Abstract
Mandibular lesions are classified as odontogenic and nonodontogenic based on the cell of origin. Odontogenic lesions are frequently encountered at head and neck imaging. However, several nonodontogenic pathologies may also involve mandible and present further diagnostic dilemma. Awareness of the imaging features of nonodontogenic lesions is crucial in order to guide clinicians in proper patient management. Computed tomography (CT) may provide key information to narrow diagnostic considerations. Nonodontogenic mandibular lesions may have lytic, sclerotic, ground-glass, or mixed lytic and sclerotic appearances on CT. In this article, our aim is to present various nonodontogenic lesions of the mandible by categorizing them according to their attenuations on CT.
Mandibular lesions may arise from both odontogenic and nonodontogenic origins (1). Odontogenic lesions are common in the mandible, and imaging features of these lesions are well described in the radiology literature. However, various nonodontogenic pathologies including primary tumors, tumor-like lesions, metastases, infection, vascular lesions, and metabolic abnormalities may also present as a mandibular lesion. Diagnosis may be challenging, because both odontogenic and nonodontogenic lesions may mimic each other with similar radiological appearances.
The purpose of this study is to describe the imaging features of the nonodontogenic lesions of the mandible using a classification based on the computed tomography (CT) appearances (lytic, sclerotic, mixed, ground-glass attenuation) and to discuss the diagnostic approach.
Differentiating nonodontogenic lesions from odontogenic lesions
Odontogenic lesions usually surround a component of the tooth (2). Periapical cyst, the most common odontogenic cyst, develops around the apex of the tooth. Dentigerous cyst and odontoma usually surround the crown of a tooth (2, 3). Dentigerous cyst, keratocystic odontogenic tumor and ameloblastoma most commonly arise from the posterior mandible adjacent to third molar tooth (3). A lesion associated with an impacted tooth frequently indicates an odontogenic origin.
Nonodontogenic lesions, however, develop from osseous origin and are not tooth-related. These lesions usually, but not always, consist of a group of pathologies which may be seen anywhere in the axial skleton. Therefore, when they present in the mandible, their imaging features are similar to those seen in other parts of the body. Nonodontogenic lesions usually do not surround the tooth. However, when they are large enough it may be difficult to determine the relationship of the lesion to the adjacent teeth (2).
Lesions with lytic pattern
Static bone cavity (Stafne cyst)
A static bone cavity appears as a well-defined lytic lesion at the angle of the mandible. It is a benign pseudocyst containing fat or submandibular salivary gland tissue with a characteristic cortical defect on the medial aspect of the mandible (3).
Solitary bone cyst (traumatic, simple, hemorrhagic bone cyst)
Solitary bone cyst is thought to be the result of a trauma which gives rise to intramedullary hemorrhage. It is a well-defined unilocular pseudocyst with typical scalloped margin between the roots of normal appearing teeth (1).
Giant cell reparative cyst (central giant cell granuloma)
Giant cell reparative cyst is believed to develop from a reparative inflammatory process most likely related to trauma. The cyst is typically seen as a unilocular or multilocular lytic lesion in young women between the second and third decades of life. It occurs most commonly in the anterior mandible and may cross the midline (Fig. 1a) (1). Bone expansion and cortical erosion may also be seen (Fig. 1b). Giant cell reparative cyst may mimic brown tumor of hyperparathyroidism both radiologically and histologically. Patient’s age and blood parathormone level are helpful in distinguishing these two lesions.
Figure 1. a, b.
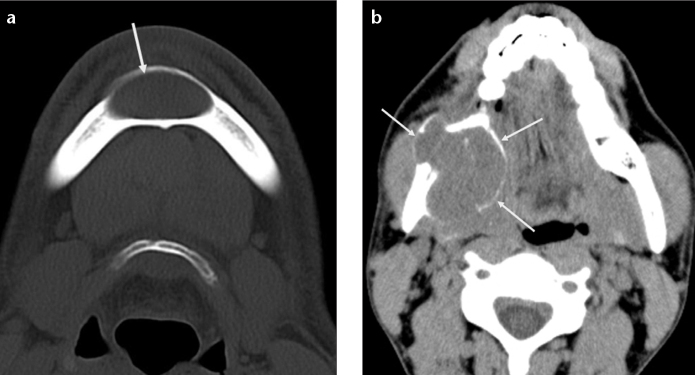
Giant cell reparative cysts in two different patients. Axial CT scan (a) shows a well-circumscribed, midline lytic lesion (arrow) in the mandibular symphysis extending to the bilateral parasymphyseal areas in a 33-year-old man. Note minimal bone expansion without cortical erosion. Axial CT image (b) of a 26-year-old man demonstrates an expansile lytic lesion (arrows) within the angle of the mandible causing bone remodeling and cortical thinning.
