethiodized oil can impact drug pharmacokinetics. A 3 mg/kg dose was injected in each rabbit; this dosing rationale was based on peak plasma concentrations (Cmax) and therapeutic drug levels for both humans and rabbits, and has been previously described (9, 13, 14). After completion of the infusion, the catheter was removed, the common femoral artery was ligated using suture to obtain hemostasis, and the groin incision was closed using absorbable Vicryl suture (Ethicon). After the procedure, the animals were aroused and recovered, returned to cages and followed up daily until time of sacrifice.
Animal necropsy and tissue harvest
For measurement of systemic sorafenib levels and liver function parameters, 2–3 mL samples of venous blood were obtained from the marginal ear vein of each rabbit at each of the following selected time points after TACE: preprocedure (labs only), and days 0 (within 30 min post-procedure), 1, 2, 3, 7, 10, and 14 until time of respective sacrifice. For labs, one sample was tested per rabbit at each time point (n=28 total samples, e.g., seven samples were tested at day 0, six samples were tested at day 1, etc.), but for serum, two samples were tested per rabbit at each time point (n=56 total samples, e.g., 14 samples were tested at day 0, 12 samples were tested at day 1, etc.). Rabbits were then sacrificed using a lethal dose of 390 mg/mL pentobarbital sodium solution (Schering-Plough) at analogous time points following TACE: day 0 (within 30 min post-procedure), 1, 2, 3, 7, 10, and 14. Within 30 min of sacrifice, rabbit necropsy was performed and livers were harvested for tissue analysis. Treated tumors were extracted and divided in half for analysis (four tumor halves per animal for total n=28; n=4 at each time point). In addition, two representative 1 cm3 samples of non-tumorous liver parenchyma—one from the left hepatic lobe and one from the right hepatic lobe—were also procured from each rabbit (two samples per animal for total n=14; n=2 at each time point). Specimens were stored in 1 mL of sterile saline and were frozen in liquid nitrogen at −80°C until the time of sorafenib level measurement. Finally, at the time of tumor extraction, a central tumor slice between tumor halves was saved for pathologic analysis in each case; the tissue was fixed in 10% neutral buffered formalin solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histopathologic analysis.
Measurement of tissue and serum sorafenib concentration
Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis was used to determine sorafenib concentrations, as previously described (9). A calibration curve was first created for the drug. Standard sorafenib solutions ranging from 0.1–100 mg/mL (0.1 0.5, 1, 2, 5, 10, 25, 50, and 100 mg/mL) were used to create the standard curve. To prepare liver and serum samples, N-(4-phenoxyphenyl)-N′-phenylurea (Sigma-Aldrich) was added at a concentration of 10 mg/mL as an internal standard to 100 mg of liver homogenized in 1 mL phosphate buffer (10 µM, pH 7.4) or 50 µL of rabbit serum. Then, 100 µL of the homogenate or serum solution was transferred to a 1.7 mL microcentrifuge tube, and 400 µL of 4:1 acetonitrile:ethanol was added for protein precipitation. The solution was then centrifuged (12,000 g for 20 min at 4°C for liver tissue, 13,000 g for 15 min at 4°C for serum), and the supernatant was removed and dried to completeness. The samples were then reconstituted in 1:4 acetonitrile:water, and the supernatant was used for sample analysis.
A Shimadzu Nexera ultrahigh pressure liquid chromatography (UHPLC) system interfaced with a Shimadzu LC-MS 8040 triple quadrupole mass spectrometer was used to carry out UHPLC-MS/MS. A Nexera LC-30AD UHPLC pump with a Nexera Sil-30AC automatic injector was used. The analytes were separated on an ACQUITY UPLC BEH Amide 1.7 mm, 2.1 mm×100 mm column applying a gradient elution with the mobile phase consisting of 0.1% formic acid in acetonitrile as solvent A and water as solvent B. Gradient elution started at 25% and increased to 75% over 3 min, changed to 95% for 2 min, then decreased to 25% for 1 min for re-equilibration. The total analysis time was 7 min, injection volume was 1 µL, flow rate was 0.6 mL/min, and column temperature was 25°C. Data acquisition was performed in multiple reaction monitoring (MRM) positive ionization mode. For sorafenib, transitions of mass-to-charge ratio (m/z) 465 to m/z 252 and m/z 465 to m/z 270 were used for quantification and qualification, respectively, and for N-(4-phenoxyphenyl)-N′-phenylurea (Sigma-Aldrich), transitions of m/z 305 to m/z 94.15 and m/z 305 to m/z 93.10 were used for quantification and qualification, respectively. Both compounds eluted with a retention time of 3.25 min.
