Abstract
PURPOSE
We aimed to evaluate the outcomes of coil embolization of true visceral artery aneurysms by three-dimensional contrast-enhanced magnetic resonance (MR) angiography.
MATERIALS AND METHODS
We used three-dimensional contrast-enhanced MR angiography, which included source images, to evaluate 23 patients (mean age, 60 years; range, 28–83 years) with true visceral artery aneurysms (splenic, n=15; hepatic, n=2; gastroduodenal, n=2; celiac, n=2; pancreaticoduodenal, n=1; gastroepiploic, n=1) who underwent coil embolization. Angiographic aneurysmal occlusion was revealed in all cases. Follow-up MR angiography was conducted with either a 1.5 or 3 Tesla system 3–25 months (mean, 18 months) after embolization. MR angiography was evaluated for aneurysmal occlusion, hemodynamic status, and complications.
RESULTS
Complete aneurysmal occlusion was determined in 22 patients (96%) on follow-up MR angiography (mean follow-up period, 18 months). Neck recanalization, which was observed at nine and 20 months after embolization, was confirmed in one of eight patients (13%) using a neck preservation technique. In this patient, a small neck recanalization covered by a coil mass was demonstrated. The complete hemodynamic status after embolization was determined in 21 patients (91%); the visualization of several collateral vessels, such as short gastric arteries, after parent artery occlusion was poor compared with that seen on digital subtraction angiography in the remaining two patients (9%). An asymptomatic localized splenic infarction was confirmed in one patient (4%).
CONCLUSION
Our study presents the follow-up results from three-dimensional contrast-enhanced MR angiography, which confirmed neck recanalization, the approximate hemodynamic status, and complications. This effective and less invasive method may be suitable for serial follow-up after coil embolization of true visceral aneurysms.
True visceral artery aneurysms are a rare and uncommon form of vascular disease often found incidentally in 0.09% to 2% of the general population (1–3). However, these true aneurysms have an incidence of rupture and mortality rate of 20% to 75% due to life-threatening hemorrhage (4, 5). Aneurysms can be saccular or fusiform. Endovascular treatment of true visceral artery aneurysms using coil embolization has been reported as an invasive and effective procedure to prevent rupture (6).
Various modalities have been used as follow-up evaluation methods after coil embolization of visceral artery aneurysms, including computed tomography (CT), magnetic resonance (MR) angiography, ultrasonography (US), and digital subtraction angiography (DSA) (6, 7). However, specific methods and the ideal follow-up times after coil embolization of visceral artery aneurysms are not well established. Coil embolization of visceral artery aneurysms occasionally result in neck recanalization, growth of a residual aneurysm neck or body remnant, organ infarction, and coil migration (7–9). In particular, one group reported that neck recanalization was effectively followed up by three-dimensional (3D) contrast-enhanced MR angiography (CEMR angiography) (8).
The aim of the present study was to evaluate the outcomes of coil embolization of true visceral artery aneurysms and to assess the role of 3D CEMR angiography as a follow-up method.
Materials and methods
Patients
CEMR angiography was performed after coil embolization of true visceral artery aneurysms. This retrospective study was approved by the review board of our institution, and written informed consent was obtained from each patient or a corresponding family member prior to coil embolization.
Twenty-three patients (male, 13; female, 10; mean age, 60 years; age range, 28–83 years) with true visceral aneurysms were treated between February 2006 and September 2012. Aneurysms in all patients were diagnosed with either CT or US. Indications for treating true aneurysms were determined based on a previous study (10) and included aneurysms measuring 1.5 to 2 cm in diameter, symptoms attributable to aneurysms, an increasing aneurysm size, and childbearing age. Aneurysms were localized within the splenic artery (n=15), hepatic artery (n=2), gastroduodenal artery (n=2), celiac artery (n=2), inferior pancreaticoduodenal artery (n=1), and right gastroepiploic artery (n=1) (Table). Aneurysms were saccular (n=21) or fusiform (n=2) (Table). Angiographic aneurysmal occlusion was revealed in all cases. An aneurysm with a mural thrombus was observed in only one patient (Patient no: 21) (Table).
Table.
