Abstract
PURPOSE
Many studies have indicated that cervicogenic headache may originate from the cervical structures innervated by the upper cervical spinal nerves. To date, no study has investigated whether narrowing of the craniovertebral angle (CVA) or cervicomedullary angle (CMA) affects the three upper cervical spinal nerves. The aim of this study was to investigate the effect of CVA and/or CMA narrowing on the occurrence of cervicogenic headache.
MATERIALS AND METHODS
Two hundred and five patients diagnosed with cervicogenic headache were included in the study. The pain scores of patients were determined using a visual analog scale. The nonheadache control group consisted of 40 volunteers. CVA and CMA values were measured on sagittal T2-weighted magnetic resonance imaging (MRI), on two occasions by two radiologists. Angle values and categorized pain scores were compared statistically between the groups.
RESULTS
Intraobserver and interobserver agreement was over 97% for all measurements. Pain scores increased with decreasing CVA and CMA values. Mean angle values were significantly different among the pain categories (P < 0.001). The pain score was negatively correlated with CMA (Spearman correlation coefficient, rs, −0.676; P < 0.001) and CVA values (rs, −0.725; P < 0.001).
CONCLUSION
CVA or CMA narrowing affects the occurrence of cervicogenic headache. There is an inverse relationship between the angle values and pain scores.
Cervicogenic headache (CH) is a referred pain from the cervical structures innervated by the three upper cervical spinal nerves. Anesthetic blocks of the cervical structures or related nerves can provide temporary pain relief, suggesting that the pain may be due to a neck disorder (1–4). Possible causes of CH are atlantooccipital joint, atlantoaxial joint, zygapophyseal joint, intervertebral disc, and upper cervical spinal nerve pathologies. However, there is agreement that degenerative changes in the cervical spine do not necessarily correlate with pain (1, 5). The pain in CH may originate from various anatomic structures in the cervical spine. The diagnostic value of such changes remains controversial, and their relevance in CH is unknown. Many studies have indicated that CH may originate from the cervical structures innervated by the upper cervical spinal nerves and trigeminal nerve branches (6, 7). To date, no study has investigated whether narrowing of the craniovertebral angle (CVA) or cervicomedullary angle (CMA) affects the trigeminal nerve branches and three upper cervical spinal nerves via stretching, thereby causing pain. Herein, we aimed to determine whether CVA and/ or CMA narrowing has any effect on CH occurrence.
Materials and methods
A total of 4122 patients were admitted to the Neurology and Neurosurgery Department of Bașkent University with a complaint of headache between January 2011 and May 2012, and 205 of them diagnosed with CH were included in the study. The diagnosis of CH was made according to the International Headache Society (IHS) diagnostic criteria for CH (1):
Pain, referred from a source in the neck and perceived in one or more regions of the head and/or face, fulfills criteria C and D.
Clinical, laboratory, and/or imaging evidence of a disorder or lesion within the cervical spine or soft tissues of the neck is known to be, or generally accepted as, a valid cause of headache.
- There is evidence that the pain can be attributed to the neck disorder or lesion based on at least one of the following:
- Demonstration of clinical signs that implicate a source of pain in the neck.
- Abolition of headache following diagnostic block of a cervical structure or its nerve supply using placebo or other adequate controls. Abolition of headache means complete relief of headache, indicated by a score of zero on a visual analog scale (VAS).
Pain resolves within three months after successful treatment of the causative disorder or lesion.
Following approval by the Institutional Review Board of our institution, the patients were evaluated for symptoms related to CH. All patients were informed about the study, and detailed informed consent was obtained from each participant.
Patient exclusion criteria were as follows: hyperlipidemia, hypertension, rheumatoid arthritis, abnormal findings on brain magnetic resonance imaging (MRI) (multiple sclerosis [MS], tumors, infarct, ischemic gliosis, vascular malformation, fracture, or infection), or a diagnosis of primary headache such as migraine, tension-type or cluster-type headache. The nonhead-ache control group (group 0) consisted of 40 volunteers with similar demographic characteristics to those of the pain group. A neurologist with 10 years of experience (approximately six years of involvement in the subspecialty of headaches, particularly migraines and four years of experience as a neurosurgeon) made the CH diagnosis in all 205 patients (pain group) and verified the lack of headache in the control group. Blockage of the occipital nerve was applied, and the patients who had responded to the medications for the last 2–3 months were included in the study. The pain scores were determined using the 100 mm VAS and categorized into six pain groups, ranging 0–5 (no pain, 0–2 mm; mild pain, 2–17 mm; moderate pain, 17–47 mm; severe pain, 47–77 mm; very severe pain, 77–96 mm; and most severe pain imaginable, 96–100 mm). There was no patient in the sixth pain group, which was thus excluded from the study. Brain MRI was performed in all patients within two days of the clinical diagnosis.