Statistical analysis
LC-MS/MS data integration was carried out by LabSolutions software (Shimadzu). Other statistical analyses were performed using SPSS Statistics version 17.0 (SPSS Inc.). Sorafenib and laboratory parameter levels were reported using descriptive statistics as mean±standard deviation for each time point.
Results
Seven rabbits (two males and five females, mean weight, 3.0±0.03 kg) underwent sorafenib TACE. Sorafenib solution was successfully prepared and a total of 9 mg was injected from the proper hepatic artery in each of the seven rabbits. Tissue harvesting and processing were successfully performed in all seven rabbits, and LC-MS/MS analysis of the tissue and serum specimens for sorafenib measurement was technically successful in all cases. Liver and systemic sorafenib pharmacokinetics are summarized in Table 1. Mean sorafenib levels within treated tumor and untreated hepatic parenchyma were highest within 30 min following TACE, with peak concentrations (Cmax) measuring 3.53 mg/mL and 0.75 mg/mL, respectively. Mean circulating serum sorafenib also peaked within 30 min after TACE, with Cmax measuring 58.58 mg/mL. Liver tumor, hepatic tissue, and serum sorafenib half-lives approximated 9.9, 18.3, and 5.2 hours, respectively. Liver tumor, hepatic tissue, and serum sorafenib area under the concentration versus time curve (AUC), calculated using the linear trapezoidal method (15), were 90.2, 54.8, and 839.4 mg/mL·h, respectively.
Table 1.
Temporal evolution of sorafenib concentrations (μg/mL)
| Time | Tumor levels n=4 | Liver tissue levels n=2 | Serum levels* |
|---|---|---|---|
| Day 0 | 3.531±1.738 | 0.753±0.075 | 58.58±37.86 |
| Day 1 | 0.490±0.291 | 0.540±0.397 | 4.867±6.812 |
| Day 2 | 0.122±0.002 | 0.122±0.004 | 0.100±0.000 |
| Day 3 | 0.132±0.021 | 0.110±0.001 | 0.088±0.025 |
| Day 7 | 0.109±0.003 | 0.108±0.000 | 0.067±0.029 |
| Day 10 | 0.108±0.001 | 0.108±0.000 | 0.075±0.035 |
| Day 14 | 0.150±0.028 | 0.112±0.004 | 0.050±0.000 |
Number of serum samples is as follows: Day 0 (n=14), Day 1 (n=12), Day 2 (n=10), Day 3 (n=8), Day 7 (n=6), Day 10 (n=4), Day 14 (n=2).
Biochemical liver function markers obtained at multiple post-procedure time points are summarized in Table 2. There was an early peak in levels of serum bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at post-procedure day 1 (mean 0.18 mg/dL, 1,114 U/L, and 565 U/L, respectively), all of which normalized by day 14, while gamma glutamyltransferase (GGT) levels peaked at day 10 (mean 31.3 U/L) and normalized by day 14.
Table 2.