Summary of patient profiles, aneurysm, embolization technique, and embolization follow-up outcome
| Patient number | Age (years), gender | Location | Form | Maximum size (mm) | Embolization technique | Follow-up period (months) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 28, F | SA | Saccular | 16 | CP (PP with NR) | 21 | Complete occlusion |
| 2 | 60, F | SA | Saccular | 17 | CP (PP with NR) | 23 | Complete occlusion |
| 3 | 67, M | SA | Saccular | 21 | Isolation | 21 | Complete occlusion |
| 4 | 44, M | SA | Saccular | 16 | CP (PP with NR) | 22 | Complete occlusion |
| 5 | 71, M | RGEA | Saccular | 13 | Isolation and CP | 25 | Complete occlusion |
| 6 | 47, M | CHA | Fusiform | 22 | Isolation and CP | 24 | Complete occlusion |
| 7 | 57, M | SA | Saccular | 38 | Isolation and CP | 22 | Complete occlusion |
| 8 | 32, F | SA | Saccular | 22 | Isolation and CP | 22 | Complete occlusiona |
| 9 | 49, M | SA | Saccular | 24 | Isolation and CP | 24 | Complete occlusion |
| 10 | 83, F | SA | Saccular | 20 | Isolation and CP | 21 | Complete occlusion |
| 11 | 69, M | IPDA | Saccular | 11 | CP | 22 | Complete occlusion |
| 12 | 80, F | SA | Saccular | 21 | CP (PP with NR) | 21 | Complete occlusion |
| 13 | 49, F | SA | Saccular | 11 | CP (PP) | 22 | Complete occlusion |
| 14 | 77, F | CHA | Saccular | 46 | Isolation | 21 | Complete occlusion |
| 15 | 47, F | SA | Saccular | 23 | CP (PP with NR) | 20 | Neck recanalizationb |
| 16 | 65, F | SA | Saccular | 20 | CP and isolation | 21 | Complete occlusion |
| 17 | 71, M | CA | Fusiform | 21 | CP | 22 | Complete occlusion |
| 18 | 65, M | GDA | Saccular | 22 | CP and isolation | 21 | Complete occlusion |
| 19 | 65, F | SA | Saccular | 20 | CP (PP with NR) | 9 | Complete occlusion |
| 20 | 52, M | SA | Saccular | 20 | CP and isolation | 9 | Complete occlusion |
| 21 | 70, M | GDA | Saccular | 30 | CP and isolation | 3 | Complete occlusion |
| 22 | 69, M | CA | Saccular | 28 | CP | 3 | Complete occlusion |
| 23 | 61, M | SA | Saccular | 23 | CP (PP) | 3 | Complete occlusion |
Splenic infarction at three months.
Neck recanalization at nine and 20 months.
CA, celiac artery; CHA, common hepatic artery; CP, coil packing; F, female; GDA, gastroduodenal artery; IPDA, inferior pancreaticoduodenal artery; M, male; MRA, magnetic resonance angiography; NR, neck remodeling technique; PP, preserving the parent artery circulation; RGEA, right gastroepiploic artery; SA, splenic artery.
Coil embolization technique
Preoperative 3D CT angiography was performed to evaluate the size and form (with or without mural thrombus) of aneurysms. Coil embolizations were performed using the common femoral artery approach. Following regional local anesthesia around the common femoral artery, a puncture was created using the Seldinger technique. Systemic heparinization was then performed after placement of the arterial introducer sheath according to our embolization protocol. Heparin (3000 IU) was administered as an intravenous bolus injection followed by an additional 1000 IU every hour. The location and form of the aneurysm was determined by DSA using a 4 F or 5 F diagnostic catheter with rotational angiographic acquisition, and selective arterial injection was performed using a flat panel angiography system (Innova 4100, GE Healthcare, Milwaukee, Wisconsin, USA). A 2 F microcatheter (Excelsior 1018, Boston Scientific, Natick, Massachusetts, USA) was inserted into the aneurysm sac or parent artery of the aneurysm using the coaxial technique. Coil embolization was performed by three qualified physicians, each with at least six years of experience performing coil embolizations with Guglielmi detachable coils (GDC, Boston Scientific), interlocking detachable coils (IDC, Boston Scientific), and/or another detachable coil (Cerecyte, Micrus Endovascular, San Jose, California, USA). Selective exclusion of the arterial lesion with microcoils deployed across the neck into the sac of the aneurysm (coil packing) to preserve the parent arterial circulation with or without the use of the neck preservation technique (neck remodeling technique) was achieved in eight patients (Fig. 1). In the remaining 15 patients, the parent arterial circulation (e.g., isolation technique at sites both distal and proximal to the aneurysm embolization) was occluded because the aneurysms had a broad neck and were fusiform, and no organ infarctions were observed by angiography (Figs. 2–4). Angiographic results of coil embolization were obtained in all patients who had complete aneurysmal occlusion and no complications (e.g., organ infarction).
