Abstract
PURPOSE
We aimed to determine the natural history of small index lesions identified on multiparametric-magnetic resonance imaging (MP-MRI) of the prostate by evaluating lesion-specific pathology and growth on serial MP-MRI.
MATERIALS AND METHODS
We performed a retrospective review of 153 patients who underwent a minimum of two MP-MRI sessions, on an institutional review board-approved protocol. Index lesion is defined as the lesion(s) with the highest cancer suspicion score based on initial MP-MRI of a patient, irrespective of size. Two study cohorts were identified: (1) patients with no index lesion or index lesion(s) ≤7 mm and (2) a subset with no index lesion or index lesion(s) ≤5 mm. Pathological analysis of the index lesions was performed following magnetic resonance/ultrasound fusion-guided biopsy. Growth rate of the lesions was calculated based on MP-MRI follow-up.
RESULTS
Patients with small index lesions measuring ≤7 mm (n=42) or a subset with lesions ≤5 mm (n=20) demonstrated either benign findings (86.2% and 87.5%, respectively) or low grade Gleason 6 prostate cancer (13.8% and 12.5%, respectively) on lesion-specific targeted biopsies. These lesions demonstrated no significant change in size (P = 0.93 and P = 0.36) over a mean imaging period of 2.31±1.56 years and 2.40±1.77 years for ≤7 mm and ≤5 mm index lesion thresholds, respectively. These findings held true on subset analyses of patients who had a minimum of two-year interval follow-up with MP-MRI.
CONCLUSION
Small index lesions of the prostate are pathologically benign lesions or occasionally low-grade cancers. Slow growth rate of these small index lesions on serial MP-MRI suggests a surveillance interval of at least two years without significant change.
Prostate cancer is the most common solid-organ malignancy in men in the western world, and it is one of the leading causes of cancer-related mortality (1). Widespread prostate specific antigen (PSA)-based screening has resulted in a marked increase in the rate of prostate cancer diagnosis, accompanied by significant downward grade and stage migration, as well as a decrease in mortality rate, though not commensurate with the detection rate (2, 3). Hence, there is a tendency to overtreat clinically-insignificant prostate cancer, leading to concerns regarding the quality of life. This has prompted renewed interest in more conservative management with active surveillance and investigational focal therapy options.
Clinically-insignificant prostate cancer has been defined as small tumors with low Gleason grade, although thresholds for these parameters are not fully agreed upon (4–6). For instance, many reports use a lesion size threshold of 0.5 cm3, which corresponds to a spherical lesion with a diameter of approximately 1 cm (7). However, some investigators have used even smaller volume thresholds of 0.2 cm3 and 0.5 cm3 based on overall prostate cancer tumor burden on radical prostatectomy specimens with clinically-insignificant disease. Due to the formalin fixation and tissue contraction of the prostatectomy specimens, these lower volume thresholds would perhaps more accurately correspond to 5 mm and 7 mm diameter spherical lesions in the setting of prostates bearing two to three cancer foci, given the commonly multifocal nature of prostate cancer (8, 9).
Multiparametric-magnetic resonance imaging (MP-MRI) of the prostate has been investigated as an anatomic and functional imaging method to aid in cancer detection (9–14). A subset of patients undergoing MP-MRI is found to have small index lesions, which would represent clinically-insignificant disease if found to harbor prostate cancer. In fact, when such lesions are biopsied they often prove to harbor benign tissue or low grade disease, and it would be tempting to use MP-MRI to monitor such patients. However, the optimal imaging interval has not been determined. Herein, we aim to define the natural history of small index lesions detected on MP-MRI, based on lesion-specific biopsy pathology and investigate subsequent growth rates determined by serial MRI studies.