Temporal evolution of liver function parameters
| Time | Bilirubin* (mg/dL) | AST* (U/L) | ALT* (U/L) | GGT* (U/L) |
|---|---|---|---|---|
| Preprocedure | 0.10±0.01 | 10±1.9 | 18±4.6 | 5.1±1.6 |
| Day 0 | 0.11±0.02 | 17±9.5 | 20±6.2 | 6.2±1.9 |
| Day 1 | 0.18±0.21 | 1.114±1.785 | 565±837 | 10.7±9.6 |
| Day 2 | 0.09±0.01 | 146±209 | 166±176 | 9.1±5.5 |
| Day 3 | 0.14±0.09 | 153±207 | 99±49 | 6.4±3.7 |
| Day 7 | 0.11±0.02 | 143±219 | 161±228 | 13.8±12.3 |
| Day 10 | 0.13±0.00 | 26±24 | 109±132 | 31.3±36.3 |
| Day 14 | 0.10±0.00 | 22±0.0 | 12±0.0 | 5.6±0.0 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase.
Number of animals tested at each time point is as follows: preprocedure (n=7), Day 0 (n=7), Day 1 (n=6), Day 2 (n=5), Day 3 (n=4), Day 7 (n=3), Day 10 (n=2), and Day 14 (n=1).
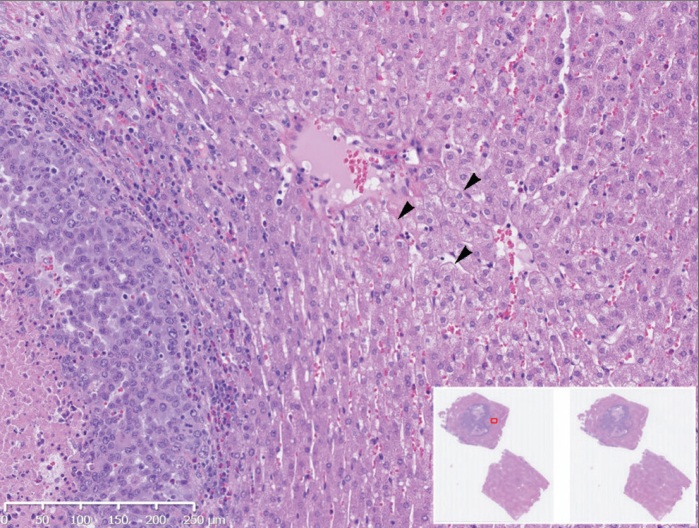
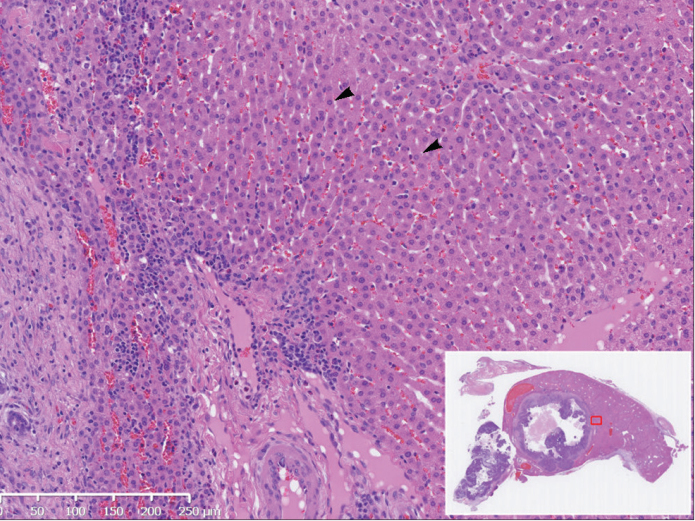
Histologic assessment of tumors, performed upon animal sacrifice at days 0, 1, 2, 3, 7, 10, and 14, showed only nonspecific hepatocyte ballooning degeneration between days 0–3 (Fig. 1), but which appeared to resolve by day 7 (Fig. 2). Of note, no clinical alterations (such as ascites development) were identified in any rabbit subjects.
Figure 1.
Day 3 histopathologic assessment (hematoxylin and eosin stain) of VX2 tumor following sorafenib TACE in a 3.2 kg rabbit. Image shows nonspecific peritumoral hepatocyte ballooning degeneration (arrowheads), demonstrating cellular enlargement and pallor (10× magnification). Inset image displays nonmagnified tumor section map.
Figure 2.