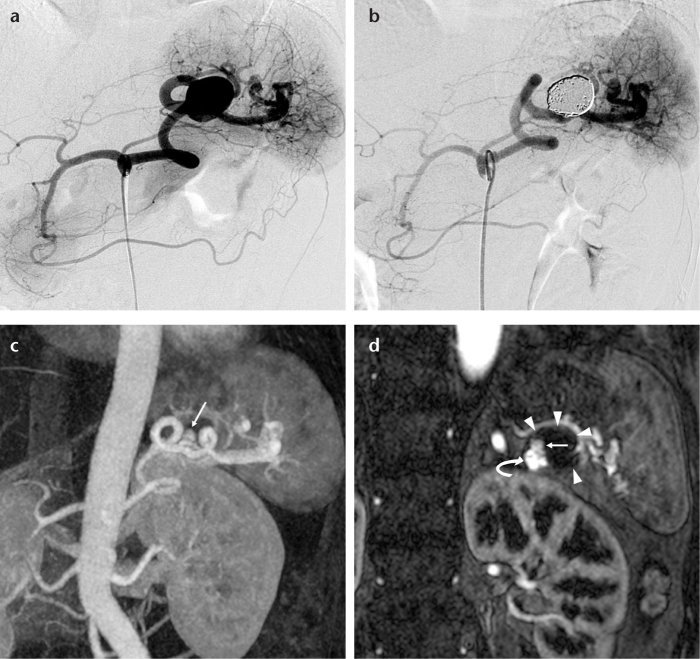
Figure 1. a–d.
A 47-year-old female with a true splenic artery aneurysm. A celiac arteriogram (a) shows a saccular splenic artery aneurysm. A celiac arteriogram after coil embolization using a neck preservation technique (b) shows occlusion of the aneurysm and patency of the splenic artery. Follow-up contrast-enhanced 1.5 T MR angiograms (maximum intensity projection and source image) performed at nine months after initial embolization (c, d) show clear enhancement of the enlarged parent artery (d, curved arrow) without an increase in aneurysm size (d, arrowheads). This suggested neck recanalization (c, d, arrows).
Figure 2. a–e.
A 69-year-old male with a true celiac artery aneurysm. A celiac arteriogram after coil embolization using the coil packing technique (a) shows complete occlusion of the aneurysm (curved arrow) and the common hepatic artery via collateral circulation by the right gastric artery from the left gastric artery (arrows). A superior mesenteric arteriogram after coil embolization (b) shows complete occlusion of the aneurysm (curved arrow). Note the arterial flow of the hepatic arteries via collateral circulation by the pancreaticoduodenal arteries (arrowheads) and the splenic artery via collateral circulation by the dorsal pancreatic artery (arrow). Unenhanced CT for detailed examination of acute cholangitis four months after coil embolization (c) shows strong metallic coil artifacts. Follow-up contrast-enhanced 3 T MRI (dynamic study; axial image) performed three months after initial embolization (d) shows complete occlusion of the aneurysm without metallic artifacts (arrowheads). Follow-up contrast-enhanced 3 T MR angiogram (maximum intensity projection; oblique view) performed three months after initial embolization (e) shows clear enhancement of the collateral circulation, such as the right gastric artery (white arrows), pancreaticoduodenal arteries (arrowheads), and dorsal pancreatic artery (black arrow).
Figure 4. a–d.