Osteitis fibrosa cystica (hyperparathyroidism)
Osteitis fibrosa cystica is a late bony complication of severe hyperparathyroidism. Imaging findings include generalized demineralization of bone, “salt and pepper” appearance of the skull, and bone cysts referred to as “brown tumors” (4). On imaging, brown tumors are usually seen as expansile osteolytic lesions mimicking metastasis (Fig. 2). Generalized demineralization of bone associated with elevated parathormone level indicates the proper diagnosis.
Figure 2. a–c.
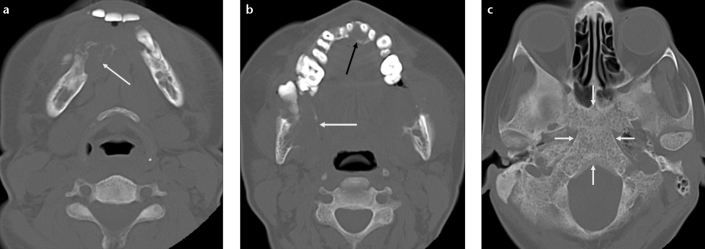
Osteitis fibrosa cystica in a 42-year-old woman. Axial consecutive CT images (a, b) show multiple expansile lytic lesions within the anterior and posterior body of the mandible (white arrows) and the anterior maxilla (black arrow) with cortical thinning and erosion. CT scan obtained at skull base level (c) reveals generalized demineralization of the clivus (arrows), a finding that helps to differentiate osteitis fibrosa cystica from osteolytic metastasis.
Aneurysmal bone cyst
Aneurysmal bone cyst is a rare non-neoplastic expansile lesion of the mandible (5). It appears as a unilocular or multilocular osteolytic lesion. Multiple cystic lesions divided by enhancing septations associated with fluid-fluid levels are characteristic features of the aneurysmal bone cyst (6).
Central mucoepidermoid carcinoma
Central mucoepidermoid carcinoma is a rare subtype of mucoepidermoid carcinoma arising from the mandible. The tumor typically develops in the posterior mandible and may be associated with an unerupted tooth (7). Medullary bone destruction with intact cortical bone is one of the diagnostic criteria for central mucoepidermoid carcinoma. However, cortical perforation may also be seen in advanced disease (Fig. 3) (7).
Figure 3. a, b.
Central mucoepidermoid carcinoma in a 53-year-old woman. Axial precontrast (a) and postcontrast (b) CT images show a homogeneously enhancing lytic lesion (arrows) with lingual cortical erosion within the right ramus of the mandible.
Langerhans cell histiocytosis (histiocytosis X)
Langerhans cell histiocytosis (LCH) is a disease of reticuloendothelial system characterized by abnormal proliferation of Langerhans cells. Bone lesions, usually affecting the craniofacial structures and skull base, are the most common manifestations of LCH. Imaging reveals single or multiple sharply defined lytic bone lesions with uniform contrast enhancement (Fig. 4) (8).
Figure 4. a, b.
Langerhans cell histiocytosis in a three-year-old boy. Axial CT image (a) shows multiple osteolytic lesions (arrows) with cortical erosions in the rami of the mandible. CT scan obtained at the skull base level (b) also reveals “punched-out” lesions (arrows) at the superior-lateral margins of the orbit.
Hematological malignancy
Lymphoma and leukemia typically cause poorly marginated osteolytic lesions (1). Multiple myeloma most commonly appears as multiple lytic lesions with nonsclerotic borders (3).
Secondary malignant invasion
Squamous cell carcinoma originating from adjacent tissues is the most common malignant mandibular lesion (Fig. 5) (1).
Figure 5.
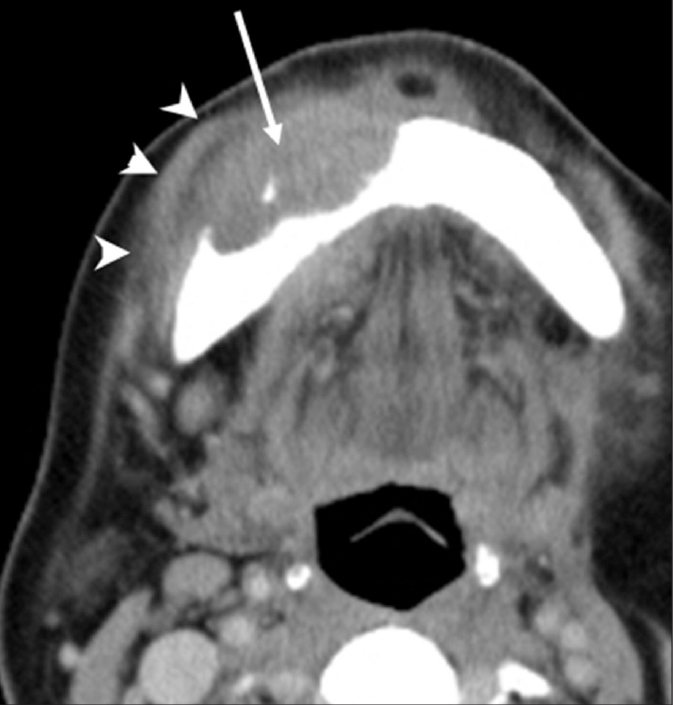
Squamous cell carcinoma of the lower lip in a 64-year-old woman. Axial CT image demonstrates a soft-tissue mass (arrow) within the anterior body of the mandible with buccal cortical destruction. Note that the mass is extending to the perimandibular region (arrowheads).