Day 7 histopathologic assessment (hematoxylin and eosin stain) of VX2 tumor following sorafenib TACE in a 2.9 kg rabbit. Image demonstrates unremarkable appearance of peritumoral hepatocytes (arrowheads) (10× magnification). Inset image exhibits nonmagnified tumor section map.
Discussion
While clinically beneficial, the practical utility of oral sorafenib for treatment of HCC is limited by a high incidence of adverse side effects, which frequently lead to dose reduction or discontinuation of therapy. In a study of oral sorafenib combined with doxorubicin drug-eluting bead therapy for treatment of advanced HCC, over 30% of patients required sorafenib dose interruptions and 20% required dose reductions (16), presenting a major detriment to drug utilization. More recently, two meta-analyses have identified higher rates of adverse events with TACE and oral sorafenib combination therapy; these adverse events are predominantly related to oral sorafenib side effects, such as hand-foot syndrome (17, 18). In contrast, TACE-mediated intrahepatic sorafenib infusion theoretically promises to counter tumor angiogenesis while limiting systemic side effects though targeted transarterial delivery, but this approach demands an understanding of the sorafenib pharmacokinetics. In the current investigation, we administered a lipid-emulsified sorafenib preparation to VX2 tumor-laden rabbits, and used LC-MS/MS methods to determine sorafenib levels in hepatic tumor and liver tissue at various time points after TACE. Immediately after TACE, mean intratumoral and nontumorous sorafenib levels were 3.5 and 0.75 mg/mL, respectively, subsequently decreasing with half-lives of 10–18 hours. The ratio of peak sorafenib concentration within tumor tissue as compared to normal liver tissue was nearly 5:1, confirming preferential tumoral drug uptake using targeted transarterial delivery to hypervascular liver tumors. This phenomenon is well-known to occur in TACE, where cancerous tissue takes up arterially injected drug in a 3:1 to 20:1 ratio as compared to normal liver tissue (5, 19). Moreover, our results are in accordance with the outcomes of a study conducted by Chatziioannou et al. (20), in which conventional TACE with sorafenib yielded intrahepatic drug levels of 0.794 mg/mL and 0.064 mg/mL at 24 and 72 hours, respectively.
The intratumoral sorafenib levels attained in the present investigation are reduced as compared to our previously reported experience, in which sorafenib oil emulsion was administered at a 3 mg/kg dose via the left hepatic artery to nontumorous left liver lobes, resulting in immediate postprocedure peak liver tissue levels on the order of 90 mg/mL (9). The basis for this inconsistency may be related to a difference in site of drug administration; namely, sorafenib injection in the current study occurred via the more proximal proper hepatic artery with drug infusion to the entire liver rather than through the more distal left hepatic artery with dispensation to the left hepatic lobe in the prior investigation (9). This difference in technique likely resulted in dilution of the same delivered sorafenib dose over a greater volume of liver tissue—the left hepatic lobe accounts for about 40% of total liver volume (21)—with an anticipated reduction in measured drug concentration. Additional explanations for the concentration discrepancy are also possible. These include inconsistency in the sorafenib emulsion (related to separation) with reduced drug administration, as well as variation related to small sample size.
In examining circulating drug levels after conventional sorafenib TACE in the current study, we found that the systemic release characteristics of sorafenib using this approach were relatively unfavorable. Measurement of dynamic serum sorafenib levels following conventional sorafenib TACE demonstrated comparatively high levels of circulating sorafenib herein, indicating early drug washout from tumor into the systemic circulation. This finding suggests that the degree of embolic occlusion of liver blood vessels, which serves to prevent drug clearance from the liver, was likely insufficient and temporary in the present study, and suggests the need for a more robust and durable extent of vessel embolization to sequester drug within liver tissue. This concept is supported by a biodistribution and pharmacokinetic study of doxorubicin TACE reported by Raoul et al. (22), which revealed an increase in the liver tissue half-life of doxorubicin from 1.8 to 2.6 days through the addition of arterial embolization after administration of doxorubicin emulsion as compared to ethiodized oil emulsion alone. Such embolization may be achieved through injection of particulate agents after ethiodized oil mixture administration, as ethiodized oil is generally considered a non-permanent embolic agent that is cleared from liver by Kupffer cells (23). Another promising avenue for potential investigation includes drug-eluting beads, an embolic platform associated with reduced systemic drug release as compared to ethiodized oil when applied for TACE (24). To this end, the feasibility of targeted delivery of sorafenib loaded poly(lactide-co-glycolide) microspheres has been recently demonstrated (25). As a final note, anatomic characteristics, such as tumor size and presence of arteriovenous communications, may also impact drug pharmacokinetics after TACE. While embolization, be it with particles for large tumors or arterioportal shunts or coils for arteriovenous fistulae, may help dampen variability in drug washout that might precipitate pharmacokinetic differences, some degree of variation is nonetheless to be expected from case to case.