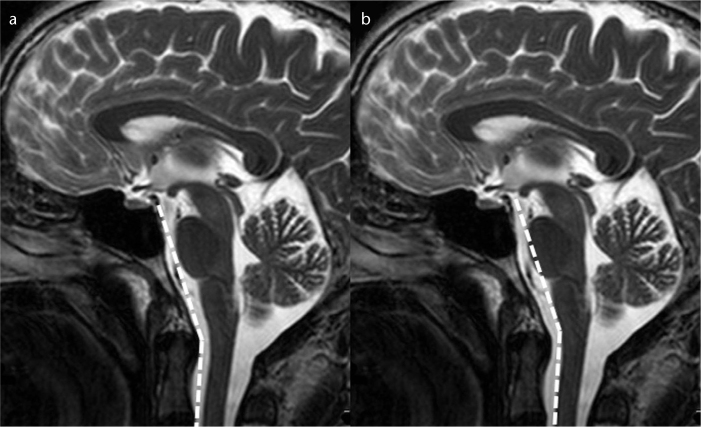
Brain MRI was performed in a routine natural supine position using a 1.5 Tesla scanner (Intera, Gyroscan, Philips Medical Systems, Best, The Netherlands). This natural position places the head in slight extension and represents the position in all static clinical MRIs that are obtained for the measurement of CVA and CMA. All images were taken according to a standard protocol using axial T2-weighted turbo spin echo (TR/TE, 4466/100 ms; slice thickness [ST], 5 mm; number of excitations [NEX], 3], coronal and sagittal T2-weighted turbo spin echo (TR/ TE, 4800/100 ms; ST, 4 mm; NEX, 3), axial fluid-attenuated inversion recovery (TR/TE, 6000/100 ms; ST, 5 mm; NEX, 3), and axial T1-weighted spin echo sequences (TR/TE, 462/11; ST, 5 mm; NEX, 3) covering the whole brain and upper cervical spine. All brain MRIs of the pain and control groups included the first two upper cervical vertebrae. Midline sagittal images of the T2-weighted series were used to obtain measurements. The CVA was constructed by drawing a line along the clivus and extrapolating it inferiorly into the upper cervical spinal canal (Fig. 1a). The angle between the two lines on the ventral side of the medulla oblongata and upper cervical spinal cord constituted the CMA (Fig. 1b). The CVA and CMA values were measured by two radiologists who were each blinded to the other’s results. CVA and CMA values were measured on two occasions by each radiologist with a four-week interval. The radiologists were also blinded to the clinical findings of the patients.
Figure 1. a, b.
T2-weighted mid-sagittal images show the normal craniovertebral angle (a) and normal cervicomedullary angle (b).
Statistical analysis
Compliance with the normal distribution of continuous variables was checked using the Shapiro-Wilk test. Levene’s test was used to analyze the homogeneity of the groups’ variances. The groups’ variances were homogeneous in terms of the pain severity groups, so group means were compared using two-factor analysis of variance, followed by the methods of multiple comparison using Bonferroni’s test. Correlations between variables were evaluated using Spearman correlation coefficient. Reliability analysis was performed, and intraclass correlation coefficients were calculated for consistency of measurements.
The Statistical Package for the Social Sciences version 17.0 software (SPSS Inc., Chicago, Illinois, USA) was used for the analysis of data sets. A P value less than 0.05 was deemed to indicate statistical significance.