A 69-year-old male with a true inferior pancreaticoduodenal artery aneurysm. A superior mesenteric arteriogram (a) shows a saccular inferior pancreaticoduodenal artery aneurysm (arrow). A superior mesenteric arteriogram after coil embolization using the coil packing technique (b) shows occlusion of the aneurysm (arrow). Follow-up contrast-enhanced 3 T MRI (dynamic study; axial image) performed 22 months after initial embolization (c) shows complete occlusion of the aneurysm without metallic artifacts (arrowheads). A follow-up contrast-enhanced MR angiogram (maximum intensity projection) using 3 T MRI performed 22 months after initial embolization shows (d) complete occlusion of the aneurysm (black arrow) and clear enhancement of the pancreaticoduodenal and hepatic arteries (white arrows).
Follow-up MRI methods and techniques
We used 3D CEMR angiography to evaluate all patients after coil embolization. Follow-up imaging was conducted with either a 1.5 Tesla (T) magnetic resonance imaging (MRI) system (n=4) (Magnetom Symphony Siemens Medical Solutions, Erlangen, Germany) or a 3 T MRI system (n=19) (Signa HDxt, GE Healthcare). Follow-up examinations were performed using 3 T MRI after April 2007. The first follow-up CEMR angiography examination was conducted three months after embolization, the second was conducted six months after the first follow-up, and the third was conducted one year after the second follow-up. That is, CEMR angiography was conducted at approximately three, nine, and 21 months after the initial embolization. However, CEMR angiography was performed every six months if abnormal findings were detected during any of the follow-up tests.
1.5 T MRI: image acquisition system, technique, and parameters
The 3D CEMR angiography imaging was performed on a 1.5 T MRI scanner using generalized autocalibrating partially parallel acquisitions and a sensitivity encoding body array coil with a six-coil element. Imaging was performed using a coronal 3D volumetric interpolated breath-hold examination (TR, 4.6 ms; TE, 1.5 ms; flip angle, 25°; slice thickness, 2.5 mm; field of view (FOV), 320×350 mm; matrix, 160×384; SENSE factor, 2). Data were acquired with a voxel size of 2×0.9×2.5 mm, and images were reconstructed with a voxel size of 1×0.45×2.5 mm through in-plane zero-filling interpolation.
3 T MRI: Image acquisition system, technique, and parameters
The 3D CEMR angiography imaging was performed on a 3 T MRI scanner using an array spatial sensitivity encoding technique and a sensitivity encoding cardiac coil with an eight-coil element. The imaging was performed with axial 3D breath-hold liver acquisition with volume acceleration (TR, 3.3 ms; TE, 1.5 ms; TI, 5 ms; flip angle, 12°; slice thickness, 2 mm; FOV, 320×380 mm; matrix, 192×320; acceleration factor, 2). Data were acquired with a voxel size of 1.7×1.2×2 mm, and images were reconstructed with a voxel size of 0.625×0.74×2 mm by in-plane zero-filling interpolation.
The breath-hold time for acquisition was 25 s for both 1.5 and 3 T MRI. Gadodiamide contrast agent (Omniscan, Daiichi Sankyo, Tokyo, Japan) was injected into a dorsal hand vein at 0.1 mmol/kg body weight (2 mL/s) for a dynamic study with an automatic infusion system (Sonic Shot GX, Nemoto Kyorindo Co., Ltd., Tokyo, Japan). A saline flush (30 mL) was also performed.
CEMR angiography image analysis
CEMR angiography image analysis was performed on a 3D workstation (Advantage Workstation, version 4.6, GE Healthcare) using subtracted source images to obtain multiplanar reformatted, maximum-intensity projection. Two interventional radiologists (M.K., A.K.) with more than six and fifteen years of experience, retrospectively analyzed the anonymized image data in a three-stage procedure. This was conducted by a side-by-side comparison with coil embolization on DSA. First, source images and 3D maximum intensity projections were evaluated for aneurysm occlusion according to Roy’s classification (class 1, complete occlusion; class 2, residual/recurrent neck; and class 3, residual/recurrent aneurysm) (11). Second, the hemodynamic status, such as the collateral and parent arterial circulation status, was evaluated. Third, post-coil embolization-related complications were evaluated (e.g., organ ischemic changes). Major and minor complications were evaluated according to the Society of Interventional Radiology reporting standards (12).
