Abstract
The Amplatzer® Vascular Plug (AVP) can be used to embolize medium-to-large high-flow vessels in various locations. Between 2009 and 2012, 41 AVPs (device size, 6–22 mm in diameter) were used to achieve occlusion in 31 patients (24 males, seven females) aged 9–92 years (mean age, 54.5 years). The locations and indications for embolotherapy were as follows: internal iliac artery embolization before stent-graft repair for aorto-iliac (n=6) and common iliac artery (n=3) aneurysms, subclavian artery embolization before stent-graft repair for thoracic aorta (n=3) and arcus aorta (n=1) aneurysms, brachiocephalic trunk embolization before stent-graft repair for a thoracic aorta aneurysm (n=1), embolization of aneurysms and pseudoaneurysms (n=5), embolization for carotid blow-out syndrome (n=3), closure of arteriovenous fistula (n=8), and closure of a portosystemic fistula (n=1). Of the 41 AVPs, 30 were AVP 2 and 11 were AVP 4. The mean follow-up duration was 4.7 months (range, 1–24 months). During follow-up, there was one migration, one insufficient embolization, and one recanalization. The remaining vascular lesions were successfully excluded from the circulation. The AVP, which can be used in a wide spectrum of pathologies, is easy to use and causes few complications. This essay presents our experience with the AVP.
The Amplatzer® Vascular Plug (AVP, AGA Medical Corp., Golden Valley, Minnesota, USA) is a good alternative to other embolic materials (1). The AVP has many advantages over embolic materials such as coils or glue. It is retractable, and it can be repositioned. Use of the AVP has cost advantages over other embolic materials. It can be used safely in trauma patients or in patients before endovascular aortic repair (EVAR). It ensures permanent occlusion, and its migration risk is less than for coils. The results of embolization with the AVP have been excellent (2), and no contraindications for its use have been reported (3). All four types of AVP have two components: a vascular plug and a delivery wire. The plugs have radiopaque platinum marker bands at both ends (4). The AVP 2 contains a more densely woven nitinol mesh and minimizes migration and recanalization (5). The AVP 4 is used mostly in small, tortuous vessels (6). The AVP is made of self-expanding material, and it returns to its original shape after release from the catheter. It has a long delivery cable, is preloaded in a loader, and is delivered through guiding catheters ranging from 5 to 8 F in size. The AVP is released by rotating the delivery cable counterclockwise.
Thirty-one patients (24 males, seven females) aged 9–92 years (mean age, 54.5 years), who underwent percutaneous occlusion with the AVP at our hospital between 2009 and 2012, were evaluated retrospectively. Patients with any pathology involving AVP use for embolization were included in this study. Patients in whom only other embolic materials were used were excluded. The study was approved by the Institutional Review Board and consent was obtained from the patients before the procedures. Most patients underwent general anesthesia. A transfemoral arterial or venous approach was used in most patients. In the patient with a portosystemic fistula, a transjugular approach was used. The procedures were performed under heparinization only in patients with a pulmonary arteriovenous fistula (AVF) or thoracic aorta aneurysm.
In 24 of the 31 patients, we used only the AVP as the embolic material. In the remaining seven patients, we used the AVP with coils. In six of the 31 patients, more than one AVP was used. In total, 41 AVPs (30 AVP 2, 11 AVP 4), 6–22 mm in diameter, were used. The diameter of the AVP was selected to be 30%–50% larger than that of the targeted location in each patient. Computed tomography (CT) angiography was used most commonly for follow-up imaging (mean follow-up duration, 4.7 months; range, 1–24 months). This pictorial essay presents our experience using the AVP in various locations as material to embolize medium-to-large vessels with high flow.
Internal iliac artery embolization to prevent endoleakage
Left internal iliac artery embolization was performed before EVAR in five males and one female with aorto-bi-iliac aneurysms: a 71-year-old male with a 16 mm AVP 2, a 68-year-old male with a 12 mm AVP 2 and coils, a 66-year-old male with a 12 mm AVP 2, a 71-year-old male with a 10 mm AVP 2 and coils, a 74-year-old male with an 18-mm AVP 2, and a 43-year-old female with a 10 mm AVP 2. Unilateral internal iliac artery embolization with a 12 mm AVP 2 was performed in a 56-year-old female and a 73-year-old male with unilateral common iliac artery aneurysms. Bilateral internal iliac artery embolization with two 12 mm AVP 2 was performed in a 59-year-old male with bilateral common iliac artery aneurysms.