Lesions with lytic or sclerotic pattern
Metastasis
Metastasis to the mandible is four times more common than those to the maxilla (1). The most common locations are the angle and posterior body of the mandible probably due to increased marrow vascularity. Kidney, lung, and breast carcinomas are the most common malignant tumors associated with the mandibular metastasis (Fig. 6). The lesions are usually lytic with ill-defined borders; however, sclerotic metastases may also be detected especially in prostate carcinoma (1).
Figure 6.
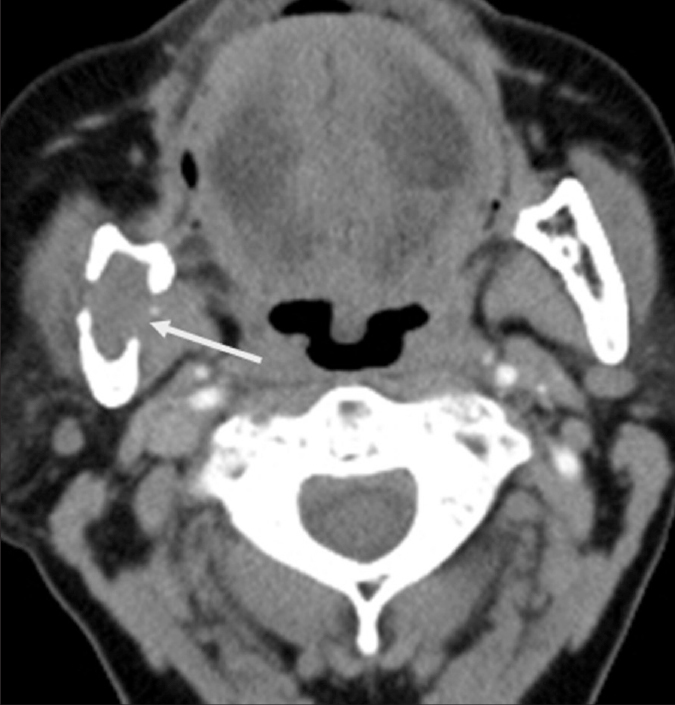
Metastatic renal cell carcinoma in a 55-year-old woman. Axial CT scan shows an osteolytic lesion (arrow) with cortical erosions on both lingual and buccal aspects of the mandibular ramus.
Lesions with sclerotic pattern
Torus mandibularis
Torus mandibularis is an asymptomatic tumor-like condition of the mandible. Exostosis, protuberance of dense cortical bone, is seen along the lingual aspect of the mandible on imaging (Fig. 7) (2).
Figure 7.
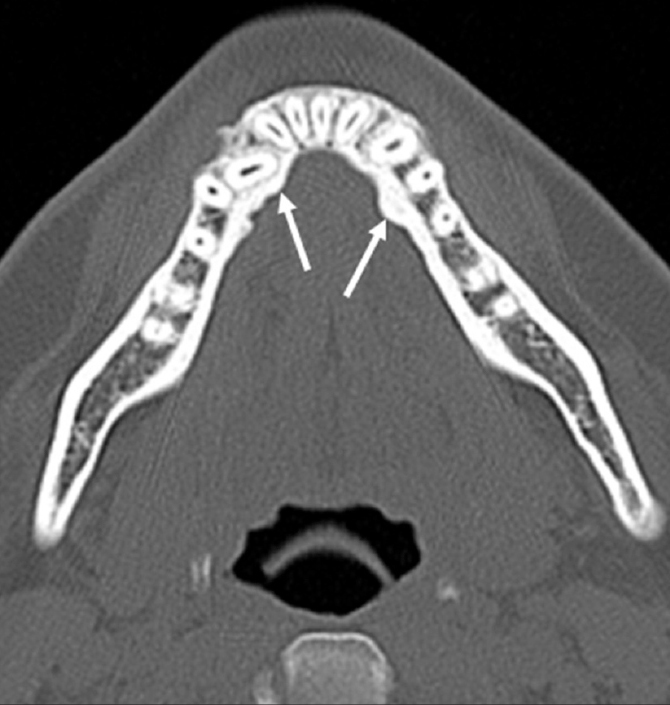
Torus mandibularis in a 27-year-old woman. Axial CT scan demonstrates bony protuberance (arrow) in the lingual aspects of the anterior mandible.