Results
Twenty-three patients with true visceral artery aneurysms were evaluated by CEMR angiography (source images included) after coil embolization; no prominent metallic artifacts were found (Figs. 1–4). The interval between coil embolization and the follow-up CEMR angiography examination was 3–25 months (mean, 18 months) (Table).
A small neck recanalization (class 3) was observed in one of eight patients (13%) using a neck preservation technique by CEMR angiography. In one patient, complete aneurysm occlusion at three months after embolization was observed; however, neck recanalization was observed between nine and 20 months after embolization (Fig. 1). Re-embolization was not performed in this patient given the small neck recanalization at nine and 20 months post-treatment; follow-up was recommended for this particular patient. The remaining seven patients using a neck preservation technique and all patients treated by parent artery occlusion (n=15) showed complete occlusion (class 1) at follow-up.
The hemodynamic status following embolization was determined in 21 patients (91%) by CEMR angiography; there were no prominent metallic artifacts (Figs. 2–4). The visualization of several small collateral vessels (short gastric arteries, pancreatic arteries, and omental arteries) after parent artery occlusion was poor compared with DSA visualization in the remaining two patients (9%) (Fig. 3). Excellent visualization of the parent artery was achieved in all patients using a neck preservation technique during the follow-up period (Fig. 1).
Figure 3. a, b.

A 57-year-old male with a true splenic artery aneurysm. A celiac arteriogram after coil embolization using coil packing and the isolation technique (a) shows complete occlusion of the aneurysm (curved arrows). Note the arterial reperfusion of the spleen via collateral circulation by the short gastric arteries from the left gastric artery (a, arrowheads) and the gastroepiploic artery from the gastroduodenal artery (a, arrows). A follow-up contrast-enhanced MR angiogram (maximum intensity projection) using 3 T MRI performed 22 months after initial embolization (b) shows complete occlusion of the aneurysm and arterial reperfusion of the spleen via collateral circulation by the gastroepiploic artery (arrows). Other collateral vessels, such as short gastric arteries, are not shown.
We also confirmed an asymptomatic localized splenic infarction at three months after embolization in one patient (4%); however, this was diagnosed as class A and thus required no therapy. No other additional complications were observed in any of the patients.
Discussion
Follow-up protocols after embolization are not yet well established and are inconsistent among previous reports. Methods for follow-up imaging after endovascular treatment of visceral and renal artery aneurysms have been reported in 14 publications (6, 7, 13–24). In regards to the follow-up imaging modality, CT examination was reported in 13 publications and was thus the most widely used (7, 13–24). In fact, CT was the only follow-up examination method used in nine of these publications (13, 14, 16, 19–24). Follow-up outcomes of completely thrombosed aneurysms were reported in 11 publications (6, 13–16, 18–20, 22–24), 10 of which were evaluated by CT (13–16, 18–20, 22–24). At present, there is no CT method that is optimal for follow-up evaluation after endovascular treatment, especially treatments using metallic embolic agents such as microcoils. CT cannot identify complete aneurysm occlusion due to metallic artifacts (15, 19). Strong artifacts were observed in the patients of this study, making it difficult to evaluate the aneurysm after coil embolization (Fig. 2c). In other words, neck recanalization/residual flow of the coiled aneurysm may be overlooked.
DSA is often used as an evaluation method after endovascular treatment (7, 15, 19). However, DSA is an invasive procedure, and follow-up with this method is not optimal. The assessment of aneurysms after coil embolization is further limited by the superimposition of arteries. Neck recanalization/coil compaction of aneurysms after embolization may be partially or totally masked by the radio-opaque coil mass and thereby missed by DSA (25). For these reasons, DSA was not performed in the follow-up in this study.
Contrast-enhanced US (CEUS) is useful for evaluation after endovascular treatment (26). CEUS is a low-cost, noninvasive, and safe technique that can evaluate the state of aneurysms after coil embolization using a small amount of contrast agent. However, this imaging technique has some disadvantages. For example, the diagnostic accuracy of CEUS strongly depends on operator experience, body habitus (e.g., obesity), arterial wall calcification, the presence of a coiled aneurysm at a position anterior to the parent artery, and air in the digestive tract.