Assessment of laboratory biochemical markers of liver function showed significant post-TACE transaminase elevation, particularly AST. Hepatic lab parameters, including bilirubin, AST, ALT, and GGT, showed transient increase to a peak at 24 hours post-TACE, with subsequent resolution over the course of two weeks. On average, AST values increased to more than 1,000 U/L. The transaminase elevation seen, corroborated by similar findings in the Chatziioannou et al. (20) study, may be suggestive of liver toxicity, hepatocyte damage, and cell death that warrant additional safety investigation, particularly because such transaminase increase has been clinically correlated with reduced survival outcomes following TACE. To this end, investigators who developed the Assessment for Retreatment with TACE (ART) score, which takes AST levels, Child-Pugh score, and radiologic tumor response into account, showed that a 25% increase in AST was independently associated with poorer survival after TACE (26, 27). While all of the rabbits treated in the current study developed an AST elevation of more than 25% baseline level, histologic assessment of tumors showed only nonspecific hepatocyte ballooning degeneration between days 0–3, similar to that seen in prior studies (9), but which appeared to resolve by day 7. Nonetheless, further exploration is necessary to ensure that the transarterial approach to sorafenib delivery is not associated with prohibitive hepatocyte toxicity.
This study has several limitations. First, sorafenib dosing was empirically based on peak and therapeutic plasma concentrations, although this dosing rationale had a strong foundation in peak plasma concentrations and therapeutic drug levels for both humans (13, 14) and rabbits (Bayer Pharmaceutical Corporation, unpublished data), and has previously been explained (9). Second, this was a small, preliminary study with a limited sample size. Third, sorafenib was administered nonselectively from the proper hepatic artery, which likely resulted in broad drug delivery and a suboptimal degree of liver embolization that contributed to high systemic drug washout; future studies should aim for more selective and occlusive injection to minimize this effect. Fourth, although widely accepted as a preclinical HCC model, the VX2 tumor is an imperfect surrogate for human HCC, and sorafenib uptake and distribution may not be analogous.
In conclusion, while transarterial delivery of a lipid-emulsified sorafenib solution showed high liver tumor to normal hepatic tissue uptake ratio, this approach resulted in high levels of circulating sorafenib while exhibiting evidence of early washout from treated tumors. Although these findings again confirm the viability of targeted intrahepatic sorafenib infusion, the degree of systemic drug release after conventional sorafenib TACE merit continued investigation into alternative delivery methods utilizing more embolic delivery platforms to improve drug sequestration and limit systemic release.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. http://dx.doi.org/10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. http://dx.doi.org/10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. http://dx.doi.org/10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Ding X, Wei D, et al. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010;21:326–332. doi: 10.1097/CAD.0b013e3283350e26. http://dx.doi.org/10.1097/CAD.0b013e3283350e26. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S211–221. doi: 10.1016/s1051-0443(07)61789-8. http://dx.doi.org/10.1016/S1051-0443(07)61789-8. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. http://dx.doi.org/10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 7.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. http://dx.doi.org/10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. http://dx.doi.org/10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 9.Gaba RC, Yap FY, Martinez EM, et al. Transarterial sorafenib chemoembolization: preliminary study of technical feasibility in a rabbit model. J Vasc Interv Radiol. 