Osteoma
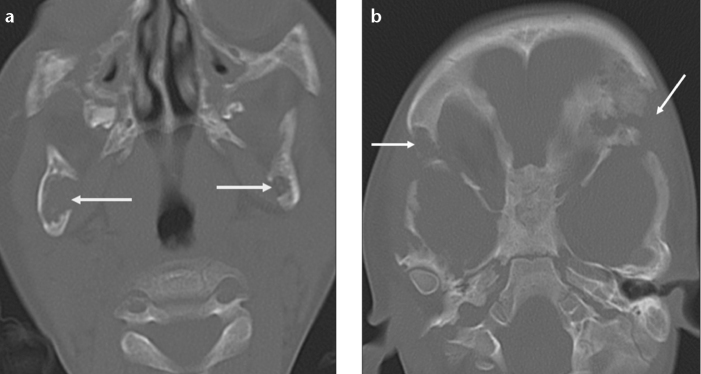
Osteomas are benign tumors composed of mature compact and/or cancellous bone (2). They typically occur in the craniofacial bones. Osteomas are most commonly seen in the posterior body or condyle of the mandible. They appear as a well-circumscribed broad-based or pedinculated sclerotic mass on imaging. Multiple osteomas in the mandible should raise the possibility of Gardner’s syndrome (Fig. 8).
Figure 8. a–c.
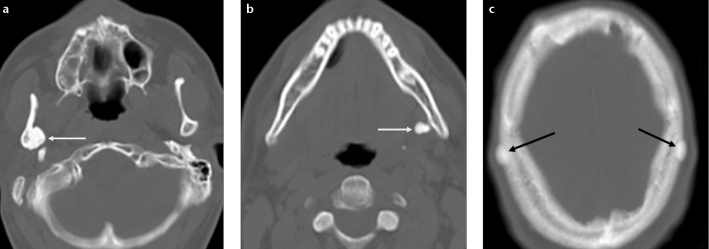
Multiple osteomas in a 37-year-old man with Gardner’s syndrome. Axial CT images (a, b) show multiple well-defined hyperdense lesions (white arrows) arising from the ramus and the angle of the mandible. CT scan obtained at the vertex of the skull (c) demonstrates additional osteomas (black arrows) in the calvarium.
Osteochondroma
Osteochondroma is a cartilage-capped exophytic tumor arising from the cortex of the bone (9). It usually occurs in the axial skeleton. Mandibular involvement is rare. The most common locations in the mandible are the condyle and the coronoid process (10). An osteochondroma appears as a sessile or pedunculated bony outgrowth (Fig. 9). CT plays a critical role in the diagnosis by revealing characteristic cortical and medullary continuity between the lesion and the parent bone.
Figure 9.
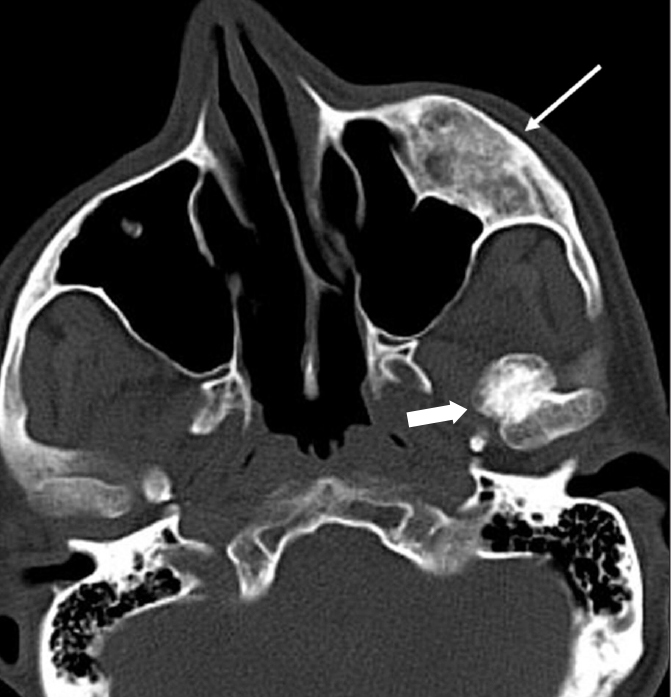
Osteochondroma in a 48-year-old man. Axial CT image shows a bony outgrowth (thick arrow) originating from the left mandiblular condyle. Note the associated fibrous dysplasia (thin arrow) within the left maxillary bone.
