Abstract
Electroporation involves applying electric field pulses to cells, leading to the alteration or destruction of cell membranes. Irreversible electroporation (IRE) creates permanent defects in cell membranes and induces cell death. By directly targeting IRE to tumors, percutaneous nonthermal ablation is possible. The history of IRE, evolution of concepts, theory, biological applications, and clinical data regarding its safety and efficacy are discussed.
A number of modalities are available for percutaneous tumor ablation. These methods primarily include thermal techniques, such as cryoablation, radiofrequency, or microwave ablation, which involve cooling or heating the tissue to induce cell death. Because these methods depend on thermal injury, they carry some risk to the adjacent extracellular environment. Consequently, treating tumors adjacent to critical vascular structures is a challenge that potentially limits the aggressiveness of the attempted ablation.
In recent years, electroporation has emerged as a new method of tumor ablation. Electroporation, also known as electropermeabilization, involves the application of short pulses of strong electric fields to cells and tissues. External electric fields increase the transmembrane potential, charging the membrane like a capacitor by moving ions from the surrounding solution. Applying electric fields to cells is thought to induce the formation of pores, which are responsible for the permeabilization effect; however, more extensive, irreversible damage from higher fields is used to ablate tumor cells (Fig. 1). By using small electrodes (∼1 mm diameter) placed close to the target and short, repetitive electric field pulses, nonthermal irreversible electroporation (IRE) appears to offer an advantage over other ablation methods that involve tissue heating.
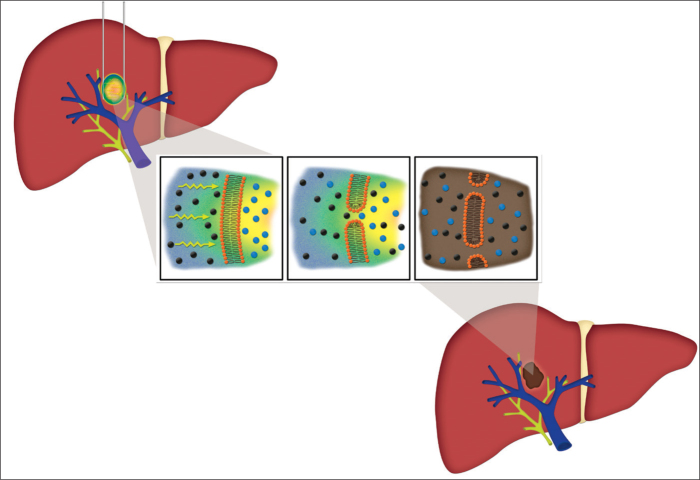
Figure 1.
Cartoon illustration of irreversible electroporation in a liver tumor. The left panel demonstrates a liver containing an ovoid tumor with surrounding two linear irreversible electroporation probes. Magnified view of the tumor reveals movement of the electric current (yellow arrows) across a cell membrane that leads to the breakdown of cell membrane integrity, the loss of cellular gradients, and the death of the cell. The image in the right panel shows an ablated tumor, depicting its preserved adjacent vasculature and ducts.
While electroporation has been studied and used for decades in the laboratory and food industry, it has not been applied to the field of interventional oncology until recently. Today, human trials utilizing electroporation to treat a variety of tumors are underway. This review describes the evolution of the technique from the bench to the bedside and highlights the remaining challenges to standardizing its use in tumor ablation.
History of electroporation science
The earliest observations of a phenomena resembling electroporation can be traced to the 1700s. The first description of IRE may have been made in 1754 by Nollet, who applied electric sparks to human and animal skin and noted resulting red spots. During the 18th century, there was increasing interest in the effect of electricity on biological systems, most of which was focused on the twitching and contraction induced by electrical currents applied to animal spinal cords and to muscle-nerve preparations (1). In the 1800s, a report of using high voltage discharges to purify river water was the first to describe the bactericidal effect of IRE, although the mechanism was not understood at that time. During the nineteenth century, there were other reports of electricity used in medicine. For example, hemolysis due to the application of pulses of electricity was reported, but the underlying mechanisms of electroporation were not known.
It was not until the twentieth century that the phenomenon of electroporation was characterized as inducing increased membrane permeability or that the thermal and nonthermal effects of electrical energy were resolved. Some of the first observations distinguished the effects of electrical burns from nonthermal electrical injuries due to lightning, which are now thought to be caused by electroporation (2). Additionally, during the early 1900s, the concept of the cell membrane as a dielectric layer was advanced. In 1925, a study accurately estimated the thickness of the cell membrane by assessing the electrical properties of red blood cells. Thus, the first half of the twentieth century saw two major breakthroughs that advanced the understanding of electroporation: 1) the demonstration that membranes are dielectric structures and 2) that electricity generates both thermal and nonthermal biological effects.