Materials and methods
Patient selection
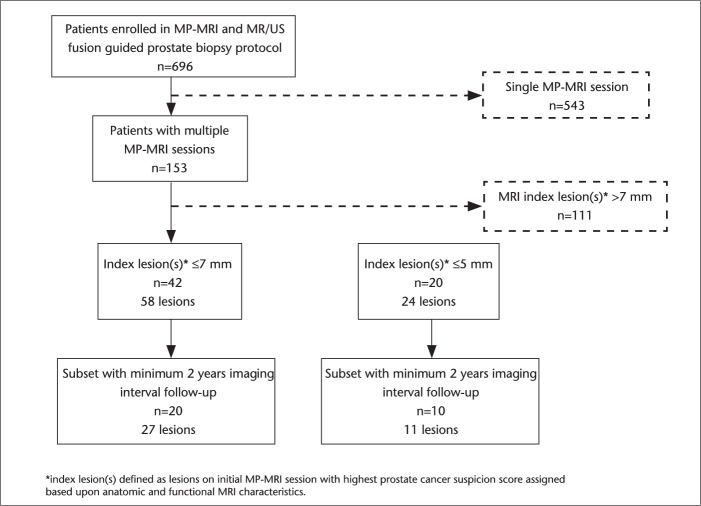
A retrospective review was performed on 696 patients who underwent MP-MRI and subsequent magnetic resonance/ultrasonography (MR/US) fusion guided prostate biopsy at the National Cancer Institute of the National Institutes of Health between August 2007 and December 2012, on institutional review board approved protocols. This patient population was evaluated to identify individuals with at least two MP-MRIs (Fig. 1). Of 153 patients with a minimum of two MPMRI studies, a study cohort was identified, consisting of patients with either no index lesion or one or more index lesions measuring ≤7 mm in greatest dimension on the initial MP-MRI study. Index lesions were defined as any lesion(s) bearing the highest prostate cancer suspicion scores based on initial MP-MRI in a patient, irrespective of lesion size. Another study cohort consisted of only the patients with either no index lesion on MP-MRI or index lesion(s) measuring ≤5 mm. The size of all lesions were measured on T2-weighted MRI sequences; thus consistency and comparability was achieved across patients as well as across serial studies on the same patient.
Figure 1.
Patient selection for analysis cohorts of small index lesions found on initial multiparametric MRI (MP-MRI).
Multiparametric MRI protocol
All MRI studies were performed using a combination of an endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania, USA) tuned to 127.8 MHz and a cardiac coil (SENSE, Philips Medical Systems, Best, the Netherlands) standardly performed on a 3 T magnet (Achieva, Philips Medical Systems, Best, the Netherlands) without prior bowel preparation. The endorectal coil was inserted coated with an anesthetic gel while the patient was positioned in the left lateral decubitus position. The balloon surrounding the coil was distended with 3 mol/L of perfluorocarbon (Fluorinert, 3M, St. Paul, Minnesota, USA) to a volume of approximately 50 mL to reduce susceptibility artifacts induced by air in the endorectal coil balloon. The MRI protocol included triplanar T2-weighted turbo-spin-echo, diffusion-weighted MRI (DW-MRI), three dimensional (3D) MR spectroscopy imaging (MRSI), axial precontrast T1-weighted MRI, axial 3D fast field-echo dynamic contrast-enhanced (DCE) MRI sequences. The specific sequence parameters have been defined in previous reports (10, 12).
Data analyses
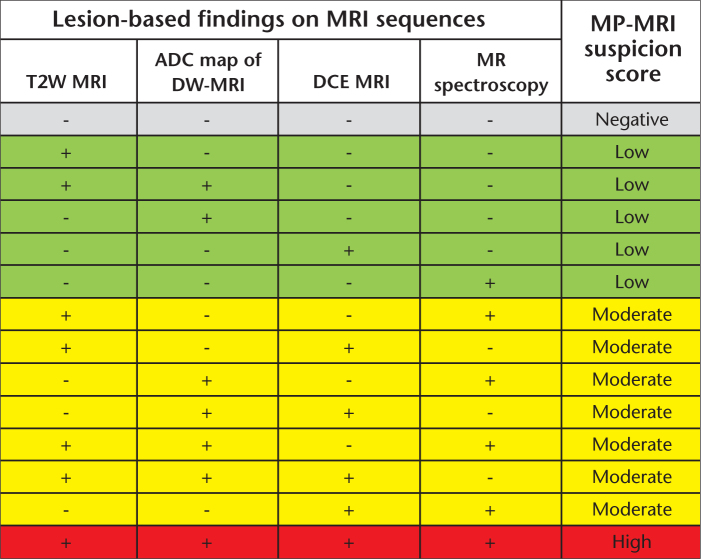
MRI analysis was performed by two experienced genitourinary radiologists (B.T. and P.L.C. with six and 12 years of experience in prostate MRI, respectively) who evaluated T2-weighted MRI, apparent diffusion coefficient (ADC) maps of DW-MRI, MRSI and DCE MRI in consensus, blinded to the clinicopathological findings (PSA, clinical staging and prior prostate biopsy pathology results). An imaging-based prostate cancer suspicion score was assigned to each lesion based on its features on different pulse sequences on MRI, as previously described and outlined in Fig. 2 (14). The criterion for a “visible” lesion on MP-MRI was a well circumscribed, round-ellipsoid low-signal-intensity region within the prostate gland on T2-weighted MR images and ADC maps of DW-MRI (10, 12). The 3D-MRSI analysis evaluated choline/citrate ratios within voxels in the target biopsy sites. Healthy choline/citrate ratio value was defined as 0.13±0.081, based on 433 biopsy-proven healthy voxels from peripheral zone regions of 44 patients referred for prostate MRI, as previously reported (10, 12). Voxels were considered abnormal when the choline/citrate ratio was three or more standard deviations above the mean healthy choline/citrate ratio value (≥0.373). DCE MR images were evaluated by direct visual interpretation of raw dynamic-enhanced T1-weighted images and the diagnostic criterion for prostate cancer was defined as a focus of early and intense enhancement with rapid wash out compared to the background (10, 12, 15).