Osteopetrosis
Osteopetrosis is a rare genetic bone disease which may also involve the mandible. An increase in bone density –osteosclerosis– is the typical imaging finding. There is an increased incidence of mandibular osteomyelitis in patients with osteopetrosis (Fig. 10) (11).
Figure 10.
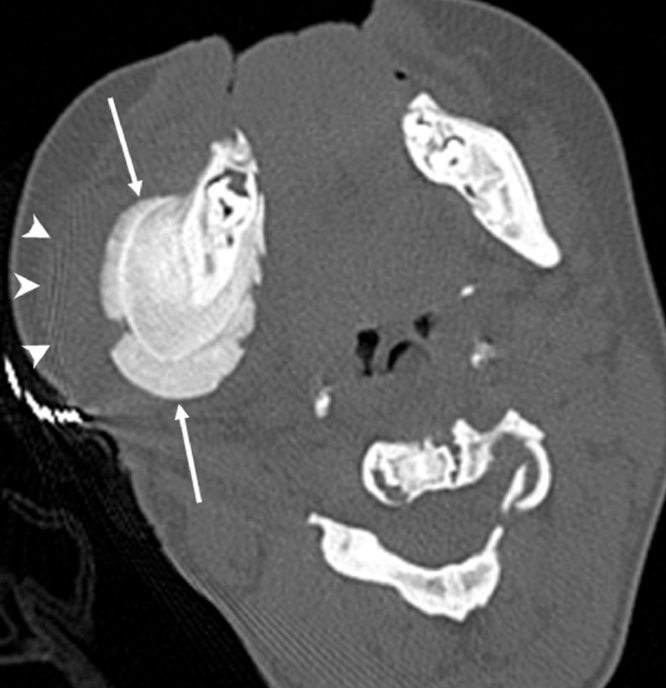
Osteopetrosis with mandibular osteomyelitis in an 11-year-old boy. Axial CT scan shows diffuse increase in bone density of the mandible and the cervical vertebra associated with periosteal new bone formation (arrows) along the cortex of the right mandibular body. Note the diffuse soft-tissue thickening (arrowheads).
Myositis ossificans of the pterygoid muscles
Myositis ossificans, either localized or progressive, may affect the pterygoid muscles and is usually related to trauma (12). Imaging reveals heterotopic ossification of the pterygoid muscles extending between the pterygoid plate and the ramus of the mandible (Fig. 11).
Figure 11.
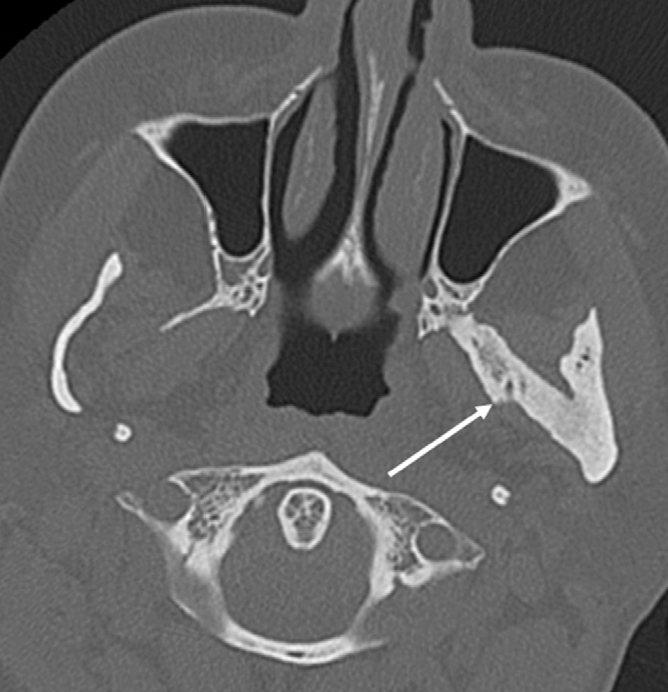
Myositis ossificans in a 41-year-old woman with a history of tooth extraction. Axial CT image demonstrates ossification of the left lateral pterygoid muscle (arrow) extending from the left lateral pterygoid plate to the ramus of the mandible.
Lesions with mixed lytic and sclerotic pattern
Osteomyelitis
Mandibular osteomyelitis is a rare entity in healthy individuals. It is usually seen in immunosuppressed or debilitated patients with a history of antecedent dental caries, surgical procedure, trauma, or radiotherapy. Imaging findings are variable depending on the type and the stage of the disease (3). No prominent abnormality is detected in acute phase. However in chronic phase, cortical plate destruction, periosteal reaction (Fig. 10), or sequestra are seen, causing lytic, sclerotic, or mixed lesions (Fig. 12) (3). Accompanying soft-tissue abnormalities such as haziness or obliteration of fat planes and also clinical findings are helpful in differentiating infection from neoplasia.