During the 1950s and 1960s, the process of electroporation involving the permeabilization of cell membranes was elucidated, primarily in experiments on nerves and in studies related to food sterilization. For example, Stämpfli and Huxley described the reversible and IRE of frog nerve membranes, assessing the effect of altering the characteristics of the electrical pulse applied to the membrane and measuring consequent changes in membrane resistance, likening the membrane to a capacitor (1). In parallel, since the initial observation of the bactericidal effect of electricity on river water in 1898, research on water and food purification has continued. By the 1960s, commercial installations utilizing electrical pulses to provide nonthermal bacterial inactivation were available (1). This effort culminated in the seminal work of Sale and Hamilton, who elucidated the nonthermal bactericidal effect of electrical pulses, described the optimal pulse variables used to accomplish this affect, and demonstrated the underlying physiology of the changes in membrane conformation and permeability (1).
During the late 1900s, reversible electroporation became a mainstream technology in medical and biological sciences. The early 1980s saw the introduction of electroporation to induce cell fusion (electrofusion) and the transfer of DNA into cells (electrotransfer). An early paper described the application of short electric pulses to mouse lyoma cells deficient in the thymidine kinase gene, permitting the uptake of plasmid DNA containing the herpes simplex thymidine kinase gene and producing stable transformants (3). The study postulated the “electroporation model,” whereby changes in the membrane lipids caused increased cell permeability and therefore trans-membrane movement. DNA electrotransfer can be performed in bacterial and eukaryotic cell lines and circumvents the need for viral delivery methods of DNA.
Electrofusion and DNA electrotransfer have long since been established as laboratory applications of reversible electroporation. In the laboratory, electroporation is performed with hand-held or bench-top electroporators. These instruments are designed to create electric fields in cell solutions. Cell suspensions are pipetted into glass or plastic cuvettes with two aluminum electrodes on either side. The application of electrotransfer has been expanded beyond DNA to the introduction of enzymes, antibodies, and particles, including viruses and other biochemical reagents, into cells for intracellular assays.
During the 1990s, laboratory techniques for gene transfection into cells and gene delivery into biological tissues were developed and honed. Early on, electroporation led to cell death in a large portion of treated cells. Because of this, electroporation was initially limited to bacterial transformation. Over the past few decades, a number of advances have improved the efficiency of electroporation and its applications. Such advances includes determining and maintaining optimal pulse parameters, particularly with the use of a square wave electroporator that can provide precise, careful control of the amplitude and duration (4).
These initial discoveries inspired the later application of reversible electroporation to induce cellular permeability in large molecules, such as the use of cytotoxic agents in the treatment of cancer, and to induce permeability in the skin, enhancing transdermal drug delivery (1). The use of electroporation to enhance the uptake of antitumoral drugs (electrochemotherapy) was further developed, leading to clinical trials, as described below. Interestingly, in the late 1990s, there were reports that cell death due to IRE came not only from necrosis but also from apoptosis, which may have been the first suggestion that electroporation could offer advantages as an ablative technique.
Physiological principles underlying electroporation
Currently, the transient aqueous pore hypothesis is the favored explanation for the physiologic effects of electroporation, and recent molecular dynamic simulations support this theory (5). By this hypothesis, electroporation entails the creation of nanoscale pores or defects in the cell membrane. Assuming that membrane hydrophobic pores are randomly and spontaneously created due to the thermal motion of phospholipid molecules, the location and size of the pores vary randomly. According to classical surface physical chemistry, the formation energy of a single pore of radius r is expressed as ΔWp(r)=2γπr−πr2Γ (6). This expression indicates that in the absence of an external electrical field, the pore formation energy for a single pore, ΔW(r), is the difference between the energy gained in the formation of the outer edge of the pore (γ) and the energy reduction due to the loss of a circular patch cut from the membrane when the pore was created (πr2Γ). This notion is derived from Deryagin’s and Gutop’s work on soap film stability proposed in the 1960s (6). The constant γ represents the energy per length at the edge of the pore; Γ is the energy per area of a flat, pore-free membrane.
The pore formation energy by definition represents the activation energy barrier that thermal fluctuations must overcome to generate a hydrophobic pore in the absence of a transmembrane voltage. The activation energy barrier is sufficiently high, such that cells do not frequently self-rupture. However, these spontaneous pores likely permit the emergence of sites where physiologic phospholipid translocations occur. If the hydrophobic core radius (r) exceeds a critical value, rt (0.3 to 0.5 nm), it overcomes the energy barrier and turns into a hydrophilic pore, which is presumed to be responsible for the initiation of electroporation. After a hydrophilic pore forms, water molecules pass into the intermembrane space during the pore transformation process. Therefore, electroporation creates a nanohydrophilic environment in a typically hydrophobic location. Nearby lipids then reorient to form more stable structures, generating a stable hydrophilic pore.