Results
Two hundred and forty-five subjects were included in the study (155 females aged 32.1±9.7 years and 90 males aged 31.7±8.2 years; overall mean age, 31.9±8.9 years; range, 14–78 years). The pain group consisted of 205 patients (135 females and 70 males; mean age, 31.8±9.3 years; range, 14–78 years), whereas the nonheadache control group consisted of 40 volunteers (20 females and 20 males; mean age, 31.2±7.3 years; range, 20–55 years) with similar demographic characteristics to those of the pain group. We divided patients into five groups according to the pain scores. There were 49 patients in pain group 1 (mild pain) (30 females and 19 males; mean age, 33.4±8.8 years; range, 14–50 years), 59 patients in pain group 2 (moderate pain) (30 females and 29 males; mean age, 33.6±10.5 years; range, 18–78 years), 52 patients in pain group 3 (severe pain) (38 females and 14 males; mean age, 31.1±8.2 years; range, 16–47 years), and 45 patients in pain group 4 (very severe pain) (37 females and 8 males; mean age, 30.0±9.8 years; range, 18–60 years). There was no patient in pain group 5 (most severe pain, sixth group of the VAS). The control group was indicated as group 0 (no pain). Ages were comparable among the four pain groups and controls (P = 0.167), and there was also no age difference between the gender groups (P = 0.773).
Intraobserver and interobserver agreement were found to be over 97% for all measurements (P < 0.001, Table 1).
Table 1.
Intraobserver and interobserver agreement for CVA and CMA measurements
| Intraclass correlation coefficient | 95% confidence interval | |
|---|---|---|
| Intraobserver agreement | ||
| CVA | ||
| 1st vs. 2nd measurement of 1st radiologist | 0.989 | 0.986–0.991 |
| 1st vs. 2nd measurement of 2nd radiologist | 0.992 | 0.990–0.994 |
| CMA | ||
| 1st vs. 2nd measurement of 1st radiologist | 0.983 | 0.978–0.987 |
| 1st vs. 2nd measurement of 2nd radiologist | 0.985 | 0.981–0.989 |
| Interobserver agreement | ||
| CVA | ||
| 1st measurement of 1st vs. 2nd radiologist | 0.989 | 0.986–0.992 |
| 2nd measurement of 1st vs. 2nd radiologist | 0.992 | 0.990–0.994 |
| CMA | ||
| 1st measurement of 1st vs. 2nd radiologist | 0.983 | 0.978–0.987 |
| 2nd measurement of 1st vs. 2nd radiologist | 0.985 | 0.981–0.988 |
CMA, cervicomedullary angle; CVA, craniovertebral angle.
Agreements were found to be over 97% for all measurements (P < 0.001).
Pain scores increased with decreasing CVA values, and the difference was statistically significant (P < 0.001). There was no difference in CVA values between the control group (group 0) and pain group 1 (P = 0.685). However, CVA values were different among all other pain groups compared with groups 0 and 1 (P < 0.01).
Pain scores also increased with decreasing CMA values, and the difference was statistically significant (P < 0.001). There was no difference in CMA values between pain groups 2 and 3 (P = 0.069). However, CMA values were different among the other pain groups and compared with pain groups 2 and 3 (P < 0.05).
The relationship between the CMA and CVA values and pain scores was statistically significant for each group (Tables 2, 3).
Table 2.
Craniovertebral angle (°) in the pain groups and significance of differences between the groups
| Pain group (n) | Mean±SD | 95% confidence interval | P | |||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |||
| Group 0 (n=40) | 153.3±4.9 | 151.6–155.0 | 0.685 | < 0.001 | < 0.001 | < 0.001 |
| Group 1 (n=49) | 150.9±5.3 | 149.5–152.7 | 0.004 | < 0.001 | < 0.001 | |
| Group 2 (n=59) | 147.2±6.2 | 145.8–148.6 | < 0.001 | < 0.001 | ||
| Group 3 (n=52) | 142.8±5.6 | 141.0–144.4 | < 0.001 | |||
| Group 4 (n=45) | 136.9±5.0 | 134.5–138.7 | ||||
SD, standard deviation.
Table 3.
Cervicomedullary angle (°) in the pain groups and significance of differences between the groups
| Pain group (n) | Mean±SD | 95% confidence interval | P | |||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |||
| Group 0 (n=40) | 159.5±4.1 | 157.9–161.1 | 0.017 | < 0.001 | < 0.001 | < 0.001 |
| Group 1 (n=49) | 155.7±5.2 | 154.4–157.4 | 0.017 | < 0.001 | < 0.001 | |
| Group 2 (n=59) | 152.6±5.3 | 151.3–154.0 | 0.069 | < 0.001 | ||
| Group 3 (n=52) | 149.7±5.7 | 148.1–151.4 | 0.026 | |||
| Group 4 (n=45) | 146.0±5.1 | 143.7–147.8 | ||||
SD, standard deviation.