Figure 12. a, b.
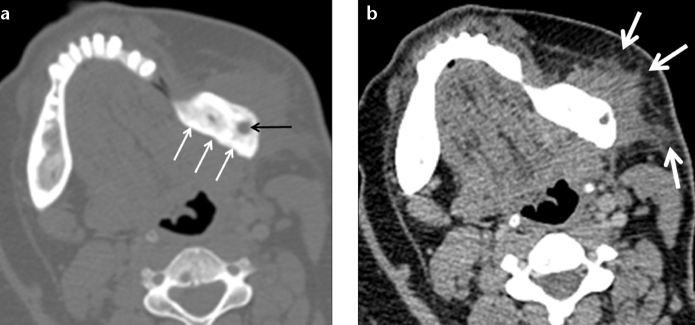
Osteomyelitis in a 23-year-old woman. Axial CT image at bone window (a) shows sclerotic changes (white arrows) associated with a focal lytic area (black arrow) within the angle of the left mandible. Axial CT image at soft-tissue window (b) also reveals surrounding fat stranding (arrows), a suggestive finding of inflammation. (Courtesy of Nezahat Erdoğan, MD).
Osteoradionecrosis
Radiation therapy for head and neck tumors may cause bone necrosis in the jaw. Osteoradionecrosis usually develops in patients with oral carcinomas between four months and three years after radiotherapy (13). The body of the mandible is the most commonly affected site. Imaging findings include ill-defined lytic and sclerotic areas with enlarged trabecular spaces, bone sequestration or fragmentation and areas of gas attenuation (1). Buccal cortical erosions and the involvement of the opposite site of the mandible are suggestive features of osteoradionecrosis (Fig. 13) (14).
Figure 13.
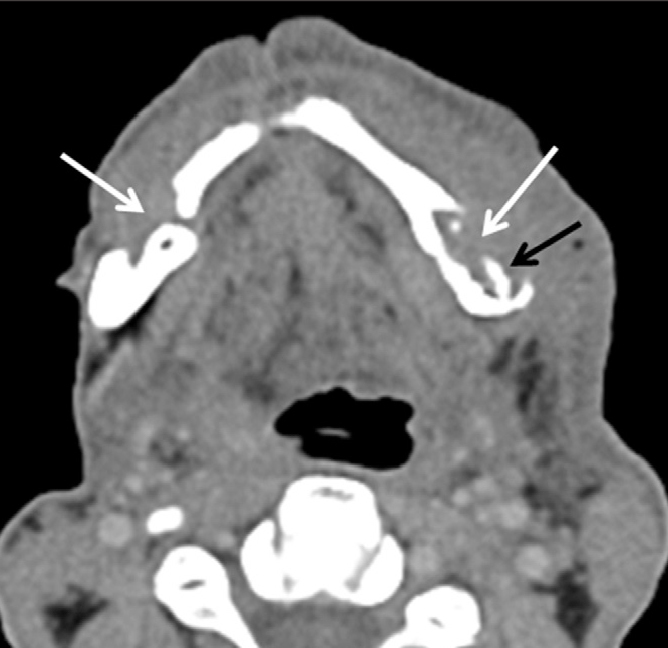
Osteoradionecrosis in a 47-year-old man with a history of operation and radiation therapy for squamous cell carcinoma of the retromolar trigone. Axial CT image demonstrates bilateral lytic lesions (white arrows) with cortical destruction on both buccal aspects of the mandibular body associated with bony sequestrum (black arrow). Note the increased attenuation of the subcutenous fat representing edema secondary to the radiation therapy. (Courtesy of Nezahat Erdoğan, MD).
Biphosphonate-related osteonecrosis of the jaw
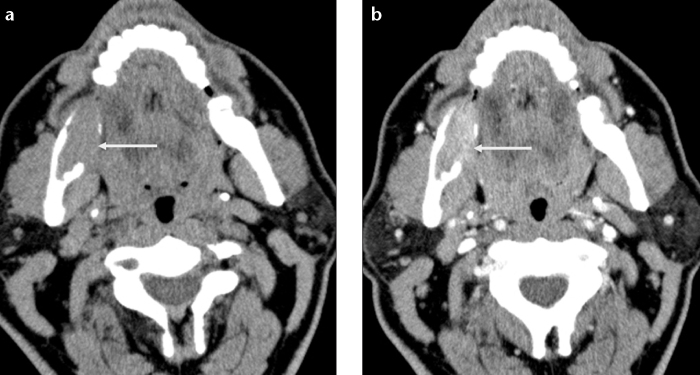
Biphosphanates are drugs that decrease bone turnover and are used to treat various diseases such as osteoporosis, multiple myeloma or metastasis. Biphosphonate-related osteonecrosis of the jaw (BRONJ) is characterized by bone necrosis that occurs secondary to biphosphonate treatment. BRONJ most commonly involves the mandibula. Imaging findings are nonspecific. Mixed, predominantly lytic or predominantly sclerotic bone changes may be seen (Fig. 14). BRONJ should be considered in patients with a history of biphosphonate therapy without jaw irradiation (15).