Figure 2.
Lesion-based multiparametric MRI cancer suspicion scoring system. T2W MRI, T2-weighted MRI; ADC, apparent diffusion coefficient; DCE MRI, dynamic contrast enhanced MRI; DW-MRI, diffusion-weighted MRI; MP-MRI, multiparametric MRI.
Demographic, imaging, and pathology data were collected and stored in a secure database. All biopsy results were reviewed by a single genitourinary pathologist blinded to the imaging characteristics. Pathologic findings from MR/US fusion guided biopsies of the index lesions were correlated with the lesions identified on MP-MRI for each patient. Also, MP-MRI findings from subsequent scans of each patient were evaluated retrospectively to assess index lesion growth rates, measured as change in the greatest diameter of the lesion over time (Fig. 3). Statistical comparisons of categorical and continuous variables were assessed with the Freeman-Halton extension of the Fisher’s exact test and paired, two-tailed Student’s t-tests, respectively. All statistical analyses were performed using JMP Pro 10.0 (SAS Institute Inc, 2012, Cary, North Carolina, USA).
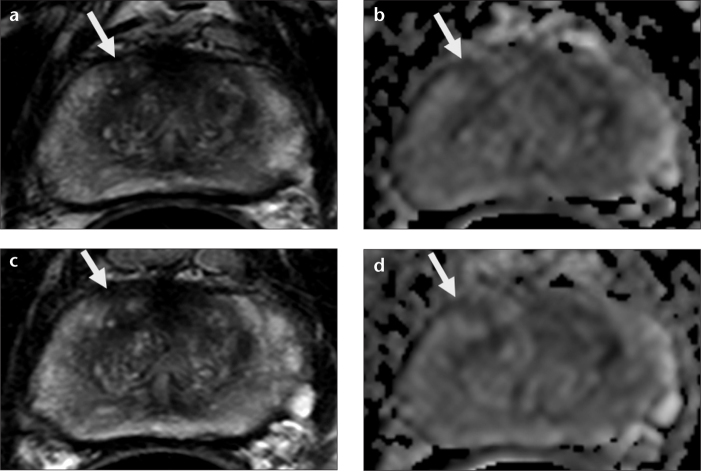
Figure 3. a–d.
Patient MRI depicting baseline T2-weighted (a) and diffusion-weighted imaging (DWI) (b) sequences which identified a right midanterior peripheral zone lesion measuring 5 mm (arrows). Subsequent T2-weighted (c) and DWI (d) sequences show no change in size or characteristics (arrows). At both time points, dynamic contrast-enhanced MRI and MR spectroscopy were negative in this area, maintaining a moderate level of suspicion. Gleason 3+3=6 was diagnosed in <5% of targeted biopsy cores.