The relationship between the pain score and CMA (Spearman correlation coefficient, rs, −0.676; P < 0.001) and the relationship between the pain score and CVA (rs, −0.725; P < 0.001) values were negatively correlated. There was an inverse relationship between the pain scores and mean angle values. In addition, the relationship between the pain score and CMA and CVA values were negatively correlated with gender (Table 4).
Table 4.
Spearman correlation coefficient (rs) for pain score-CVA, pain score-CMA, and CVA-CMA correlations
| Total | Female | Male | ||||
|---|---|---|---|---|---|---|
| rs | P | rs | P | rs | P | |
| Pain score vs. CVA | −0.725 | < 0.001 | −0.717 | < 0.001 | −0.679 | < 0.001 |
| Pain score vs. CMA | −0.676 | < 0.001 | −0.647 | < 0.001 | −0.678 | < 0.001 |
| CVA vs. CMA | 0.834 | < 0.001 | 0.836 | < 0.001 | 0.817 | < 0.001 |
CMA, cervicomedullary angle; CVA, craniovertebral angle.
In 245 subjects, there was an 83.4% correlation between CVA and CMA values (rs, 0.834; P < 0.001) according to gender.
Ninety five patients showed a restricted range of motion on physical examination, and all were in the severe pain (50 patients in group 3) and very severe pain (45 patients in group 4) groups.
Discussion
Our study showed that CVA and CMA narrowing affects the occurrence of CH. The average CMA and CVA values in CH patients were significantly narrower than those in controls, and there was an inverse relationship between the pain scores and CVA and CMA values (Figs. 2–4).
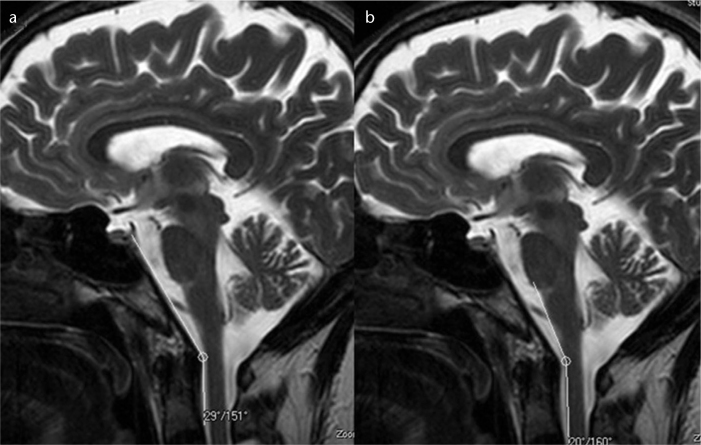
Figure 2. a, b.
T2-weighted mid-sagittal images show the normal craniovertebral angle (a) and normal cervicomedullary angle (b) in a normal participant with a pain score of 0.
Figure 4. a, b.
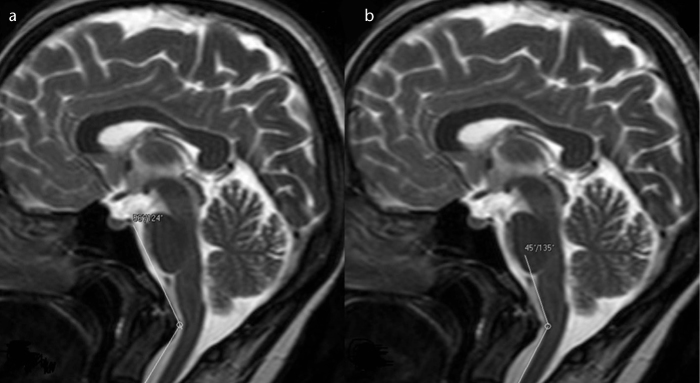
T2-weighted mid-sagittal images show the prominently narrowed craniovertebral (a) and cervicomedullary (b) angles in a patient with a pain score of 4.
The differential diagnosis of CH is difficult in practice. Cervical structures facilitate the pain; however, CH is not limited to neck pain. The pain in CH radiates from the back to frontal regions and is also a referred pain from the cervical structures innervated by the three upper cervical spinal nerves (3). Considering the difficulty in the clinical diagnosis, the IHS developed new diagnostic criteria for CH in 2004 (1). Tumors, fractures, infections, rheumatoid arthritis, posterior cranial fossa lesions, and vertebral artery dissection or aneurysms should be ruled out (7).