One study reported the use of MRI for follow-up examination, but did not disclose the details of the image acquisition techniques or evaluation methods used (6). The present study introduced a 3D CEMR angiography protocol in which pulse sequence parameters were optimized for acquisition time and imaging FOV, and a contrast-enhanced dynamic study was added for evaluation of aneurysms after coil embolization. The imaging FOV of MR angiography is sufficient to assess coiled aneurysms, hemodynamic status, and organ infarction. Detachable microcoils are composed of platinum and are non-ferromagnetic. Therefore, platinum microcoils can generate a small artifact in MRI. However, there were no perceptible artifacts in MRI in this study.
Embolization preserves the parent artery flow with aneurysmal occlusion and thus avoids organ infarction. One disadvantage of this technique is the potential for neck recanalization/ coil compaction (8, 27). Neck recanalization was observed within the aneurysm neck and coil mesh. The change was considered to be a neck recanalization accompanying coil compaction given the lack of change in aneurysm size at nine months after the initial embolization. An aneurysm with a mural thrombus is regarded as a risk factor for neck recanalization (28), but a mural thrombus was not observed in this patient. Embolization was performed using a neck preservation technique with a balloon catheter. Therefore, the coiled aneurysm was continuously affected by arterial blood flow. In the present study, we identified neck recanalization/coil compaction in 13% of patients using this technique. Previous studies have reported neck recanalization/coil compaction rates ranging from 2% to 12.5% (7, 15, 17, 19). These studies involved CT evaluation and reported possibly inaccurate incidence rates for neck recanalization due to metallic coil artifact. Our results showed a slightly higher neck recanalization rate, which can be further improved using CEMR angiography.
Parent artery embolization (i.e., isolation technique) always results in collateral circulation, which is easily detected by DSA, and the hemodynamic status in associated organs can be readily monitored. Observation of collateral circulation is crucial because increased blood flow after embolization results in higher arterial wall stress and may lead to the formation of secondary aneurysms (29). By carefully observing the collateral circulation after coil embolization, we found a tendency of the collateral circulation path to expand, but with no formation of secondary aneurysms. Follow-up examinations require the evaluation of collateral circulation as well as aneurysm occlusion. In some patients, the collateral circulation was poorly imaged. However, a clear diagnosis was difficult to obtain because the hemodynamics were altered, possibly due to narrow paths and low blood flow, thereby resulting in the loss of collateral flow.
Complications associated with endovascular treatment of visceral artery aneurysms arise from the infarction of an end-organ due to a distal thromboembolic event (e.g., nontarget vessel embolization). Such complications occur in approximately 21%–24% of endovascular treatment cases (7, 17). Therefore, image assessment is also considered to be an important aspect of follow-up procedures after endovascular treatment.
We speculated that the minimal follow-up period might be longer than nine months, and recommendation of a two-year follow-up period might be ideal if the coil occlusion is stable. If neck recanalization and other controversial clinical complications are observed, follow-up by CEMR angiography is necessary. In addition, the contrast agent used has several disadvantages, including a risk of renal dysfunction and a potential for allergic reactions. Moreover, gadolinium is associated with higher costs. However, 3D CEMR angiography is an accurate and excellent noninvasive technique for the detection of neck recanalization, assessment of hemodynamic status, and identification of complications after coil embolization. In recent years, the utility of unenhanced MR angiography has been reported even in the abdominal region (30, 31). It is anticipated that this method can be utilized in the future for follow-up after coil embolization of visceral artery aneurysms.
Our study had some limitations worth noting. First, its retrospective nature might have resulted in a selection bias. Furthermore, the follow-up period was relatively short. Second, the MRI and imaging parameters used varied among patients. However, we ensured that patients with 3D CEMR angiography were included in this study. Third, it is difficult to measure the technical outcomes of endovascular treatment, which include coil packing attenuation and the percentage of occlusion. CEMR angiography does not expose the patient to radiation and can thus be used safely for repeated evaluations after embolization.