Results
Of 153 patients who underwent multiple MP-MRI and MR/US fusion guided biopsy, 42 patients were determined to have either no identifiable lesion or index lesions all measuring ≤7 mm. A total of 58 index lesions (0–3 index lesions per patient) were identified in this cohort of 42 patients. A second cohort included a subset of patients with no MRI lesions or index lesions all measuring ≤5 mm; 20 patients harboring 24 index lesions (0–2 index lesions per patient) were identified in this cohort. The majority of patients in both cohorts had index lesions that were assigned moderate or low suspicion based on MP-MRI findings. There was a categorical shift observed towards higher suspicion scores in the patient cohort with index lesions ≤7 mm (1.7% high, 53.4% moderate, 37.9% low, 6.9% no suspicion) versus those with index lesions ≤5 mm (0% high, 50% moderate, 33.3% low, and 16.7% no suspicion), though no statistically significant differences were observed (P = 0.59). Targeted biopsy showed benign findings in the majority of patients in both cohorts (86.2% in the ≤7 mm cohort and 87.5% in the ≤5 mm cohort). Furthermore, targeted biopsy of the index lesions consistently revealed the lowest grade of disease assigned to biopsy specimens (Gleason 3+3=6 disease) in patients identified with prostate cancer. Patient demographics, characteristics of the index lesions as identified on the initial MP-MRI study, biopsy pathology results for the MR/US fusion guided biopsies targeting these index lesions, and follow-up data regarding most recent PSA and subsequent MP-MRI studies are summarized in Table 1.
Table 1.
Patient demographics, index lesion characteristics on initial MP-MRI, corresponding lesion-specific biopsy pathology, and serial MP-MRI follow-up data
| ≤7 mm index lesion(s) | ≤5 mm index lesion(s) | |
|---|---|---|
| Number of patients | 42 | 20 |
| Age (years) | 62.14±6.94 (47–77) | 62.85±6.29 (52–75) |
| Prebiopsy PSA (ng/dL) | 4.88±2.92 (0.2–12.79) | 5.42±3.22 (0.2–12.79) |
| Follow-up PSA (ng/dL) | 5.22±3.59 (0.39–15.5) | 5.55±3.35 (0.39–13.2) |
| Total number of index lesions, n (range per patient) | 58 (0–3 per patient) | 24 (0–2 per patient) |
| Index lesion MP-MRI prostate cancer suspicion scores, n (%) | ||
| High | 1 (1.8) | 0 (0) |
| Moderate | 31 (53.4) | 12 (50.0) |
| Low | 22 (37.9) | 8 (33.3) |
| None | 4 (6.9) | 4 (16.7) |
| Index lesion-specific biopsy pathology, n (%) | ||
| No cancer | 50 (86.2) | 21 (87.5) |
| Gleason =6 | 8 (13.8)a | 3 (12.5)a |
| Gleason ≥7 | 0 (0) | 0 (0) |
| Number of MP-MRI sessions | 2.81±0.97 (2–6) | 2.70±0.73 (2–4) |
| Longest imaging interval (years) | 2.31±1.56 (0.25–7.97) | 2.40±1.77 (0.25–7.97) |
Unless otherwise specified, data are given as mean±standard deviation (range).
PSA, prostate specific antigen; MP-MRI, multiparametric-magnetic resonance imaging.
All patients with no more than two cores involved and ≤20% of any core involved.
Table 2a depicts the qualitative and quantitative change in size noted on follow-up MP-MRI studies. Analysis of 58 index lesions in the cohort of patients with index lesions ≤7 mm demonstrated no significant change in index lesion size over a period of 2.31±1.56 years (P = 0.93). Similarly, analysis of 24 lesions in patients who harbored index lesions ≤5 mm had no significant change in size over 2.40±1.77 years (P = 0.36). Qualitatively, the majority of lesions remained stable in both cohorts (68.9% and 62.5% for patient cohorts with ≤7 mm and ≤5 mm index lesions, respectively).
Table 2. a, b.