Our pain groups (205 patients) were selected according to the following diagnostic criteria: patients with hyperlipidemia, hypertension, rheumatoid arthritis, abnormal findings on brain MRI (such as MS, tumors, infarct, ischemic gliosis, vascular malformation, fracture, infection) and those diagnosed with primary headache such as migraine, tension-type or cluster-type headaches were excluded from the study.
CH shows a female predominance (8), which was also observed in our study. Trauma is a predisposing factor (8). In our study, 12 patients (5%) had a history of head trauma; no patient in the control group suffered neck trauma.
Craniovertebral junction (CVJ) alignment is altered when cervical spine degeneration occurs, and CMA and CVA values also change with advancing age. The pathogenesis of CH is unclear and starts earlier in life (at approximately 32–35 years of age) (9, 10); thus, most cases are not caused by spondylosis. In our study, the mean age of the pain group was 31 years.
The exact pathophysiology of CH is unknown, but dysfunction, weakness, and spasms of the muscles may lead to CH. Some authors have suggested that mechanical cervical spine pathologies and dysfunction in neck muscles may produce painful and limited neck motion (11, 12).
Trigger points in the posterior neck muscles (trapezius, sternocleidomastoid, and splenius capitis) and pericranial tenderness have been associated with CH (1). Many authors have indicated muscle imbalance, such as muscle tightness and weakness, and others have confirmed decreased strength and endurance of the deep neck flexors in CH patients (11, 13).
Schellhas et al. (14) mapped extraspinal pain with a radicular distribution as well as distant or referred pain related to different levels of the cervical spine. It was concluded that trigeminal pain is infrequently related to cervical disc pathology (15). Fukui et al. (16) showed that the C2/3 to C7/Th1 joints are related to a pain distribution pattern that does not involve trigeminal areas. C2/3 and C3 stimulation can produce occipital pain but no headache at the forehead (16). All these studies indicate that most neck lesions do not produce CH and cannot explain the CH source.
The most common cause of spinal dysfunction in the elderly is spondylotic myelopathy. In that group of patients, symptoms include weakness of the arms and legs, upper motor neuron dysfunction, and sensory symptoms; headache is usually not among the symptoms. The clinical observations show that spondylosis is not an important cause of headache (15). Knackstedt et al. (17) indicated no difference in disc degeneration or MRI signal changes in the transverse and alar ligaments among patients with CH, migraine, and whiplash-associated headaches. Therefore, CH might not be due to the neck lesions, as indicated in such studies (1, 5, 15), and it would not be appropriate to focus only on the structural changes.
The source of CH remains crucial for detecting its main pathology. Like most authors, we suggest that the spinal nucleus of the trigeminal nerve can be highly suspected as the pain source in CH (7, 17, 18). The pain originating from the cervical structures innervated by the upper cervical spinal nerves might be sensed in areas innervated by the trigeminal nerve branches. Convergence between the cervical afferents and nervus trigeminus may result in CH (7, 18, 19). In our study, we focused on this pathway and investigated whether narrowing of the CVA and CMA affects the trigeminal nerve branches and three upper cervical spinal nerves via stretching, thereby causing pain.
The CVJ refers to the occiput, atlas, axis, and supporting ligaments. It also includes the cervicomedullary junction (medulla, spinal cord, and lower cranial nerves). Signs and symptoms are variable in CVJ abnormalities, although most signs and symptoms may lead the patient to consult a neurologist or neurosurgeon. Presently, with the large number of patients undergoing MRI, it is easy to routinely evaluate the CVJ on sagittal MRI of the brain (20).
The CMA was first described by Bundschuh et al. (21) as the angle subtended by the lines drawn parallel to the ventral surfaces of the medulla and upper cervical cord on MRI. Bundschuh et al. (21) reported that there was a strong correlation between CMA values less than 135° and clinical evidence of cervical myelopathy, brainstem compression, or C2 root pain. In some current studies, the average CMA values were reported to be 155.2°, 163°, and 158.4°, respectively (21–23).
The CVA is formed with a line constructed along the posterior surface of the axis body and the odontoid process (Wackenheim clivus baseline or clivus-canal angle) (24). The CVA normally ranges from 150° in flexion to 180° in extension (25). Ventral spinal cord compression may occur when the angle is less than 150° (25).