Figure 14. a, b.
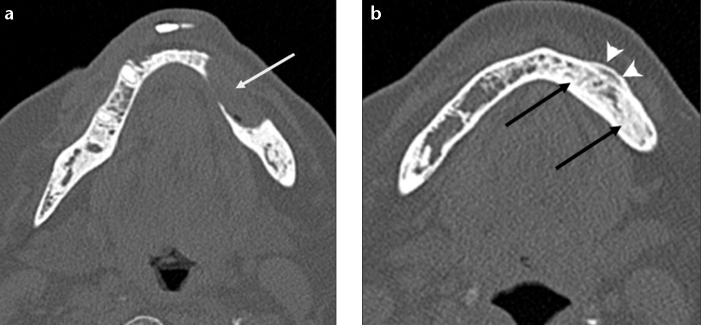
Biphosphonate-related osteonecrosis of the mandible in a 64-year-old woman with a history of biphosphonate treatment for rheumatoid arthritis. Axial CT image (a) shows a lytic lesion (arrow) with cortical defect within the anterior body of the left mandible. CT scan obtained at a lower level (b) also shows sclerotic changes (black arrows) within the mandible associated with periosteal reaction (arrowheads) adjacent to the lytic lesion.
Lesions with lytic, sclerotic, mixed pattern, or ground-glass attenuation
Ossifying fibroma (cemento-ossifying fibroma)
Ossifying fibroma contains varying amounts of fibrous tissue, bone trabeculae, and cementum like spherules (2). Most of these tumors develop in the posterior mandible during the third and fourth decades of life. Ossifying fibroma is a well-defined, focal expansile lesion with a variable appearance depending on the degree of calcification. The lesion is lytic initially. However, with maturation, it appears as a lesion of mixed density, as a lesion with ground-glass attenuation, or as a sclerotic lesion (1, 2). The presence of a sharply defined narrow transition zone, a growth pattern perpendicular to the long axis of the bone and tooth displacement indicates a diagnosis of ossifying fibroma (2). Juvenile ossifying fibroma is an aggressive variant of the tumor that typically occurs in boys younger than 15 years (Fig. 15).
Figure 15.
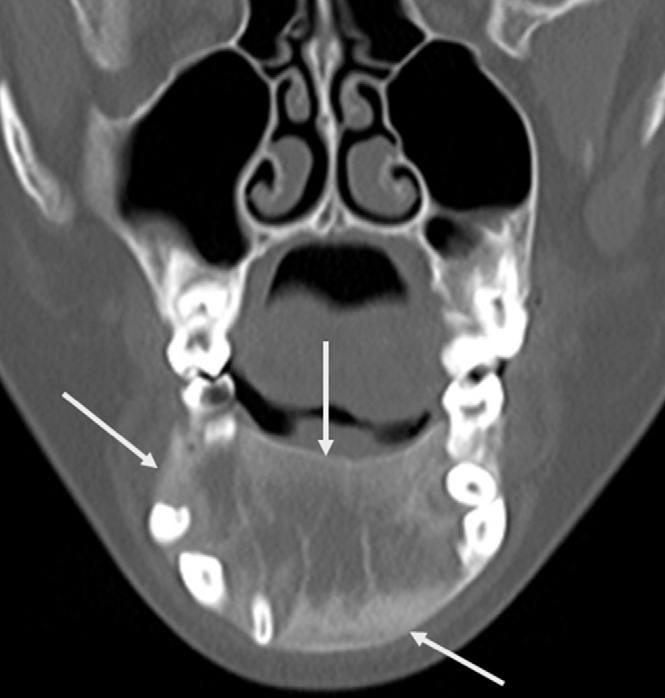
Juvenile ossifying fibroma in a 10-year-old girl. Coronal CT image demonstrates a well-defined, expansile lesion (arrows) with predominantly ground-glass attenuation in the body of the mandible.
Fibrous dysplasia
Fibrous dysplasia contains cellular fibrous tissue and woven bone trabeculae (2). At imaging, most cases demonstrate ground-glass attenuation; however, mixed, predominantly lytic, or sclerotic pattern may also be seen. Ill-defined transition zone and longitudinal growth pattern without displacement of the teeth are suggestive features of fibrous dysplasia and are helpful to differentiate it from ossifying fibroma (2).