In conclusion, in the present study we demonstrated that 3D CEMR angiography achieves moderate to high diagnostic performance for the detection of neck recanalization, approximate hemodynamic status, and organ infarction during follow-up evaluation in patients treated by coil embolization. Our findings collectively suggest that follow-up evaluations of true visceral artery aneurysms can be carried out by 3D CEMR angiography after coil embolization.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Carmeci C, McClenathan J. Visceral artery aneurysms as seen in a community hospital. Am J Surg. 2000;179:486–489. doi: 10.1016/s0002-9610(00)00380-9. [DOI] [PubMed] [Google Scholar]
- 2.Kalko Y, Ugurlucan M, Basaran M, et al. Visceral artery aneurysms. Heart Surg Forum. 2007;10:E24–29. doi: 10.1532/HSF98.20061130. [DOI] [PubMed] [Google Scholar]
- 3.Henke PK, Cardneau JD, Welling TH, 3rd, et al. Renal artery aneurysms: a 35-year clinical experience with 252 aneurysms in 168 patients. Ann Surg. 2001;234:454–462. doi: 10.1097/00000658-200110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanley CJ, Shah NL, Messina LM. Common splanchnic artery aneurysms: splenic, hepatic, and celiac. Ann Vasc Surg. 1996;10:315–322. doi: 10.1007/BF02001900. [DOI] [PubMed] [Google Scholar]
- 5.Wagner WH, Allins AD, Treiman RL, et al. Ruptured visceral artery aneurysms. Ann Vasc Surg. 1997;11:342–347. doi: 10.1007/s100169900058. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda O, Tamura Y, Nakasone Y, Iryou Y, Yamashita Y. Nonoperative management of unruptured visceral artery aneurysms: treatment by transcatheter coil embolization. J Vasc Surg. 2008;47:1212–1219. doi: 10.1016/j.jvs.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Etezadi V, Gandhi RT, Benenati JF, et al. Endovascular treatment of visceral and renal artery aneurysms. J Vasc Interv Radiol. 2011;22:1246–1253. doi: 10.1016/j.jvir.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Koganemaru M, Abe T, Uchiyama D, et al. Detection of neck recanalization with follow-up contrast-enhanced MR angiography after renal artery aneurysm coil embolization. J Vasc Interv Radiol. 2010;21:298–300. doi: 10.1016/j.jvir.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Shah NA, Akingboye A, Haldipur N, Mackinlay JY, Jacob G. Embolization coils migrating and being passed per rectum after embolization of a splenic artery pseudoaneurysm, “the migrating coil”: a case report. Cardiovasc Intervent Radiol. 2007;30:1259–1262. doi: 10.1007/s00270-007-9166-7. [DOI] [PubMed] [Google Scholar]
- 10.Ferrero E, Ferri M, Viazzo A, et al. Visceral artery aneurysms, an experience on 32 cases in a single center: treatment from surgery to multilayer stent. Ann Vasc Surg. 2011;25:923–935. doi: 10.1016/j.avsg.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 12.Drooz AT, Lewis CA, Allen TE, et al. Quality improvement guidelines for percutaneous transcatheter embolization. J Vasc Interv Radiol. 2003;14:S237–242. [PubMed] [Google Scholar]
- 13.Kasirajan K, Greenberg RK, Clair D, Ouriel K. Endovascular management of visceral artery aneurysm. J Endovasc Ther. 2001;8:150–155. doi: 10.1177/152660280100800209. [DOI] [PubMed] [Google Scholar]
- 14.Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol. 2003;26:256–260. doi: 10.1007/s00270-003-1948-y. [DOI] [PubMed] [Google Scholar]