Subclavian artery and brachiocephalic trunk embolization to prevent endoleakage
The left subclavian artery was embolized before thoracic EVAR in three patients with a thoracic aorta aneurysm (a 62-year-old male with a 14 mm AVP 2, a 78-year-old female with a 12 mm AVP 2, and a 67-year-old male with a 10 mm AVP 2) and in a 73-year-old male with an arcus aorta aneurysm (with a 14 mm AVP 2). In a 63-year-old male with a thoracic aorta aneurysm, the brachiocephalic trunk was embolized with a 12 mm AVP 2, and because of insufficient embolization, re-embolized with a 16 mm AVP 2 after one month.
Aneurysm or pseudoaneurysm embolization
In a 56-year-old male with an aberrant right subclavian artery aneurysm and type 3 aortic dissection, the aneurysm was occluded with an 18 mm AVP 2. The right cavernous internal carotid artery aneurysm of an 18-year-old female was treated with a 7 mm AVP 4, an 8 mm AVP 4, and coils. In a 63-year-old male, the proximal and distal parts of the common hepatic artery aneurysm were occluded with a 10 and 2 mm AVP 2, respectively (Fig. 1). In a 46-year-old male with a left deep femoral artery pseudoaneurysm and traumatic AVF, the AVF was embolized with coils, and the pseudoaneurysm was embolized with an 8 mm AVP 4. In a 32-year-old male with a traumatic thyrocervical trunk pseudoaneurysm, embolization with a 6 mm AVP 4 was performed.
Figure 1. a, b.
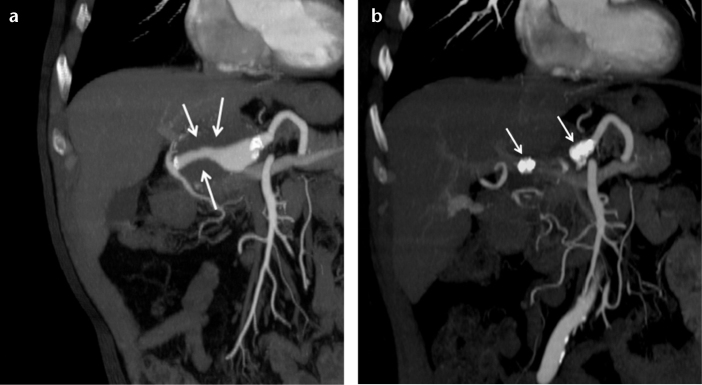
Coronal CT angiography reconstruction images of a 63-year-old male show a partially thrombosed common hepatic artery aneurysm (a, arrows) and two AVPs placed in the proximal and distal parts of the aneurysm (b, arrows) one month after the occlusion.
Embolization for carotid blow-out syndrome
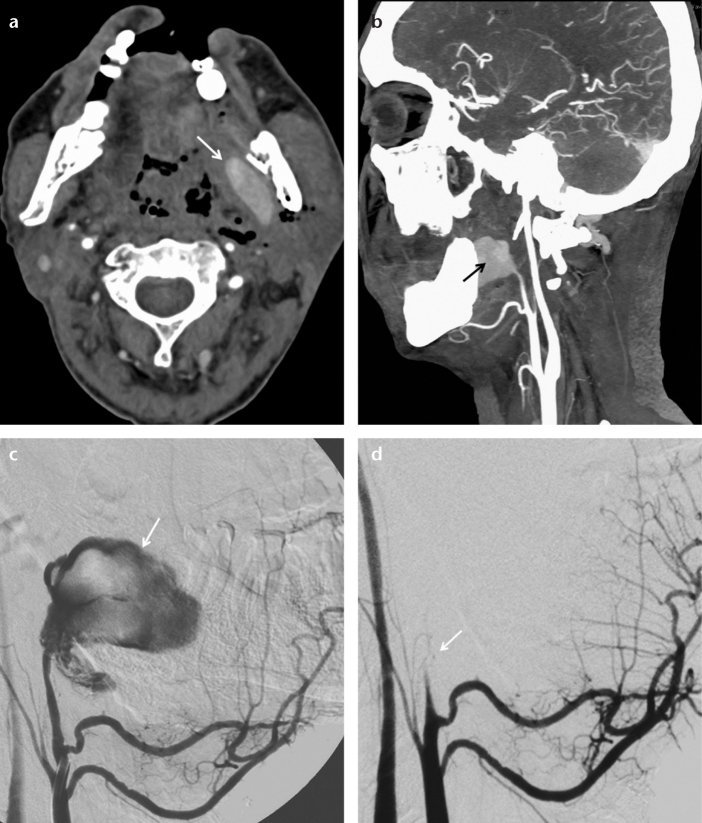
Two patients with inoperable larynx cancer underwent common carotid artery embolization for carotid blow-out syndrome: a 68-year-old male with a 12 mm AVP 2 and coils and a 58-year-old male with a 14 mm AVP 2 and coils. A 47-year-old male with hypopharynx cancer and a left internal maxillary artery pseudoaneurysm underwent internal maxillary artery embolization for carotid blow-out syndrome with an 8 mm AVP 2 (Fig. 2).