2013;24:744–750. doi: 10.1016/j.jvir.2013.01.488. http://dx.doi.org/10.1016/j.jvir.2013.01.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvinian A, Casadaban LC, Gaba RC. Development, growth, propagation, and angiographic utilization of the rabbit VX2 model of liver cancer: a pictorial primer and “how to” guide. Diagn Interv Radiol. 2014;20:335–340. doi: 10.5152/dir.2014.13415. http://dx.doi.org/10.5152/dir.2014.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaba RC, Baumgarten S, Omene BO, et al. Ethiodized oil uptake does not predict doxorubicin drug delivery after chemoembolization in VX2 liver tumors. J Vasc Interv Radiol. 2012;23:265–273. doi: 10.1016/j.jvir.2011.10.022. http://dx.doi.org/10.1016/j.jvir.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10:425–434. doi: 10.3348/kjr.2009.10.5.425. http://dx.doi.org/10.3348/kjr.2009.10.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. http://dx.doi.org/10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 14.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. http://dx.doi.org/10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 15.Bourget P, Delouis JM. Review of a technic for the estimation of area under the concentration curve in pharmacokinetic analysis. Therapie. 1993;48:1–5. [PubMed] [Google Scholar]
- 16.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 29:3960–3967. doi: 10.1200/JCO.2011.37.1021. http://dx.doi.org/10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e100305. doi: 10.1371/journal.pone.0100305. http://dx.doi.org/10.1371/journal.pone.0100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang M, Yuan JQ, Bai M, Han GH. Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Mol Biol Rep. 2014;41:6575–6582. doi: 10.1007/s11033-014-3541-7. http://dx.doi.org/10.1007/s11033-014-3541-7. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. http://dx.doi.org/10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Chatziioannou AN, Siskos AP, Loxas D, et al. Transarterial embolization with sorafenib in animal livers: a pharmacokinetics study. J Vasc Interv Radiol. 2013;24:1657–1663. doi: 10.1016/j.jvir.2013.08.007. http://dx.doi.org/10.1016/j.jvir.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Kamel IR, Kruskal JB, Warmbrand G, Goldberg SN, Pomfret EA, Raptopoulos V. Accuracy of volumetric measurements after virtual right hepatectomy in potential donors undergoing living adult liver transplantation. AJR Am J Roentgenol. 2001;176:483–487. doi: 10.2214/ajr.176.2.1760483. http://dx.doi.org/10.2214/ajr.176.2.1760483. [DOI] [PubMed] [Google Scholar]
- 22.Raoul JL, Heresbach D, Bretagne JF, et al. Chemoembolization of hepatocellular carcinomas. A study of the biodistribution and pharmacokinetics of doxorubicin. Cancer. 1992;70:585–590. doi: 10.1002/1097-0142(19920801)70:3<585::aid-cncr2820700308>3.0.co;2-#. http://dx.doi.org/10.1002/1097-0142(19920801)70:3<585::AID-CNCR2820700308>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Kan Z, McCuskey PA, Wright KC, Wallace S. Role of Kupffer cells in iodized oil embolization. Invest Radiol. 1994;29:990–993. doi: 10.1097/00004424-199411000-00007. http://dx.doi.org/10.1097/00004424-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. http://dx.doi.org/10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Sheu AY, Li W, et al. Poly(-lactide-co-glycolide) microspheres for MRI-monitored transcatheter delivery of sorafenib to liver tumors. J Control Release. 2014;184:10–17. doi: 10.1016/j.jconrel.2014.04.008. http://dx.doi.org/10.1016/j.jconrel.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261–2273. doi: 10.1002/hep.26256. http://dx.doi.org/10.1002/hep.26256. [DOI] [PubMed] [Google Scholar]
- 27.Hucke F, Sieghart W, Pinter M, et al. The ART-strategy: sequential assessment of the ART score predicts outcome of patients with hepatocellular carcinoma re-treated with TACE. J Hepatol. 2014;60:118–126. doi: 10.1016/j.jhep.2013.08.022. http://dx.doi.org/10.1016/j.jhep.2013.08.022. [DOI] [PubMed] [Google Scholar]