Size change of small index lesions in patients with serial MP-MRI follow-up (a) and serial MP-MRI follow-up with a minimum two-year interval (b)
| a. | ≤7 mm index lesion(s) | P | ≤5 mm index lesion(s) | P |
|
| ||||
| Number of patients | 42 | - | 20 | - |
| Number of index lesions | 58 | - | 24 | - |
| Index lesion size on initial MP-MRI (mm) | 4.88±1.98 (0–7) | 0.93 | 3.48±1.78 (0–5) | 0.36 |
| Index lesion size on most recent MP-MRI (mm) | 4.91±3.17 (0–20) | 3.08±2.35 (0–8.5) | ||
| Longest imaging interval (years) | 2.31±1.56 (0.25–7.97) | - | 2.40±1.77 (0.25–7.97) | - |
| Qualitative change in lesion size since first MP-MRI session, n (%) | ||||
| Increase | 10 (17.2) | 4 (16.6) | ||
| Decrease | 8 (13.8) | - | 5 (20.8) | - |
| No change | 40 (68.9) | 15 (62.5) | ||
|
| ||||
| b. | ≤7 mm index lesion(s) | P | ≤5 mm index lesion(s) | P |
|
| ||||
| Number of patients | 20 | - | 10 | - |
| Number of lesions | 27 | - | 11 | - |
| Lesion size on initial MP-MRI (mm) | 4.81±1.91 (0–7) | 0.29 | 3.49±1.94 (0–5) | 0.16 |
| Lesion size on most recent MP-MRI (mm) | 4.40±2.82 (0–9.5) | 2.23±2.89 (0–8.5) | ||
| Longest imaging interval (years) | 3.51±1.48 (2.16–7.97) | - | 3.63±1.74 (2.31–7.97) | - |
| Qualitative change in lesion size since first MP-MRI session, n (%) | ||||
| Increase | 6 (22.2) | 1 (9.1) | ||
| Decrease | 6 (22.2) | - | 4 (36.4) | - |
| No change | 15 (55.6) | 6 (54.5) | ||
Unless otherwise specified, data are given as mean±standard deviation (range).
MP-MRI, multiparametric magnetic resonance imaging.
Through subset analysis of both study cohorts, we identified patients who had a minimum two-year interval between their initial and their most recent MP-MRI sessions. Results of parallel analyses performed for these subsets are depicted in Table 2b. Again, no significant change in size was noted after a mean follow-up of 3.51±1.48 years within the patient cohort with ≤7 mm index lesions (P = 0.29) and a mean follow-up of 3.63±1.74 years within the patient cohort with ≤5 mm index lesions (P = 0.16). Qualitative changes in lesion size over the same period of MP-MRI follow-up in these subsets conferred similar results with 55.6% and 54.5% of lesions demonstrating no change in size in the ≤7 mm and ≤5 mm cohorts, respectively.
Discussion
Screening protocols incorporating serum PSA evaluation and DRE have resulted in a well-recognized downward stage migration of organ-confined prostate cancer (2, 16). Moreover, most tumors have been staged as cT1c disease representing non-palpable disease due to small tumor size, topographical location away from the surface along the rectal vault, or both. Concurrently, there has been recognition that there is overdiagnosis and overtreatment of clinically-insignificant prostate cancer (3, 17). Seminal investigations defined clinically-insignificant prostate cancer based on thresholds of tumor size and Gleason grade utilizing radical prostatectomy specimens (4, 5). Size and Gleason grade determinations have been used to enroll patients in active surveillance protocols, employing biopsy pathology from systematic TRUS-guided biopsies as the surrogate for tumor burden. With improved sensitivity and proven correlation to histopathology, MP-MRI followed by MR/US fusion-guided prostate biopsy may provide a better surrogate of tumor burden over systematic template TRUS or other historical techniques of prostate biopsy.
Recently, the US Preventative Services Task Force recommended against PSA screening for prostate cancer, which has prompted primary care physicians and urologists to strongly question the value of the widespread PSA-based screening protocols (18, 19). If PSA is to be less commonly used, alternative screening methods must be sought.
MP-MRI of the prostate has emerged as an anatomic and functional evaluation of the prostate which aids in overall prostate cancer detection and characterization of lesions suspicious for harboring higher-risk disease (11, 20, 21). Furthermore, lesion localization and size measurements from T2-weighted and DW sequences of the MP-MRI have been highly correlated to final pathologic findings on radical prostatectomy specimens (10, 12). The use of MR/US fusion-guided biopsy provides targeted sampling of suspected lesions, more closely paralleling the size and volume of disease found on radical prostatectomy. This is of importance given the methods by which the volumes defining clinically-insignificant disease were determined in landmark studies.
The current study provides quantitative measurement of lesion change in patients with clinically-insignificant disease, using MP-MRI technology. We show that small index lesions on MPMRI assessment are either benign, too small to accurately target (based on margin of error coregistering the MRI to real-time US using the software fusion platforms), or bear only low-grade prostate cancer. Our imaging data confirms the radical prostatectomy findings that McNeal et al. (22) reported over 20 years ago. These index lesions did not demonstrate a significant change in size over an average observation period of more than two years. Subset analysis of patients with a minimum of two-year interval imaging follow-up confirmed these findings. Furthermore, none of the patients with no index lesions on their initial MP-MRI developed identifiable lesions over the period of serial imaging available for analysis.