Several mathematical techniques are used to model the effect of electrical pulses on cell membranes. Most simply, the cell can be modeled as a sphere composed of concentric shells, including the interior cytoplasm, the membrane sheath, and the extracellular space. The transmembrane voltages and potential distributions can be estimated by solving the Laplace equation for each space, with the membrane assumed to be nonconducting. Transmembrane potential difference, ΔVM, may be estimated by the equation, ΔVM=1.5×r×Eext cos θ, where r is the cell radius; Eext is the external electric field strength; and θ is the polar angle relative to the direction of the electric field. If a critical transmembrane potential is reached (∼1 V), the polarity of the cell membrane is temporarily reversed, inducing local rapid rearrangement of the membrane lipid morphology and creating a pore. More complex mathematical models may be used for non-spherical cells to estimate the pore size and the likelihood that pores are temporary and heal or are permanent.
Conductive pores can either heal, as in reversible electroporation, or expand in size, as in IRE. Whether the process is reversible or irreversible depends on whether the critical defect size in the membrane is reached. This is thought to be determined by local mechanical stresses, membrane bilayer edge energy, and the nature of the applied field. Adjustable parameters of the applied electric field include pulse amplitude, duration, and frequency. Beyond the parameters of the electrical pulses used, the cell type, age of the cell, morphology, and size determine how the cells respond to the electrical pulses. For instance, Jurkat cells, an immortalized line of T lymphocyte cells, are readily electroporated, whereas human promyelocytic leukemia (HL-60) cells are not (7). More research is needed to understand which cell parameters are the strongest determinants of electroporation efficiency and whether these variables can be exploited to target neoplastic cells.
Compared with other ablation techniques, IRE is nonthermal (8, 9) when designed appropriately. However, applied electric fields can involve thermal fluctuations that may lead to tissue heating. This is known as the Joule effect after Joule’s first law, which expresses that the heat generated by current flowing through a conductor as a function of the strength and time the current is applied as the resistance of the conductor. When used as an ablation technique, the thermal effects of electroporation are ideally minimized, with the specific goal of targeting cells and avoiding the nonspecific heating of surrounding normal tissue. Applying shorter pulses minimizes the probability of a thermal effect and permits cell-specific, nonthermal ablation.
Clinical applications of reversible electroporation
Reversible electroporation has multiple applications in human patients. One active area under investigation is the enhanced delivery of drugs to increase cellular uptake following local or intravenous administration. For example, electrochemotherapy is used to increase the uptake of bleomycin and cisplatin, to which plasma membranes are relatively impermeable. Electrical pulses are delivered with needle or plate electrodes. Endoscopic, expandable soft tissue, and bone pin electrodes have also been used (4). The first clinical trial of electrochemotherapy in head and neck squamous cancer patients demonstrated the clear antitumor effects of bleomycin; this treatment was well tolerated (10). A similar trial was reported in 1993, involving the treatment of head and neck metastases (11), and later studies have reported the use of bleomycin for melanoma metastases and basal cell carcinomas (12, 13). Several other clinical trials are underway investigating the efficacy and tolerability of electrochemotherapy (14).
In addition to chemotherapy agents, the intracellular delivery of other drugs and compounds can also be enhanced with electroporation. Molecules, including oligonucleotides, ions, dyes, and radioactive tracers, can be delivered with promising clinical applications (4). Electroporation may also be applied to lipid-based barriers in human tissues, generating new aqueous pathways and hence facilitating local drug delivery. For example, the stratum corneum of the skin and other tissue monolayers in which cells are connected by tight junctions are lipid-based barriers that can be targeted with electrical pulses. The transdermal transport of large compounds, such as lidocaine, insulin, heparin, and vaccines, is therefore possible (15).
Gene electrotransfer also has potential clinical uses in human patients. In a phase I trial, plasmid interleukin (IL)-12 electroporation was performed in 24 metastatic melanoma patients, demonstrating that IL-12 protein levels and the extent of tumor necrosis increased proportionally to the plasmid doses in post-treatment biopsies. Most patients demonstrated a clinical response (16).
Mechanisms of cell death in IRE
It is thought that the primary mechanism of cell death from IRE is apoptosis (programmed cell death), in contrast to coagulative necrosis (death by ischemia or infarction), which is the primary mechanism in radiofrequency and microwave ablation. However, the exact molecular mechanism of cell death after IRE is unknown, and both necrosis and apoptosis are likely to occur. Initially, it was thought that the poration of the membrane, which leads to increased permeability, was the primary trigger of cell death. After the application of the electric field, the enhanced transmembrane molecular transport with rapid membrane discharge induces the cell to attempt to recover its membrane potential by conducting small ions, including sodium and chloride, through the transient pores. Cells under extensive chemical or osmotic stress from excessive molecular transport may then undergo lysis. Additionally, if one or more critical pores expand, the membrane may be destroyed, leading to complete dissolution of the cell.