Figure 2. a–d.
Axial CT angiography image (a) of a 47-year-old male shows a pseudoaneurysm (arrow) in the left parapharyngeal space. A sagittal CT angiography reconstruction image (b) shows a left internal maxillary artery pseudoaneurysm (arrow). Selective left external carotid angiography (c) shows active contrast extravasation from the pseudoaneurysm (arrow). Selective left external carotid angiography after the deployment of an 8 mm AVP 2 (d) shows total embolization of the left internal maxillary artery (arrow).
Arteriovenous and portosystemic fistulas embolization
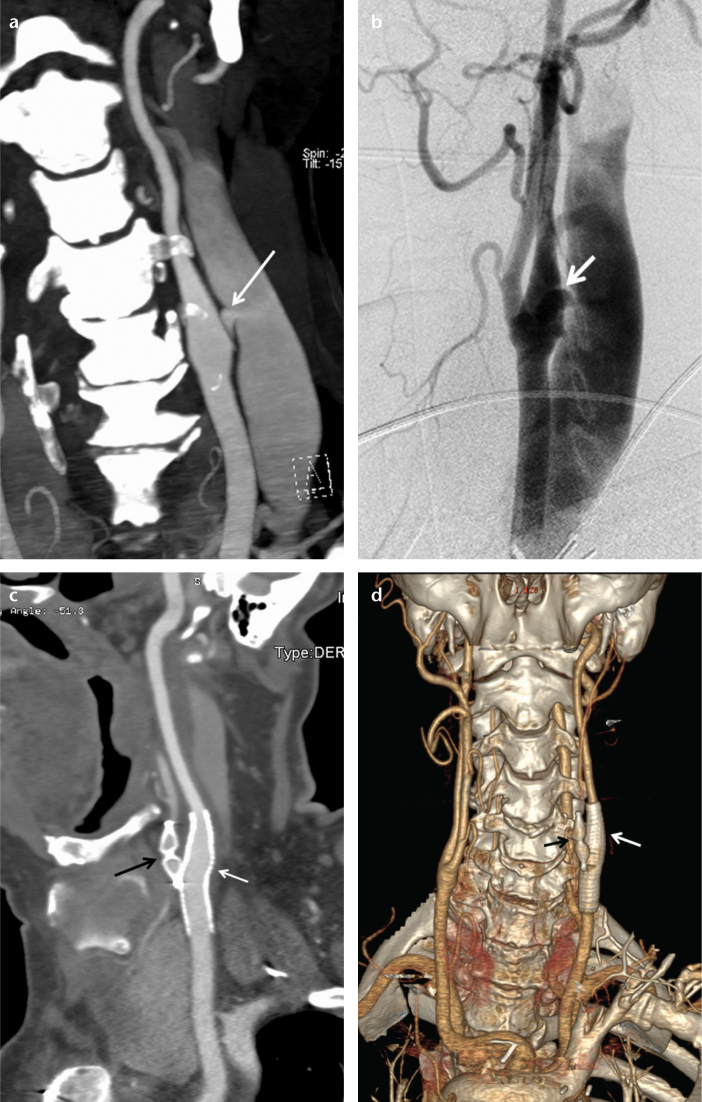
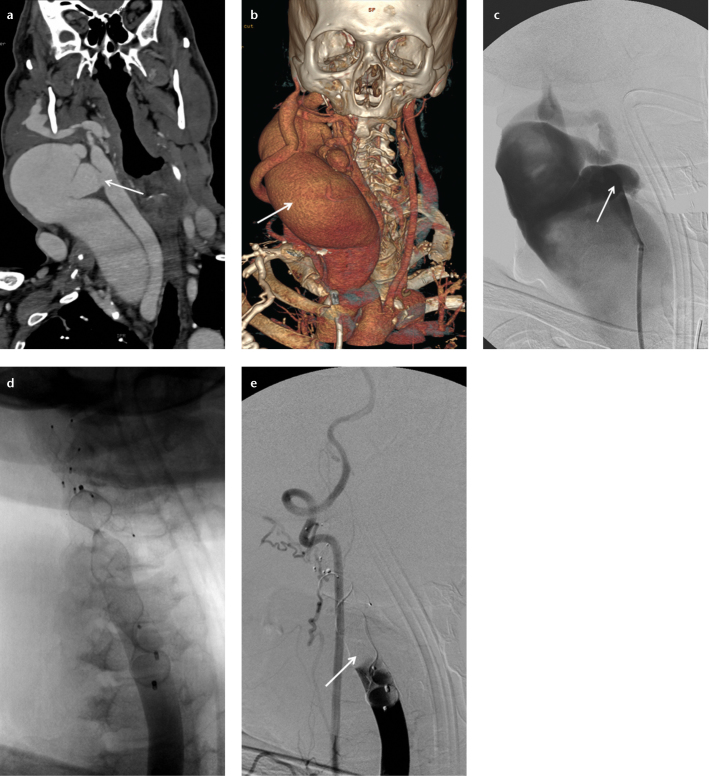
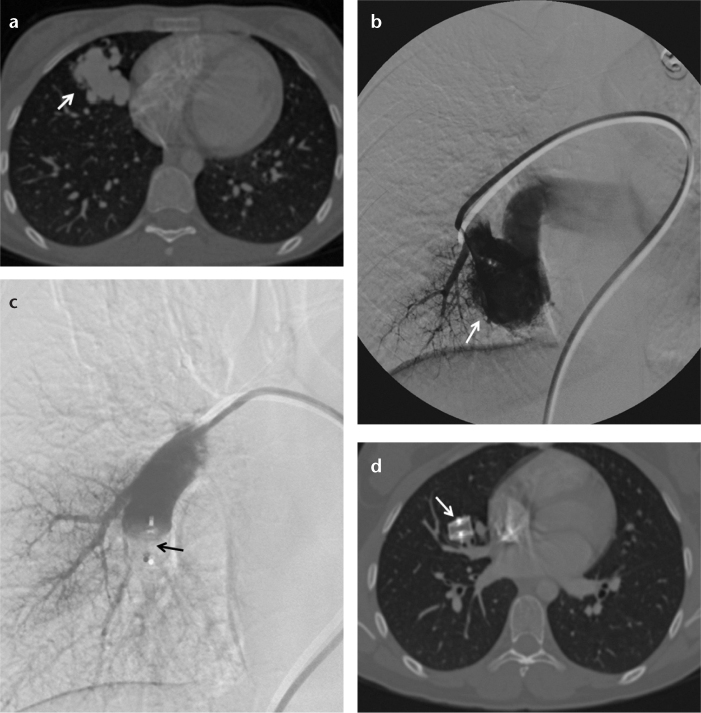
In a 62-year-old male with a left traumatic high-flow caroticojugular fistula, the left external carotid artery was embolized with an 8 mm AVP 4 to prevent retrograde filling from the external carotid artery, and subsequently the fistula between the internal carotid artery and internal jugular vein was occluded with a stent-graft (Fig. 3). In a 57-year-old male with a right traumatic high-flow caroticojugular fistula between the common carotid artery and internal jugular vein, the right internal carotid artery and external carotid artery were embolized with three AVPs to prevent retrograde filling, and then three AVPs were placed in the right facial artery and common carotid artery to occlude the fistula (with a 12 mm and a 20 mm AVP 2 and two 6, a 7, and an 8 mm AVP 4) (Fig. 4). In a 17-year-old female with a pulmonary AVF secondary to Osler-Weber-Rendu syndrome, the right-middle lobe segment pulmonary artery was embolized with a 16 mm AVP 2 (Fig. 5). The renal AVF of an 11-year-old male, who underwent a renal biopsy one year earlier following renal transplantation, was occluded with an 8 mm AVP 4 (Fig. 6). A 14 mm and a 22 mm AVP 2 were used to occlude a dysfunctional brachiocephalic hemodialysis fistula in a 92-year-old female. A 14 mm AVP 2, a 6 mm AVP 4, and coils were used to occlude a left traumatic internal iliac AVF in a 32-year-old male. A 22 mm (the largest diameter AVP) AVP 2 was placed between the left portal vein and left hepatic vein for the congenital high-flow portosystemic fistula of a nine-year-old male.