- 15.Tulsyan N, Kashyap VS, Greenberg RK, et al. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276–283. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Bageacu S, Cuilleron M, Kaczmarek D, Porcheron J. True aneurysms of the pancreaticoduodenal artery: successful non-operative management. Surgery. 2006;139:608–616. doi: 10.1016/j.surg.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Laganà D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol. 2006;59:104–111. doi: 10.1016/j.ejrad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda O, Tamura Y, Nakasone Y, Kawanaka K, Yamashita Y. Coil embolization of pancreaticoduodenal artery aneurysms associated with celiac artery stenosis: report of three cases. Cardiovasc Intervent Radiol. 2007;30:504–507. doi: 10.1007/s00270-006-0083-y. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto S, Hirota S, Maeda H, et al. Transcatheter coil embolization of splenic artery aneurysm. Cardiovasc Intervent Radiol. 2008;31:527–534. doi: 10.1007/s00270-007-9237-9. [DOI] [PubMed] [Google Scholar]
- 20.Dave B, Sharma A, Kwolek C, Demoya M, Wicky S, Kalva S. Percutaneous transcatheter arterial embolization of inferior pancreatico-duodenal artery aneurysms associated with celiac artery stenosis or occlusion. Catheter Cardiovasc Interv. 2010;75:663–672. doi: 10.1002/ccd.22395. [DOI] [PubMed] [Google Scholar]
- 21.Seo JM, Park KB, Kim KH, et al. Clinical and multidetector CT follow-up results of renal artery aneurysms treated by detachable coil embolization using 3D rotational angiography. Acta Radiol. 2011;52:854–859. doi: 10.1258/ar.2011.110063. [DOI] [PubMed] [Google Scholar]
- 22.Paci E, Mincarelli C, Fichetti M, Alborino S, Antico E, Candelari R. Stent-assisted coil embolization of renal artery bifurcation aneurysm using the kissing stent technique. J Vasc Interv Radiol. 2011;22:1485–1487. doi: 10.1016/j.jvir.2011.01.456. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi M, Nakata M, Kawai A, Suzuki K, Morita T, Sugimoto H. Ruptured renal artery aneurysm: coil packing with GDCs. Jpn J Radiol. 2012;30:442–445. doi: 10.1007/s11604-012-0057-8. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Kerim A, Cassagnes L, Alfidja A, et al. Endovascular treatment of eight renal artery aneurysms. Acta Radiol. 2012;53:430–434. doi: 10.1258/ar.2012.110458. [DOI] [PubMed] [Google Scholar]
- 25.Farb RI, Nag S, Scott JN, et al. Surveillance of intracranial aneurysms treated with detachable coils: a comparison of MRA techniques. Neuroradiology. 2005;47:507–515. doi: 10.1007/s00234-005-1375-7. [DOI] [PubMed] [Google Scholar]
- 26.Piscaglia F, Gualandi S, Galassi M, Giampalma E, Golfieri R, Bolondi L. Contrast enhanced ultrasonography for the evaluation of coil embolization of splenic artery aneurysm. Circulation. 2010;122:e451–454. doi: 10.1161/CIRCULATIONAHA.110.955518. [DOI] [PubMed] [Google Scholar]
- 27.Uchiyama D, Koganemaru M, Abe T, Hirose Y, Hayabuchi N, Akashi H. Coil embolization of splenic artery aneurysm with preservation of the parent artery using a neck remodeling technique. J Vasc Interv Radiol. 2007;18:447–450. doi: 10.1016/j.jvir.2006.12.727. [DOI] [PubMed] [Google Scholar]
- 28.Cho YD, Park JC, Kwon BJ, Hee Han M. Endovascular treatment of largely thrombosed saccular aneurysms: follow-up results in ten patients. Neuroradiology. 2010;52:751–758. doi: 10.1007/s00234-009-0622-8. [DOI] [PubMed] [Google Scholar]
- 29.Weber CH, Pfeifer KJ, Tato F, Reiser M, Rieger J. Transcatheter coil embolization of an aneurysm of the pancreatico-duodenal artery with occluded celiac trunk. Cardiovasc Intervent Radiol. 2005;28:259–261. doi: 10.1007/s00270-004-0116-3. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki M, Isoda H. Non-contrast-enhanced MR angiography of the abdomen. Eur J Radiol. 2011;80:9–23. doi: 10.1016/j.ejrad.2011.01.093. [DOI] [PubMed] [Google Scholar]
- 31.Maruno M, Kiyosue H, Tanoue S, Hongo N, Kashiwagi J, Mori H. Unenhanced magnetic resonance angiography with time-spatial labeling inversion pulse for evaluating visceral artery aneurysms after endosaccular packing with detachable coils: preliminary results. J Vasc Interv Radiol. 2013;24:289–293. doi: 10.1016/j.jvir.2012.11.001. [DOI] [PubMed] [Google Scholar]