Newer studies have shown that apoptosis after the application of electrical fields can occur without pore formation, although when pores are induced, apoptotic markers appear faster (7). Short electrical pulses induce an intracellular calcium release, most likely by the poration of the endoplasmic reticulum membrane, which in turn may initiate apoptosis. Electrical pulses may also increase the mitochondrial permeability, releasing cytochrome c, which is a small protein involved in the initiation of apoptosis. Finally, electric pulses may cause DNA damage and elevated levels of reactive oxygen species, inducing oxidative stress-mediated apoptosis.
Reports on the pathways that lead to cell death are contradictory. For instance, one study reported extensive caspase-3 activation 24 hours after the IRE of rat hepatocellular carcinoma, suggesting apoptosis (17), while another did not detect any caspase-3 positive cells in the treated area of pancreatic carcinoma (18). Contradictory reports may reflect differences in the tumor model used, among other experimental factors.
In summary, the proposed mechanisms of apoptotic cell death due to IRE include cell rupture from pore expansion and osmotic/chemical stress, intracellular calcium release from the endoplasmic reticulum, cytochrome c release from mitochondria, and oxidative stress. Irreversibly permeabilized cells that undergo apoptosis are then removed by the immune system, and the IRE may potentiate an immune reaction to the ablated tissue, enhancing the treatment effect. However, the question of whether IRE’s effects on the cell membrane serve as a final event or whether they also induce long-term programmed cell death is still open for further study.
Biological effect of IRE in live animals
The ability of IRE to induce nonthermal cell death and target the cellular membrane is the primary motivation behind applying this technology to tumor ablation. Numerous experiments in live mammals have demonstrated specific features of the IRE ablation zone that make it unique among the available ablation modalities.
Because of the relative specificity for the cell membrane, IRE has been shown in animal studies to spare tissue scaffolds, in contrast to other modalities causing thermal ablation. For example, experimental studies examining the histologic changes after IRE performed on rat carotid arteries showed that, at four weeks postablation, the vascular connective matrix remained intact. The number of arterial wall vascular smooth muscle cells was decreased, but no thrombosis, necrosis, or aneurysm was present (19). Studies of IRE ablation in the pig liver demonstrated that the complete ablation of tissue up to the blood vessel margins may be accomplished without compromising the structural integrity or functionality of blood vessels, bile ducts, or connective tissue, all of which remain intact (20, 21). Histologically, some have suggested that IRE produces an ablation zone with sharper demarcations that can be observed both micro- and macroscopically, enabling better post-treatment assessment compared with other ablation modalities (22). However, mathematical modeling and recent experimental data show that IRE has a probabilistic nature, and careful design is required to include the entire tumor in the ablated zone.
IRE ablation zones appear to be blood-flow independent and are therefore not susceptible to the heat sink effect, a phenomenon that arises in thermal ablation techniques (radiofrequency, microwave) whereby heat is dissipated by blood flow in adjacent vessels. The efficacy of thermal ablation is diminished for tumors adjacent to large vessels, most likely because of the heat sink effect (23, 24). Correspondingly, the cold sink effect in cryotherapy has been reported for lesions adjacent to blood vessels (25). In contrast, IRE has been shown to create more complete perivascular tumor ablations, even when a large vessel traverses the ablation zone (22).
Imaging findings of IRE in liver lesions have been described in animal models. On ultrasound (US) imaging, treated areas become hyperechoic with minimal formation of hyperechoic air bubbles observed in the thermal ablation techniques (20). Immediate contrast-enhanced computed tomography (CT) images demonstrate a hypoenhancing zone of ablation. For perivascular tumors, mild hepatic venous narrowing may be observed in vessels traversing the lesion, but they otherwise remain patent (26). CT imaging 48 hours after the IRE shows that the liver ablation zones centrally hypoattenuate, with mild hyperattenuation surrounding the zone of ablation; periablation arterial, but not venous, enhancement is also observed (27). The magnetic resonance imaging findings are similar, with a centrally low T1 signal and peripheral enhancement.
Application of electroporation to percutaneous tumor ablation
The concept of using IRE for tumor ablation was formally introduced less than a decade ago in a theoretical paper using a mathematical analysis to show that IRE can ablate a volume of tissue comparable to other ablation modalities without detrimental thermal effects (8). As mentioned above, electropermeabilization has been used since the 1960s in the food industry for microbial inactivation (28). Surprisingly, it was not until the theoretical paper published in 2005 that the connection between IRE and tumor ablation was made (8).