Figure 3. a–d.
Coronal CT angiography reconstruction image (a) of a 62-year-old male shows a caroticojugular fistula (arrow) between the internal carotid artery and internal jugular vein. Carotid angiography (b) shows the caroticojugular fistula (arrow). One month following the embolization, a sagittal CT angiography reconstruction image (c) shows the occluded left external carotid artery with an 8 mm AVP 4 (black arrow) and the occluded fistula with a stent-graft (white arrow). One month following the embolization, a coronal CT angiography reconstruction image (d) shows the occluded left external carotid artery with the AVP 4 (black arrow) and the occluded fistula with the stent-graft (white arrow).
Figure 4. a–e.
Coronal CT angiography reconstruction image (a) of a 57-year-old male shows a caroticojugular fistula (arrow) between the common carotid artery and internal jugular vein. A coronal CT angiography reconstruction image (b) shows the dilated internal jugular vein (arrow) secondary to the caroticojugular fistula. Carotid angiography (c) shows the caroticojugular fistula (arrow). Carotid angiography after deployment of the AVPs (d) shows multiple AVPs. Carotid angiography after deployment of the AVPs (e) shows total embolization of the common carotid artery (arrow).
Figure 5. a–d.
Axial CT angiography (a) and pulmonary angiography (b) images of a 17-year-old female show a pulmonary arteriovenous fistula (arrow) at the right middle lobe. Pulmonary angiography after deployment of a 16 mm AVP 2 (c) shows total occlusion of the feeding artery and the AVP 2 (arrow) in the right middle lobe segment pulmonary artery. Six months later, an axial CT angiography image (d) shows the AVP 2 (arrow) in the right middle lobe segmental pulmonary artery.
Figure 6. a, b.
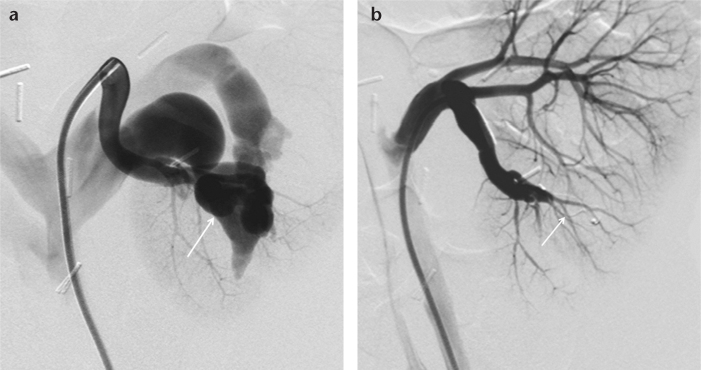
Renal angiography image (a) of an 11-year-old male shows a renal arteriovenous fistula (arrow) at the mid to lower third of the kidney. Renal angiography after deploying an 8 mm AVP 4 (b) shows total occlusion (arrow) of the fistula.
Two patients with fistulas had complications. In a 70-year-old male with a ruptured abdominal aortic aneurysm and aortocaval fistula, a 20 mm AVP 2 was used to occlude the fistula. Twelve hours later, the AVP 2 migrated to the right-lower lobe segment pulmonary artery without clinical consequences. Three months following the migration, the fistula was re-embolized with coils, and the migrated AVP 2 was left in the pulmonary artery. In a 28-year-old female with a pulmonary high-flow AVF secondary to Osler-Weber-Rendu syndrome, the left-lower lobe segment pulmonary artery was occluded with a 14 mm AVP 2; because of recanalization in the pulmonary AVF, embolization with a 12 mm AVP 2 and coils was performed 16 months after the first embolization.
Conclusion
In the embolization of arteries, which are high-flow vessels, migration of the AVP is very rare (7). Internal iliac artery embolization is one of the most commonly reported applications of the AVP in arterial embolization, because if a common iliac artery aneurysm extends to the common iliac artery bifurcation in a patient with or without abdominal aortic aneurysm, simply extending the endograft into the external iliac artery beyond the internal iliac artery orifice could result in type 2 endoleakage (8). The AVP has been used in neurointervention (9–11), venous pathologies (12, 13), and abnormal vascular (14) and nonvascular connections.
Use of the AVP has some limitations. First, a 5–8 F guiding catheter is required. In addition, the AVP is unsuitable for vessels that are too small or too large. Nineteen patients had no complications, and three patients had complications. The remaining nine patients were followed only clinically.