Based on these findings we can suggest that the subset of patients with small MRI index lesions, measuring ≤7 mm in greatest dimension, confirmed with an initial MR/US fusion-guided biopsy could forego any additional screening or active surveillance testing (PSA measurements, MP-MRI, or additional biopsy sessions) for at least two years. Given the high fidelity of MP-MRI in accurately delineating the size of lesions and data suggesting the indolent nature of these small index lesions, it may be possible to monitor patients with MP-MRI alone in the future. Though the current data-set is limited in size and duration of follow-up, there were no MRI index lesions measuring ≤7 mm harboring Gleason 7 prostate cancer, and the vast majority of patients who fit the imaging criteria for inclusion had benign prostatic tissue on targeted biopsies. It is unclear how many of these benign biopsies were due to the small diameters of the MRI lesions used as biopsy targets which approach the margin of error inherent to the MR/US fusion biopsy system. Perhaps larger series could provide further confirmation of these data and obviate the need for biopsies in this population of patients without compromising early diagnosis of clinically-significant cases of prostate cancer.
Limitations of our study include the cost of MRI which makes it currently impractical for routine use. This can potentially be overcome by eliminating use of an endorectal coil and performing a minimum number of sequences in a 10- to 15-minute time slot. Furthermore, if such imaging can obviate the frequency of widespread bio-marker testing, serial biopsy sessions, and the associated downstream costs of overtreating clinically-insignificant, indolent prostate cancer, its relative cost differential could be further minimized. Additional limitations of this study include a small sample size of patients who fit the inclusion criteria for analysis and the retrospective nature of the data analysis. Future studies with larger patient cohorts compiled from multiple centers can be used to validate these findings and perhaps broaden the evidence-based recommendations for interval imaging to include screening and surveillance of patients suspected to have prostate cancer and active surveillance of those who harbor clinically-insignificant disease.
In conclusion, small index lesions detected on MP-MRI of the prostate are pathologically confirmed to be benign or indolent cancers based on grade and size. Slow growth rate of these small index lesions on serial MP-MRI suggests that the interval imaging follow-up can span a minimum of two years.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and the Center for Interventional Oncology. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field.
This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://www.fnih.org/work/programs-development/medical-research-scholars-program.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez J, Thompson IM. Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer. 2004;101:894–904. doi: 10.1002/cncr.20480. [DOI] [PubMed] [Google Scholar]
- 3.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19:175–181. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamey TA, Freiha FS, McNeal JE, Redwine EA, Whittemore AS, Schmid HP. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71:933–938. doi: 10.1002/1097-0142(19930201)71:3+<933::aid-cncr2820711408>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 6.Goto Y, Ohori M, Arakawa A, Kattan MW, Wheeler TM, Scardino PT. Distinguishing clinically important from unimportant prostate cancers before treatment: value of systematic biopsies. J Urol. 1996;156:1059–1063. [PubMed] [Google Scholar]
- 7.Wolters T, Roobol MJ, van Leeuwen PJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011;185:121–125. doi: 10.1016/j.juro.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 8.Turkbey B, Mani H, Aras O, et al. Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol. 2012;188:1157–1163. doi: 10.1016/j.juro.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190:1721–1727. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818–1824. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yerram NK, Volkin D, Turkbey B, et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int. 2012;110:E783–E788. doi: 10.1111/j.1464-410X.2012.11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turkbey B, Mani H, Aras O, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268:144–152. doi: 10.1148/radiol.13121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turkbey B, Thomasson D, Pang Y, Bernardo M, Choyke PL. The role of dynamic contrast-enhanced MRI in cancer diagnosis and treatment. Diagn Interv Radiol. 2010;16:186–192. doi: 10.4261/1305-3825.DIR.2537-08.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derweesh IH, Kupelian PA, Zippe C, et al. Continuing trends in pathological stage migration in radical prostatectomy specimens. Urol Oncol. 2004;22:300–306. doi: 10.1016/j.urolonc.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force. Screening for Prostate Cancer: Clinical Summary of U.S. Preventive Services Task Force Recommendation. May, 2012. AHRQ Publication No. 12-05160-EF-3.
- 19.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 20.Rastinehad AR, Baccala AA, Jr, Chung PH, et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011;185:815–820. doi: 10.1016/j.juro.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119:3359–3366. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer. 1990;66:1225–1233. doi: 10.1002/1097-0142(19900915)66:6<1225::aid-cncr2820660624>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]