The first use of IRE in human patients was performed for prostate cancer (29). In this study, 16 patients were treated using four electrodes, separated 1.0–1.5 cm, and applying 90 pulses of 70–100 μs duration at 1500 V and 10 Hz. All patients tolerated the procedure well, with no postprocedural impotence or incontinence and no reported complications. Postoperative biopsies performed three weeks after ablation demonstrated no residual cancer. In one patient, a micro-focus of cancer was found outside the treated area (29).
Another early clinical trial was a single-center, nonrandomized, prospective cohort study in Australia that included 38 patients with advanced liver, kidney or lung tumors, with 69 total tumors treated (30). Treatments typically utilized unipolar or bipolar electrodes, with 90 pulses of 70 μs high-voltage (1500–3000 V) direct current (25–45 A) delivered in nine sets of 10 pulses per treatment site. Complete tumor ablation was observed in 66% of tumors, with kidney and lung tumors comprising the largest failure rate. In cirrhotic patients with hepatocellular carcinoma, 15 of 18 lesions had complete target tumor ablation, although one patient required five treatments. Large liver metastases (> 5 cm) were not controlled with IRE. No patients with lung tumors had a treatment response. Notable complications of IRE included ventricular and other arrhythmias. After four patients experienced arrhythmias, ECG-synchronized delivery was then used. Despite this, two additional patients experienced arrhythmias. Additionally, complications during renal ablations included nontarget ablation of the adrenal gland, leading to severe hypertension, and of the ureter, later causing ureteral obstruction. Other complications were related to general anesthesia.
The published experience has been slowly growing since that initial study, and the literature suggests that liver lesions may be the optimal target for IRE ablation. For example, a recent prospective study of 44 patients undergoing 48 IRE ablations for colorectal metastases (20 lesions), hepatocellular carcinoma (14 lesions), and other metastases (10 lesions) demonstrated a local, recurrence-free survival of 97.4% at three months, 94.6% at six months and 59.5% at 12 months, with a trend for higher recurrence rates for lesions larger than 4 cm (31).
A smaller series investigated the safety of treated liver tumors near vascular structures, describing IRE treatment of 65 tumors found in 28 patients, 21 of whom had colorectal metastases (32). None of the 25 treated tumors located within 1 cm of a major hepatic vein had venous thrombosis; one treatment of the 16 tumors within 1 cm of a major portal pedicle resulted in portal vein thrombosis. The authors suggest that IRE can be safely performed on liver lesions adjacent to major vascular structures, consistent with the theoretical benefits of nonthermal ablation. After six months of follow up, there was persistent disease in 1.9% of tumors and local recurrence in 5.7%, yielding an overall 7.5% treatment failure rate.
Beyond liver tumors, IRE has also been applied in the treatment of locally advanced unresectable pancreatic cancer for downstaging and control (Fig. 2). In a recent retrospective case series, IRE was performed in 14 patients, with one patient receiving two treatments; three patients had meta-static disease; and the mean tumor size was 3.3 cm (33). Only one IRE-related complication, pancreatitis, was encountered. Postprocedure scans performed immediately after IRE and 24 hours later demonstrated patent vasculature. All patients with metastatic disease eventually died from progression of disease, but two other patients who were successfully treated remained disease-free for 11 and 14 months.
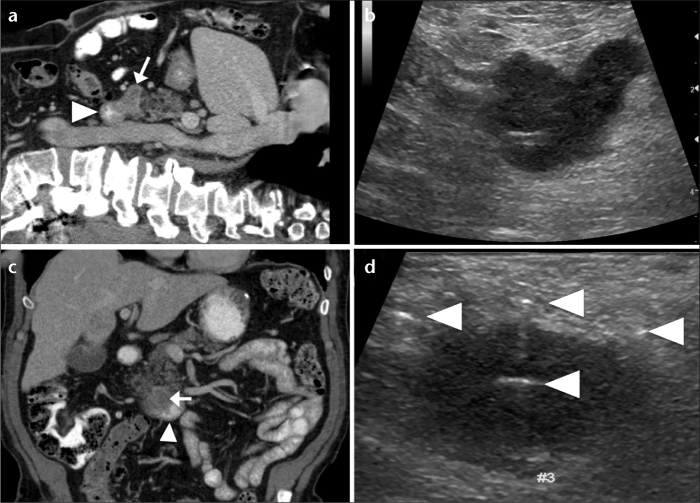
Figure 2. a–d.
Irreversible electroporation (IRE) of a locally advanced unresectable pancreatic adenocarcinoma. Sagittal reformatted contrast-enhanced CT image of the abdomen (a) reveals a pancreatic adenocarcinoma (arrow) abutting the duodenum (arrowhead). Corresponding intraoperative sagittal US image (b) shows the hypoechoic pancreatic adenocarcinoma. Coronal reformatted contrast-enhanced CT image of the abdomen (c) shows the pancreatic adenocarcinoma (arrow) and the adjacent duodenum (arrowhead). Intraoperative coronal US image (d) shows the pancreatic adenocarcinoma with four IRE probes in place (arrowheads), prior to ablation.