In conclusion, we ensured controlled, permanent occlusions in our cases with the use of functional and effective AVP, which costs less than other embolic materials (8). With improvements in its design, the clinical indications for the AVP will increase.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ong CK, Lam DV, Ong MT, Power MA, Parkinson RJ, Wenderoth JD. Neuroapplication of Amplatzer Vascular Plug for therapeutic sacrifice of major craniocerebral arteries: an initial clinical experience. Ann Acad Med Singap. 2009;38:763–768. [PubMed] [Google Scholar]
- 2.Hill SL, Hijazi ZM, Hellenbrand WE, Cheatham JP. Evaluation of the Amplatzer Vascular Plug for embolization of peripheral vascular malformations associated with congenital heart disease. Catheter Cardiovasc Interv. 2006;67:113–119. doi: 10.1002/ccd.20555. [DOI] [PubMed] [Google Scholar]
- 3.Mangini M, Lagana D, Fontana F, et al. Use of Amplatzer Vascular Plug (AVP) in emergency embolisation: preliminary experience and review of literature. Emerg Radiol. 2008;15:153–160. doi: 10.1007/s10140-007-0696-8. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz M, Glatz AC, Rome JJ, Gillespie MJ. The Amplatzer vascular plug and Amplatzer Vascular Plug II for vascular occlusion procedures in 50 patients with congenital cardiovascular disease. Catheter Cardiovasc Interv. 2010;76:411–417. doi: 10.1002/ccd.22370. [DOI] [PubMed] [Google Scholar]
- 5.Tabori NE, Love BA. Transcatheter occlusion of pulmonary arteriovenous malformations using the Amplatzer Vascular Plug II. Catheter Cardiovasc Interv. 2008;71:940–943. doi: 10.1002/ccd.21474. [DOI] [PubMed] [Google Scholar]
- 6.Yildiz AE, Peynircioglu B, Cil BE. Applications of the Amplatzer Vascular Plug 4. Diagn Interv Radiol. 2012;18:225–230. doi: 10.4261/1305-3825.DIR.4410-11.1. [DOI] [PubMed] [Google Scholar]
- 7.Cho YK, Chang NK, Ma JS. Successful transcatheter closure of a large patent ductus venosus with the Amplatzer Vascular Plug II. Pediatr Cardiol. 2009;30:540–542. doi: 10.1007/s00246-009-9397-2. [DOI] [PubMed] [Google Scholar]
- 8.Vandy F, Criado E, Upchurch GR, Jr, Williams DM, Rectenwald J, Eliason J. Transluminal hypogastric artery occlusion with an Amplatzer Vascular Plug during endovascular aortic aneurysm repair. J Vasc Surg. 2008;48:1121–1124. doi: 10.1016/j.jvs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Mordasini P, Schroth G, Brekenfeld C, Fung C, Hoppe H, Gralla J. Endovascular treatment of a traumatic direct carotid-cavernous fistula using the Amplatzer Vascular Plug II. J Endovasc Ther. 2010;17:564–568. doi: 10.1583/10-3022.1. [DOI] [PubMed] [Google Scholar]
- 10.Hoit DA, Schirmer CM, Malek AM. Use of the Amplatzer Vascular Plug as an anchoring scaffold for coil-mediated parent vessel occlusion: technical case report. Neurosurgery. 2006;59:171–172. doi: 10.1227/01.NEU.0000219856.66842.EF. [DOI] [PubMed] [Google Scholar]
- 11.Shankar JJ, Maloney WJ, Vandorpe R. Amplatzer Vascular Plug for occlusion of parent artery in carotid blowout with active extravasation. Interv Neuroradiol. 2011;17:224–227. doi: 10.1177/159101991101700214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cil B, Peynircioglu B, Canyigit M, Geyik S, Ciftci T. Peripheral vascular applications of the Amplatzer Vascular Plug. Diagn Interv Radiol. 2008;14:35–39. [PubMed] [Google Scholar]
- 13.Yoo H, Ko GY, Gwon DI, et al. Preoperative portal vein embolization using an Amplatzer Vascular Plug. Eur Radiol. 2009;19:1054–1061. doi: 10.1007/s00330-008-1240-2. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie MJ, Golden A, Sivarajan VB, Rome JJ. Transcatheter closure of patent ductus venosus with the Amplatzer vascular plug in twin brothers. Pediatr Cardiol. 2006;27:142–145. doi: 10.1007/s00246-005-1081-6. [DOI] [PubMed] [Google Scholar]