Conclusion
Mandibular lesions have a broad spectrum of differential diagnosis. Although odontogenic lesions are common, possible diagnosis of a nonodontogenic pathology should also be kept in mind. It should be remembered that a mandibular lesion may be the initial presentation of a metastatic malignancy, a metabolic abnormality (i.e., hyperparathyroidism), or a syndrome (i.e., osteomas in Gardner’s syndrome). Specific diagnosis based solely on imaging is not always possible. However, CT features of a mandibular lesion along with the patient’s age and clinical history may help the radiologist to narrow the differential diagnosis.
Acknowledgments
The authors gratefully acknowledge Nezahat Erdoğan, MD for her contribution to the article.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Dunfee BL, Sakai O, Pistey R, Gohel A. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. Radiographics. 2006;26:1751–1768. doi: 10.1148/rg.266055189. [DOI] [PubMed] [Google Scholar]
- 2.Curé JK, Vattoth S, Shah R. Radioopaque jaw lesions: an approach to the differential diagnosis. Radiographics. 2012;32:1909–1925. doi: 10.1148/rg.327125003. [DOI] [PubMed] [Google Scholar]
- 3.Devenney-Cakir B, Subramaniam RM, Reddy SM, Imsande H, Gohel A, Sakai O. Cystic and cystic appearing lesions of the mandible: review. AJR Am J Roentgenol. 2011;196:66–77. doi: 10.2214/AJR.09.7216. [DOI] [PubMed] [Google Scholar]
- 4.Selvi F, Cakarer S, Tanakol R, Guler SD, Keskin C. Brown tumour of the maxilla and mandible: a rare complication of tertiary hyperparathyroidism. Dentomaxillofac Radiol. 2009;38:53–58. doi: 10.1259/dmfr/81694583. [DOI] [PubMed] [Google Scholar]
- 5.Theodorou SJ, Theodorou DJ, Sartoris DJ. Imaging characteristics of neoplasms and other lesions of the jawbones: part 2. Odontogenic tumor-mimickers and tumor-like lesions. Clin Imaging. 2007;31:120–126. doi: 10.1016/j.clinimag.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Asaumi J, Konouchi H, Hisatomi M, et al. MR features of aneursymal bone cyst of the mandible and characteristics distinguishing it from other lesions. Eur J Radiol. 2003;45:108–112. doi: 10.1016/s0720-048x(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 7.Chiu GA, Woodwards RT, Benatar B, Hall R. Mandibular central mucoepidermoid carcinoma with distant metastasis. Int J Oral Maxillofac Surg. 2012;41:361–363. doi: 10.1016/j.ijom.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Raybaud C, Barkovich AJ. Intracranial, orbital, and neck masses of childhood. In: Barkovich AJ, Raybaud C, editors. Pediatric neuroimaging. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2012. pp. 684–687. [Google Scholar]
- 9.Avinash KR, Rajagopal KV, Ramakrishnaiah RH, Carnelio S, Mahmood NS. Computed tomographic features of mandibular osteochondroma. Dentomaxillofac Radiol. 2007;36:434–436. doi: 10.1259/dmfr/54329867. [DOI] [PubMed] [Google Scholar]
- 10.Meng Q, Chen S, Long X, Cheng Y, Deng M, Cai H. The clinical and radiographic characteristics of condylar osteochondroma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:66–74. doi: 10.1016/j.oooo.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 11.García CM, García MA, García RG, Gil FM. Osteomyelitis of the mandible in a patient with osteopetrosis. Case report and review of the literature. J Maxillofac Oral Surg. 2013;12:94–99. doi: 10.1007/s12663-011-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho DR, Farage L, Martins BJ, Speck-Martins CE. Craniofacial findings in fibrodysplasia ossificans progressiva: a computerized tomography evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:499–502. doi: 10.1016/j.tripleo.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Chrcanovic BR, Reher P, Sousa AA, Harris M. Osteoradionecrosis of the jaws–a current overview–part 1: Physiopathology and risk and predisposing factors. Oral Maxillofac Surg. 2010;14:3–16. doi: 10.1007/s10006-009-0198-9. [DOI] [PubMed] [Google Scholar]
- 14.Hermans R, Fossion E, Ioannides C, Van den Bogaert W, Ghekiere J, Baert AL. CT findings in osteoradionecrosis of the mandible. Skeletal Radiol. 1996;25:31–36. doi: 10.1007/s002560050028. [DOI] [PubMed] [Google Scholar]
- 15.Morag Y, Morag-Hezroni M, Jamadar DA, et al. Biphosphonate-related osteonecrosis of the jaw: a pictorial review. Radiographics. 2009;29:1971–1984. doi: 10.1148/rg.297095050. [DOI] [PubMed] [Google Scholar]