Another multicenter clinical trial included 27 patients with locally advanced pancreatic cancer who received IRE either with or without concurrent partial resection (34). All but one patient received surgical IRE, as opposed to the final patient, who underwent percutaneous IRE. In four cases, there were possible IRE device-related complications. There was one 90-day mortality due to hepatic and renal failure, but no cases of pancreatitis or fistula formation. Several other complications, such as deep venous thrombosis, ileus, and bile leak, were attributed to surgery. In the 26 of 27 patients who were alive at the 90-day follow-up, there was no evidence of recurrent disease. Furthermore, there was a significant decrease in patient pain and narcotics use compared with before IRE. The authors concluded that IRE is efficacious and relatively safe. These initial studies are promising and suggest that IRE may be a safe ablation modality for locally advanced pancreatic cancer. Larger trials are pending.
Still in its infancy as an ablation modality, IRE may be efficacious for other tumors. For instance, there are limited data supporting its potential use in sarcoma (35, 36). Animal models have shown efficacy of IRE for breast cancer (37) and possibly brain tumors (38), although human trials are pending. Though initial clinical trials have suggested that the use of IRE for renal cell carcinoma may be relatively safe (39), data are lacking supporting its use in place of cryotherapy or radiofrequency ablation (30). Similarly, limited data for lung cancer have failed to provide evidence supporting its use, with high recurrence rates (30, 40). The NanoKnife IRE system (Angiodynamics, Latham, New York, USA) has been commercially available since 2009 and is approved by US Food and Drug Administration for ablation of soft tissue tumors.
Other clinical applications of IRE
Beyond tumor ablation, IRE could be used to ablate other structures for nononcologic applications. For example, renal sympathetic nerve denervation with IRE is currently under investigation for the treatment of hypertension, based on initial successes with percutaneous radiofrequency ablation (41). In this technique, renal sympathetic denervation is performed via the main renal artery lumen with a catheter connected to a radiofrequency generator. By ablating these sympathetic nerves, norepinephrine levels are reduced, and hypertension is ameliorated. A small trial of 50 patients reported good outcomes, with decreased blood pressure of nearly 30 points at nine months; one renal artery dissection was noted (41). Larger trials are warranted to assess procedural safety (42). Hypothetically, using IRE rather than radiofrequency ablation could avoid any heat sink effect and diminish unwanted renovascular complications; still, others have reported that IRE relatively spares nerves. For example, a rat’s sciatic nerve treated with IRE demonstrated a full recovery of neural function after seven weeks (43). Further work is necessary to ascertain whether IRE can be applied in this field safely and efficaciously.
Another application of electroporation is wound healing. Gene therapy is an emerging field for nonhealing wounds that focuses on recombinant growth factors that can promote wound healing (44). Multiple methods of gene insertion exist, including gene guns and viral vectors; electroporation may emerge as a dominant modality.
IRE may also be applied in vascular medicine to prevent arterial restenosis after angioplasty. The medial layer of the arteries contains vascular smooth muscle cells (VSMC) that proliferate after angioplasty, causing significant unwanted luminal loss (45, 46). Various proposed methods to prevent VSMC proliferation have been suggested, including brachytherapy, cryoplasty, gene therapy, drug-eluting stents, photodynamic therapy, and more recently, electroporation (47). Studies in rat and rabbit models demonstrated that arteries treated by endovascular IRE cause the ablation of VSMCs, which persisted for over a month; the elastic lamina remained intact, while the endothelial layer regenerates (19, 47, 48). The number of VSMCs decreased without the formation of an aneurysm or thrombus and without necrosis. The vascular connective tissue matrix remained intact. At five weeks, wall fibrosis with regenerated endothelium was observed. These findings have clear implications for nonthermal endovascular ablation using IRE in cardiovascular medicine.
Special considerations for performing IRE compared with other tumor ablation modalities
The primary consideration for performing IRE is that the procedure must be performed under general anesthesia, in contrast to thermal ablation techniques that are frequently performed under conscious sedation. The principal reasons for this include muscle stimulation and cardiac arrhythmias due to the strong, pulsed electric fields. Muscle stimulation, including the diaphragm, necessitates the use of paralytic agents. Muscular contractions are typically localized to the treatment area but can still involve the diaphragm, even when patients are sufficiently paralyzed. The administration of paralytic agents necessitates neuromuscular monitoring.
Cardiac arrhythmias were previously noted in animal models and subsequently in early clinical trials (30); they are more likely to occur when treating lesions close to the heart. These observations led to the routine use of cardiac synchronization during IRE, which necessitates cardiac monitoring. While IRE necessitates neuro-muscular and cardiac monitoring, it does not require the grounding pads needed in radiofrequency ablation, the cooling system (e.g., ice bucket) needed in radiofrequency and microwave ablation, or the gas tank and delivery system needed for cryotherapy.