The mean CMA and CVA values in our control group were 159.5°±4.2° and 153.3°±4.9°, respectively. However, in the pain groups, the mean CMA (152.9°±6.6°) and CVA (146.6°±7.6°) values were significantly narrower. An inverse and statistically significant relationship was demonstrated between pain scores and CVA and CMA.
Some authors have suggested that the range of motion decreases in CH patients (26, 27). Hall et al. (27) indicated that range of motion restriction can be related to headache intensity. This relationship may explain why all 95 patients were in the severe pain (50 patients in group 3) and very severe pain (45 patients in group 4) groups in our study.
One limiting factor in our study was the insufficient number of subjects in the control group. If the subject number in the control group was close to that in the patient group (165 patients), we could have assigned cutoff values for the CVA and CMA for the occurrence of pain. The CMA and CVA values might change following physiotherapy, and medication might not be needed. However, in our study, none of the patients received physiotherapy, and we also did not monitor the CMA and CVA values after the medication; thus, further studies are needed.
In conclusion, our study showed that narrowing of the CVA and CMA affects the occurrence of CH. There was an inverse relationship between the angle values and the pain scores (Figs. 2–4). Brain MRI is applied in patients presenting with headache to exclude organic causes. During the evaluation of the brain MRI in these patients, CVA and CMA measurements obtained from the sagittal images should be mentioned in the report.
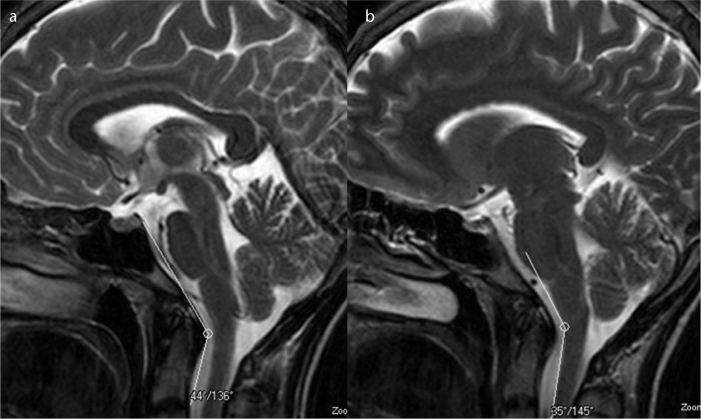
Figure 3. a, b.
T2-weighted mid-sagittal images show the narrowed craniovertebral (a) and cervicomedullary (b) angles in a patient with a pain score of 3.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Sjaastad O, Fredriksen TA, Pfaffenrath V. The Cervicogenic Headache International Study Group. Cervicogenic headache: diagnostic criteria. Headache. 1998;38:442–445. doi: 10.1046/j.1526-4610.1998.3806442.x. [DOI] [PubMed] [Google Scholar]
- 3.Sjaastad O, Fredriksen TA. Cervicogenic headache: criteria, classification and epidemiology. Clin Exp Rheumatol. 2000;18:3–6. [PubMed] [Google Scholar]
- 4.Merskey H, Bogduk N. Description of pain syndromes and definitions of pain terms. 2nd ed. Seattle: IASP Press; 1994. Classification of chronic pain. [Google Scholar]
- 5.Pollmann W, Keidel M, Pfaffenrath V. Headache and the cervical spine: a critical review. Cephalalgia. 1997;17:801–816. doi: 10.1046/j.1468-2982.1997.1708801.x. [DOI] [PubMed] [Google Scholar]
- 6.Kerr FW. Central relationships of trigeminal and cervical primary afferents in the spinal cord and medulla. Brain Res. 1972;43:561–572. doi: 10.1016/0006-8993(72)90408-8. [DOI] [PubMed] [Google Scholar]
- 7.Bogduk N. The neck and headaches. Neurol Clin. 2004;22:151–171. doi: 10.1016/S0733-8619(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 8.Vincent MB. Cervicogenic headache: clinical aspects. Clin Exp Rheumatol. 2000;18:7–10. [PubMed] [Google Scholar]
- 9.Vincent MB, Luna RA. Cervicogenic headache: a comparison with migraine and tension-type headache. Cephalalgia. 1999;19:11–16. doi: 10.1177/0333102499019s2503. [DOI] [PubMed] [Google Scholar]
- 10.Sjaastad O, Bakketeig LS. Migraine without aura: comparison with cervicogenic headache. Vaga study of headache epidemiology. Acta Neurol Scand. 2008;117:377–383. doi: 10.1111/j.1600-0404.2007.00966.x. [DOI] [PubMed] [Google Scholar]