On a practical level, ablation with IRE is performed with two to six monopolar probes or a single bipolar probe with a generator. Ablation zones of various sizes and shapes tailored for specific organs can be created depending on probes and settings used (Table). Careful mathematical modeling can predict ablation zone characteristics. CT or US guidance is often used during IRE ablation and is typically short in duration. For example, roughly one minute is required to ablate a 3 cm liver lesion.
Table.
Typical irreversible electroporation treatment parameters
In summary, while many of the procedural details are similar between IRE and other ablation modalities, the need for general anesthesia and more involved intraprocedural monitoring is an additional consideration that may be most appropriate for tumors adjacent to vessels and other critical structures because this is the setting where IRE has its strongest theoretical benefit.
Conclusion
Electroporation is a technique with a long history of application in the laboratory. It has been slowly developed, initially from observations on the effects of electricity on biological systems, in parallel with the study of electrical pulses on sterilized food and water. Once the underlying mechanisms of nonthermal electropermeabilization were better understood, applications for human patients emerged. While IRE for the treatment of hepatic, pancreatic and possibly other tumors is a promising new interventional oncology tool, more work is needed to improve its safety and delineate expected outcomes.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ivorra A, Rubinsky B. History review of irreversible electroporation in medicine. In: Rubinsky B, editor. Irreversible electroporation, series in biomedical engineering. Berlin: Chennai Springer-Verlag; 2010. pp. 1–21. [Google Scholar]
- 2.Lee RC, Gaylor DC, Bhatt D, Israel DA. Role of cell membrane rupture in the pathogenesis of electrical trauma. J Surg Res. 1988;44:709–719. doi: 10.1016/0022-4804(88)90105-9. [DOI] [PubMed] [Google Scholar]
- 3.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehl J. Electroporation for medical use in drug and gene electrotransfer. In: Alkire RC, Dieter MK, Lipkowski J, editors. Advances in electrochemical science and engineering. Weinheim: Wiley-VCH Verlag GmgH & Co.; 2011. pp. 369–388. [Google Scholar]
- 5.Bockmann RA, de Groot BL, Kakorin S, Neumann E, Grubmuller H. Kinetics, statistics, and energetics of lipid membrane electroporation studied by molecular dynamics simulations. Biophys J. 2008;95:1837–1850. doi: 10.1529/biophysj.108.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochem Bioenerg. 1996;42:135–160. [Google Scholar]
- 7.Beebe SJ, Fox PM, Rec LJ, Willis EL, Schoenbach KH. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003;17:1493–1495. doi: 10.1096/fj.02-0859fje. [DOI] [PubMed] [Google Scholar]
- 8.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 9.Golberg A, Laufer S, Rabinowitch H, Rubinsky B. In vivo nonthermal irreversible electroporation impact on rat liver galvanic apparent internal resistance. Phys Med Biol. 2011;56:951. doi: 10.1088/0031-9155/56/4/005. [DOI] [PubMed] [Google Scholar]
- 10.Mir LM, Belehradek M, Domenge C, et al. Electrochemotherapy, a new antitumor treatment: first clinical trial. C R Acad Sci III. 1991;313:613–618. [PubMed] [Google Scholar]
- 11.Belehradek M, Domenge C, Luboinski B, Orlowski S, Belehradek J, Jr, Mir LM. Electrochemotherapy, a new antitumor treatment. First clinical phase I–II trial. Cancer. 1993;72:3694–3700. doi: 10.1002/1097-0142(19931215)72:12<3694::aid-cncr2820721222>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Glass LF, Fenske NA, Jaroszeski M, et al. Bleomycin-mediated electrochemotherapy of basal cell carcinoma. J Am Acad Dermatol. 1996;34:82–86. doi: 10.1016/s0190-9622(96)90838-5. [DOI] [PubMed] [Google Scholar]
- 13.Heller R, Jaroszeski MJ, Glass LF, et al. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer. 1996;77:964–971. doi: 10.1002/(sici)1097-0142(19960301)77:5<964::aid-cncr24>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther. 2009;17:585–592. doi: 10.1038/mt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 16.Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Zhang Y, Klein R, et al. Irreversible electroporation therapy in the liver: longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer R. 2010;70:1555–1563. doi: 10.1158/0008-5472.CAN-09-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jose A, Sobrevals L, Ivorra A, Fillat C. Irreversible electroporation shows efficacy against pancreatic carcinoma without systemic toxicity in mouse models. Cancer Lett. 2012;317:16–23. doi: 10.1016/j.canlet.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6:307–312. doi: 10.1177/153303460700600407. [DOI] [PubMed] [Google Scholar]
- 20.Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007;6:287–294. doi: 10.1177/153303460700600404. [DOI] [PubMed] [Google Scholar]
- 21.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol Cancer Res Treat. 2007;6:37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 22.Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver. 2010;4(Suppl 1):S99–S104. doi: 10.5009/gnl.2010.4.S1.S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 24.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 25.Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology. 2009;41:168–172. doi: 10.1080/00313020802579292. [DOI] [PubMed] [Google Scholar]
- 26.Lee YJ, Lu DS, Osuagwu F, Lassman C. Irreversible electroporation in porcine liver: short- and long-term effect on the hepatic veins and adjacent tissue by CT with pathological correlation. Invest Radiol. 2012;47:671–675. doi: 10.1097/RLI.0b013e318274b0df. [DOI] [PubMed] [Google Scholar]
- 27.Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255:426–433. doi: 10.1148/radiol.10090337. [DOI] [PubMed] [Google Scholar]
- 28.Toepfl S, Mathys A, Heinz V, Knorr D. Potential of high hydrostatic pressure and pulsed electrics fields for energy efficient and environmentally friendly food processing. Food Reviews International. 2006;22:405–423. [Google Scholar]
- 29.Onik G, Rubinsky B. Irreversible electroporation: first patient experience focal therapy of prostate cancer. In: Rubinsky B, editor. Irreversible electroporation, series in biomedical engineering. Berlin: Chennai Springer-Verlag; 2010. pp. 235–247. [Google Scholar]
- 30.Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611–621. doi: 10.1016/j.jvir.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC., 2nd Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107:544–549. doi: 10.1002/jso.23280. [DOI] [PubMed] [Google Scholar]
- 32.Kingham TP, Karkar AM, D’Angelica MI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012;215:379–387. doi: 10.1016/j.jamcollsurg.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23:1613–1621. doi: 10.1016/j.jvir.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Martin RCG, McFarland K, Ellis S, Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215:361–369. doi: 10.1016/j.jamcollsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Neal RE, 2nd, Rossmeisl JH, Jr, Garcia PA, Lanz OI, Henao-Guerrero N, Davalos RV. Successful treatment of a large soft tissue sarcoma with irreversible electroporation. J Clin Oncol. 2011;29:e372–377. doi: 10.1200/JCO.2010.33.0902. [DOI] [PubMed] [Google Scholar]
- 36.Yu Z, Zhang X, Ren P, Zhang M, Qian J. Therapeutic potential of irreversible electroporation in sarcoma. Expert Rev Anti-cancer Ther. 2012;12:177–184. doi: 10.1586/era.11.211. [DOI] [PubMed] [Google Scholar]
- 37.Neal RE, 2nd, Singh R, Hatcher HC, Kock ND, Torti SV, Davalos RV. Treatment of breast cancer through the application of irreversible electroporation using a novel minimally invasive single needle electrode. Breast Cancer Res Treat. 2010;123:295–301. doi: 10.1007/s10549-010-0803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis TL, Garcia PA, Rossmeisl JH, Jr, Henao-Guerrero N, Robertson J, Davalos RV. Nonthermal irreversible electroporation for intracranial surgical applications. Laboratory investigation. J Neurosurg. 2011;114:681–688. doi: 10.3171/2010.5.JNS091448. [DOI] [PubMed] [Google Scholar]
- 39.Pech M, Janitzky A, Wendler JJ, et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol. 2011;34:132–138. doi: 10.1007/s00270-010-9964-1. [DOI] [PubMed] [Google Scholar]
- 40.Usman M, Moore W, Talati R, Watkins K, Bilfinger TV. Irreversible electroporation of lung neoplasm: a case series. Med Sci Monit. 2012;18:CS43. doi: 10.12659/MSM.882888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 42.Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv. 2013;6:1–9. doi: 10.1016/j.jcin.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Fan Q, Ji Z, Qiu X, Li Z. The effects of irreversible electroporation (IRE) on nerves. PLoS One. 2011;6:e18831. doi: 10.1371/journal.pone.0018831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrie NC, Yao F, Eriksson E. Gene therapy in wound healing. Surg Clin North Am. 2003;83:597–616. doi: 10.1016/S0039-6109(02)00194-9. [DOI] [PubMed] [Google Scholar]
- 45.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 46.Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling. Mechanisms and clinical implications. Circulation. 2000;102:1186–1191. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
- 47.Maor E, Ivorra A, Rubinsky B. Nonthermal irreversible electroporation: novel technology for vascular smooth muscle cells ablation. PLoS One. 2009;4:e4757. doi: 10.1371/journal.pone.0004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maor E, Ivorra A, Mitchell JJ, Rubinsky B. Vascular smooth muscle cells ablation with endovascular nonthermal irreversible electroporation. J Vasc Interv Radiol. 2010;21:1708–1715. doi: 10.1016/j.jvir.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]