- 11.Watson DH, Trott PH. Cervical headache: an investigation of natural head posture and upper cervical flexor muscle performance. Cephalalgia. 1993;13:272–284. doi: 10.1046/j.1468-2982.1993.1304272.x. [DOI] [PubMed] [Google Scholar]
- 12.Knackstedt H, Bansevicius D, Aaseth K, Grande RB, Lundqvist C, Russell MB. Cervicogenic headache in the general population: The Akershus study of chronic headache. Cephalalgia. 2010;30:1468–1476. doi: 10.1177/0333102410368442. [DOI] [PubMed] [Google Scholar]
- 13.Janda V. Muscles and motor control in cervicogenic disorders. In: Grant R, editor. Physical therapy of the cervical and thoracic spine. Edinburgh: Churchill Livingstone; 1994. pp. 195–215. [Google Scholar]
- 14.Schellhas KP, Smith MD, Gundry CR, Pollei SR. Cervical discogenic pain. Prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine. 1996;21:300–311. doi: 10.1097/00007632-199602010-00009. [DOI] [PubMed] [Google Scholar]
- 15.Vincent MB. Cervicogenic headache: the neck is a generator: con. Headache. 2010;50:706–709. doi: 10.1111/j.1526-4610.2010.01643.x. [DOI] [PubMed] [Google Scholar]
- 16.Fukui S, Ohseto K, Shiotani M, et al. Referred pain distribution of the cervical zygapophyseal joints and cervical dorsal rami. Pain. 1996;68:79–83. doi: 10.1016/S0304-3959(96)03173-9. [DOI] [PubMed] [Google Scholar]
- 17.Knackstedt H, Kråkenes J, Bansevicius D, Russell MB. Magnetic resonance imaging of craniovertebral structures: clinical significance in cervicogenic headaches. J Headache Pain. 2012;13:39–44. doi: 10.1007/s10194-011-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126:1801–1813. doi: 10.1093/brain/awg190. [DOI] [PubMed] [Google Scholar]
- 19.Kerr FW. Central relationships of trigeminal and cervical primary afferents in the spinal cord and medulla. Brain Res. 1972;43:561–572. doi: 10.1016/0006-8993(72)90408-8. [DOI] [PubMed] [Google Scholar]
- 20.Smoker WR. Craniovertebral junction: normal anatomy, craniometry, and congenital anomalies. Radiographics. 1994;14:255–277. doi: 10.1148/radiographics.14.2.8190952. [DOI] [PubMed] [Google Scholar]
- 21.Bundschuh C, Modic MT, Kearney F, Morris R, Deal C. Rheumatoid arthritis of the cervical spine: surface-coil MR imaging. Am J Roentgenol. 1988;151:181–187. doi: 10.2214/ajr.151.1.181. [DOI] [PubMed] [Google Scholar]
- 22.Abumi K, Takada T, Shono Y, Kaneda K, Fujiya M. Posterior occipitocervical reconstruction using cervical pedicle screws and plate-rod systems. Spine. 1999;24:1425–1434. doi: 10.1097/00007632-199907150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Wang C, Passias PG, Li G, Yan M, Zhou H. Interobserver and intraobserver reliability of the cervicomedullary angle in a normal adult population. Eur Spine J. 2009;18:1349–1354. doi: 10.1007/s00586-009-1112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wackenheim A. Roentgen diagnosis of the craniovertebral region. New York, NY: Springer Verlag; 1974. [Google Scholar]
- 25.VanGilder JC, Menezes AH, Dolan KD. The craniovertebral junction and its abnormalities. New York, NY: Futura; 1987. [Google Scholar]
- 26.Zito G, Jull G, Story I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Man Ther. 2006;11:118–129. doi: 10.1016/j.math.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Hall TM, Briffa K, Hopper D, Robinson KW. The relationship between cervicogenic headache and impairment determined by the flexion rotation test. J Manipulative Physiol Ther. 2010;33:666–671. doi: 10.1016/j.jmpt.2010.09.002. [DOI] [PubMed] [Google Scholar]